Carbon-Based Thermal Management Solutions and Innovations for Improved Battery Safety: A Review
Abstract
1. Introduction
2. Fundamentals of Thermal Runaway and Fire Hazards in Batteries
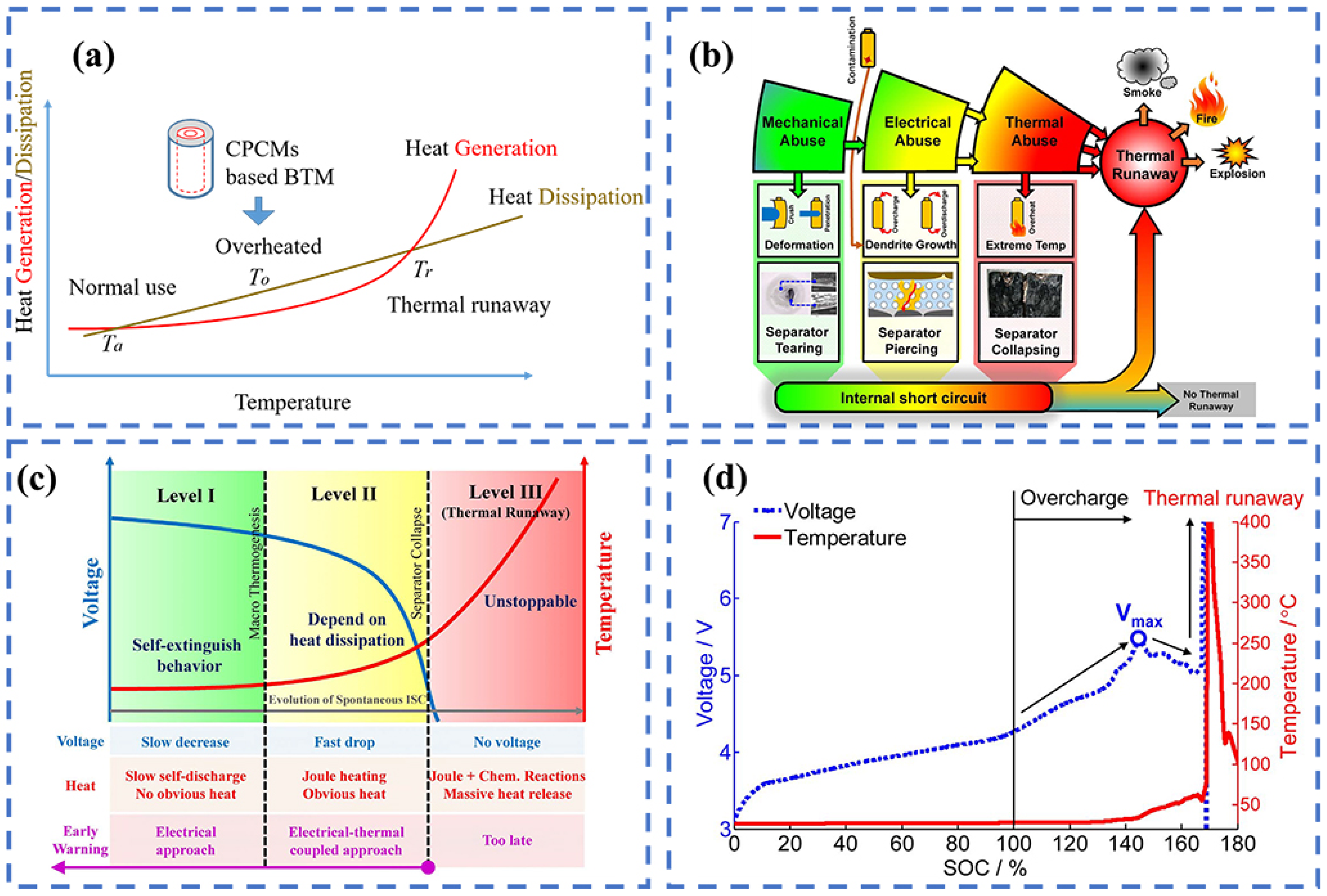
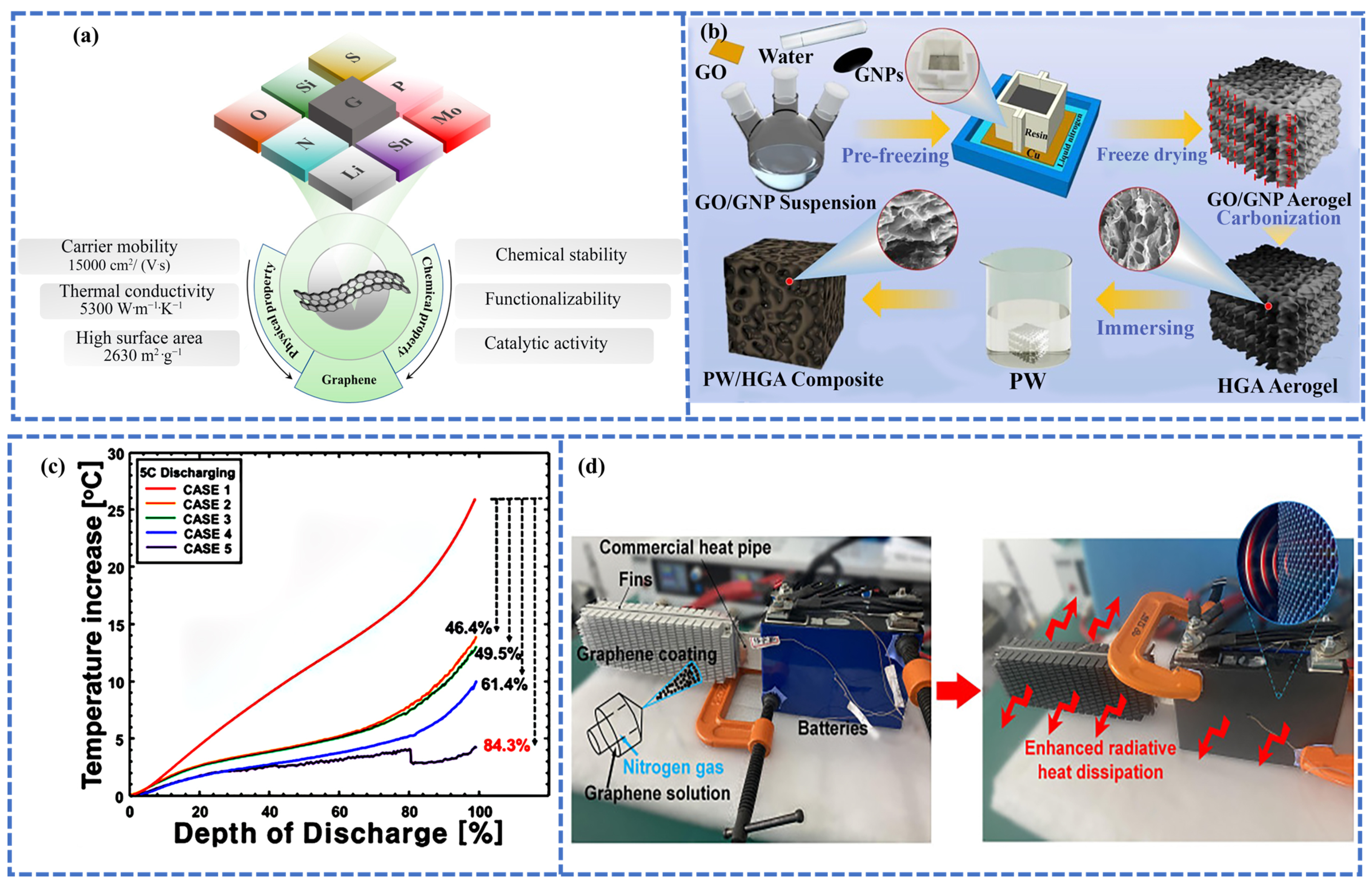
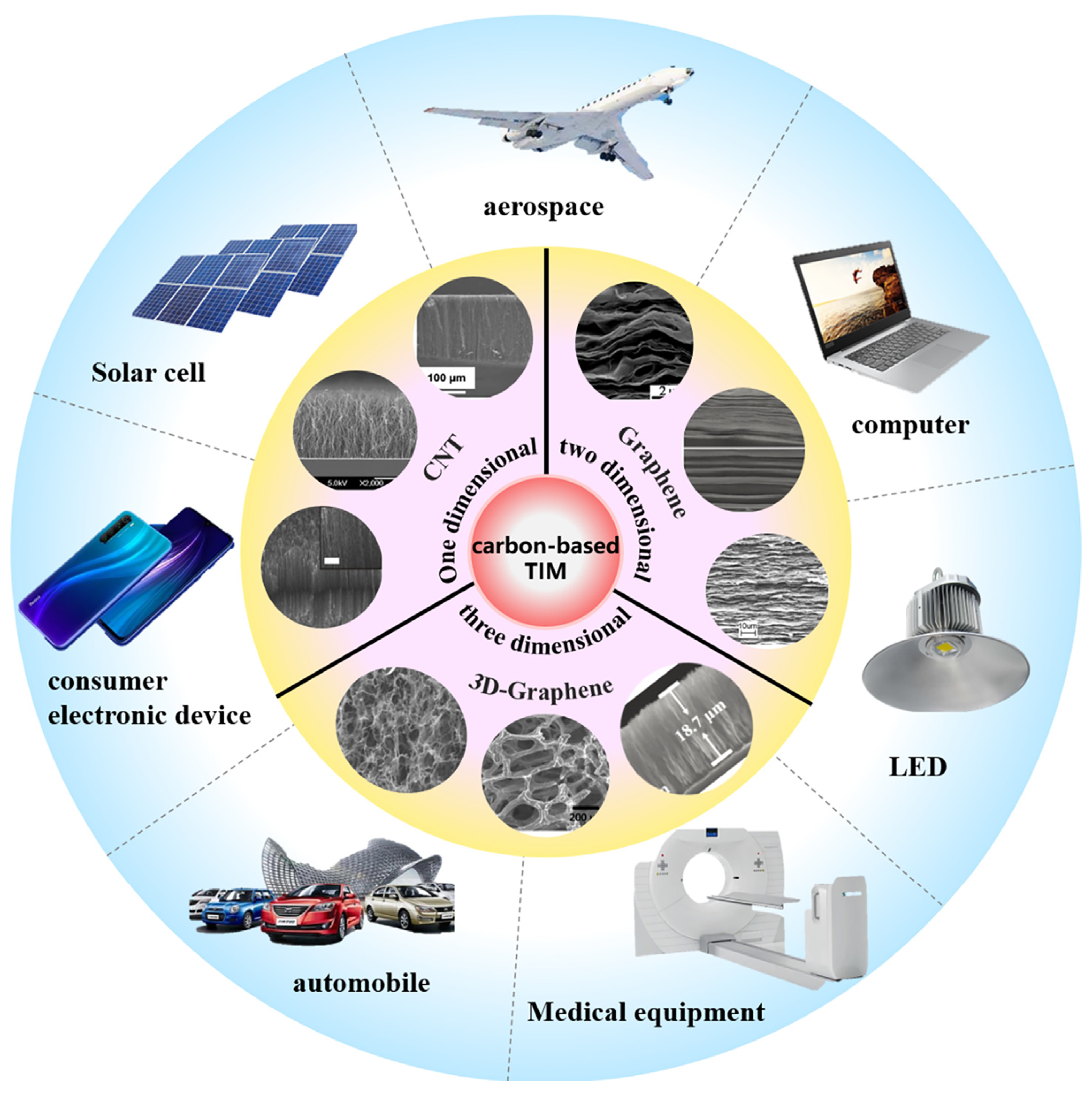
3. Carbon-Based Thermal Management Materials
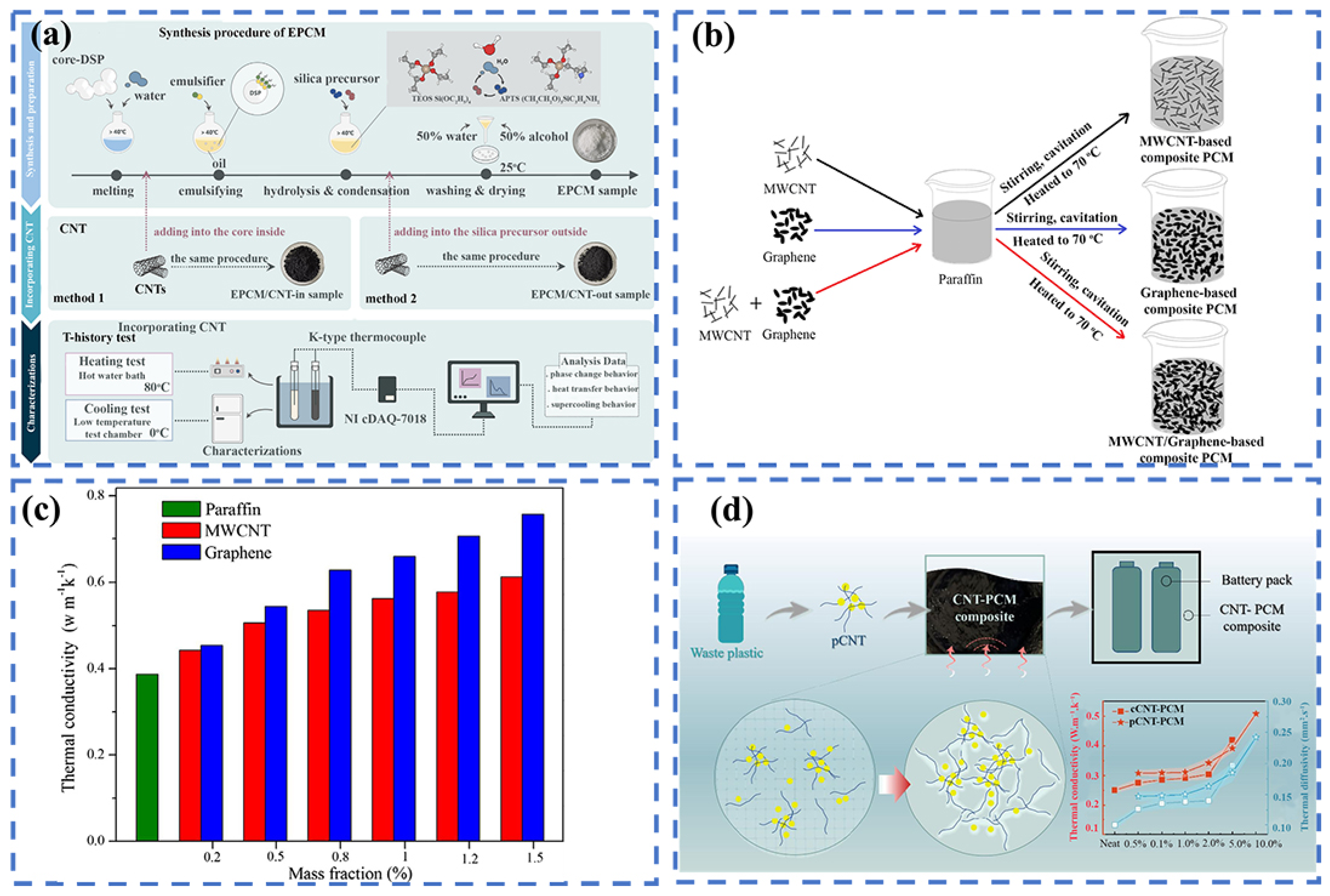
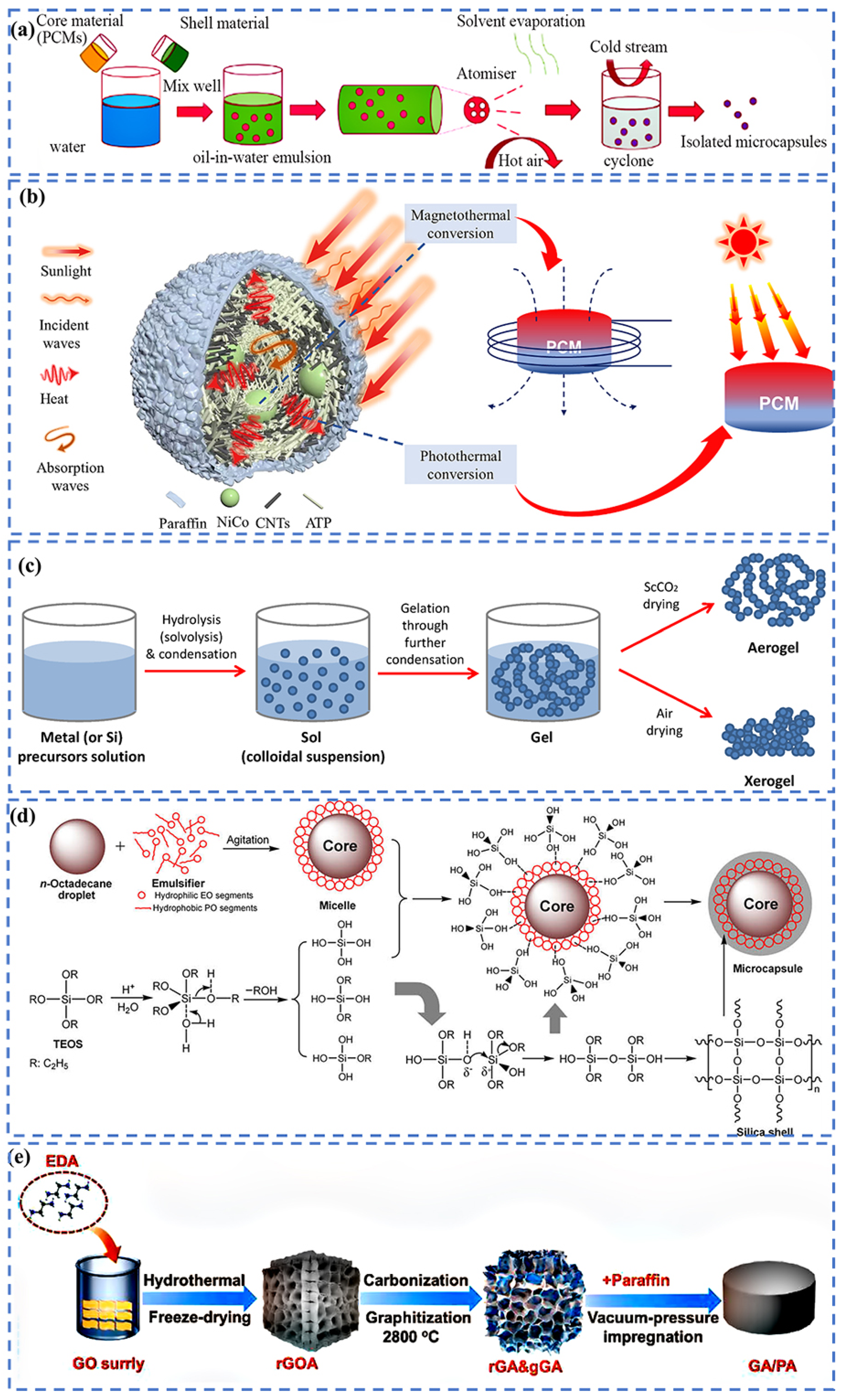
Fabrication Strategies of C-bMs for BTMS

4. Recent Innovations in C-bMs BTMS Solutions
4.1. Carbon-Based Phase Change Composites
| PCM | Type of C-bM | Thermal Conductivity | Latent Heat J/g | Melting Enthalpy | Solidification Enthalpy | Cycling Stability | Ref. |
|---|---|---|---|---|---|---|---|
| Liquid paraffin | MWCNT | 0.2 | - | 133.60 | 33.64 | - | [201] |
| CA-TD binary eutectic mixtures | Expanded graphite | 6.131 | 157.3 | 167.5 | 158.7 | 155.4 after 200 cycles | [202] |
| Polyethylene glycol (PEG) | Graphene oxide | 0.628 | 168.72 | 153.52 | - | 100 cycles | [203] |
| Stearic acid | NPC | 1.873 | 137.89 | 137.89 | 136.42 | - | [204] |
| Paraffin | Carbon fibre | 1.73 | 192.2 | 60.0 | 50.0 | 100 after 100 cycles | [205] |
| Paraffin | Graphene, EG carbon tube, | 5.1 | 131.9 | 178.5 | 171.5 | - | [206] |
4.2. Carbon-Based Heat Pipes
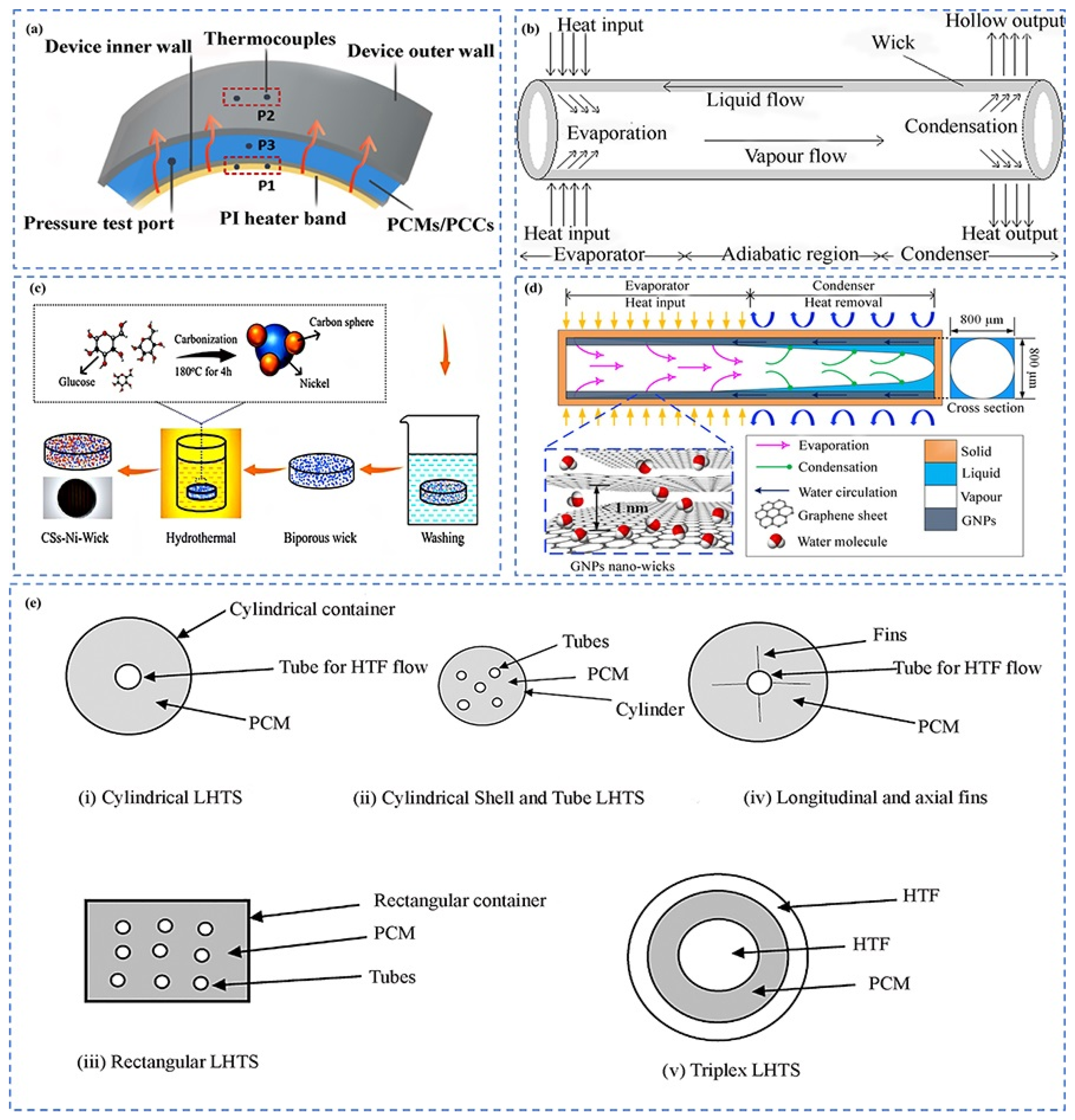
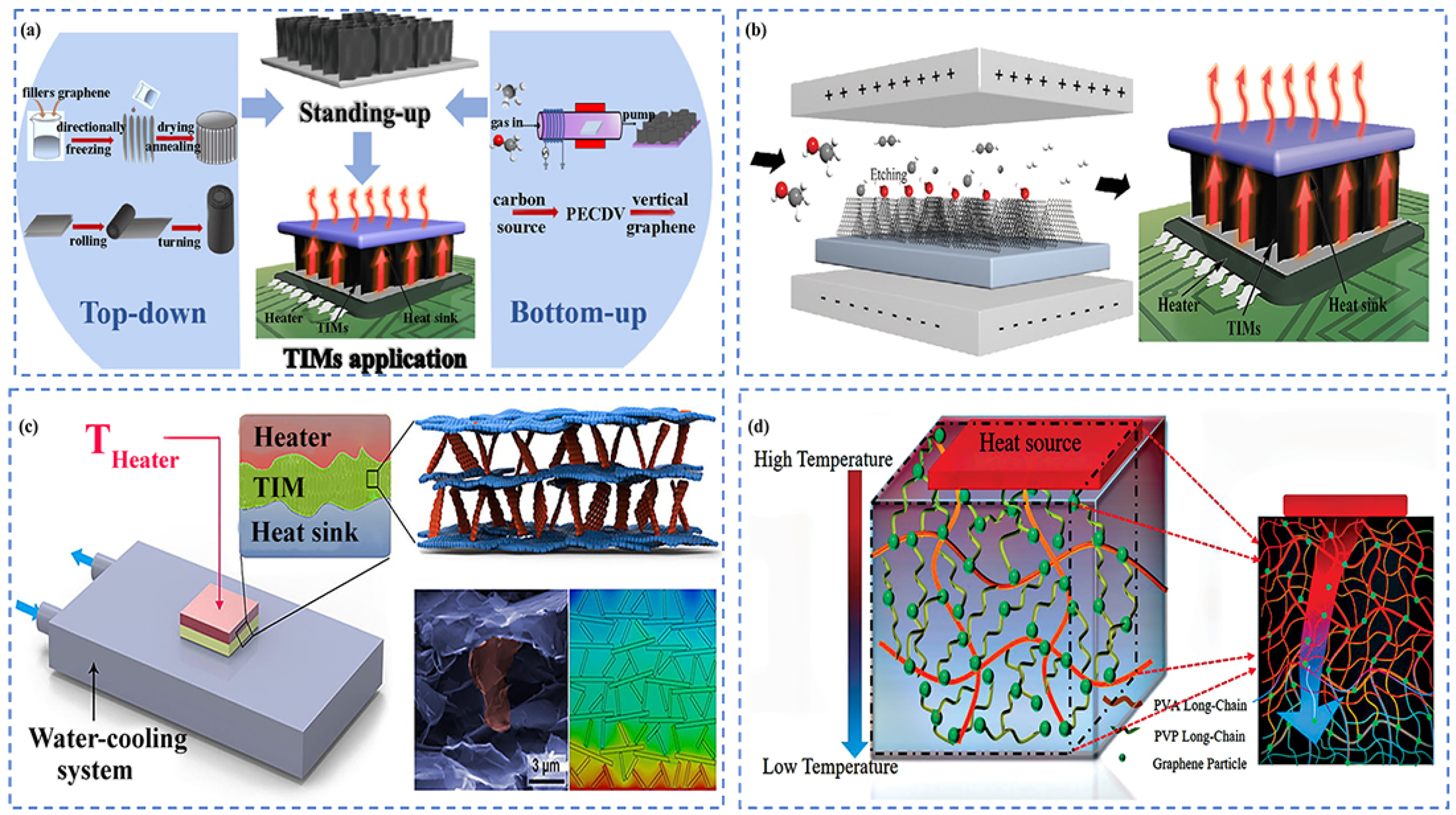
4.3. Carbon-Based Thermal Interface Materials
4.3.1. Graphene-Based TIMs
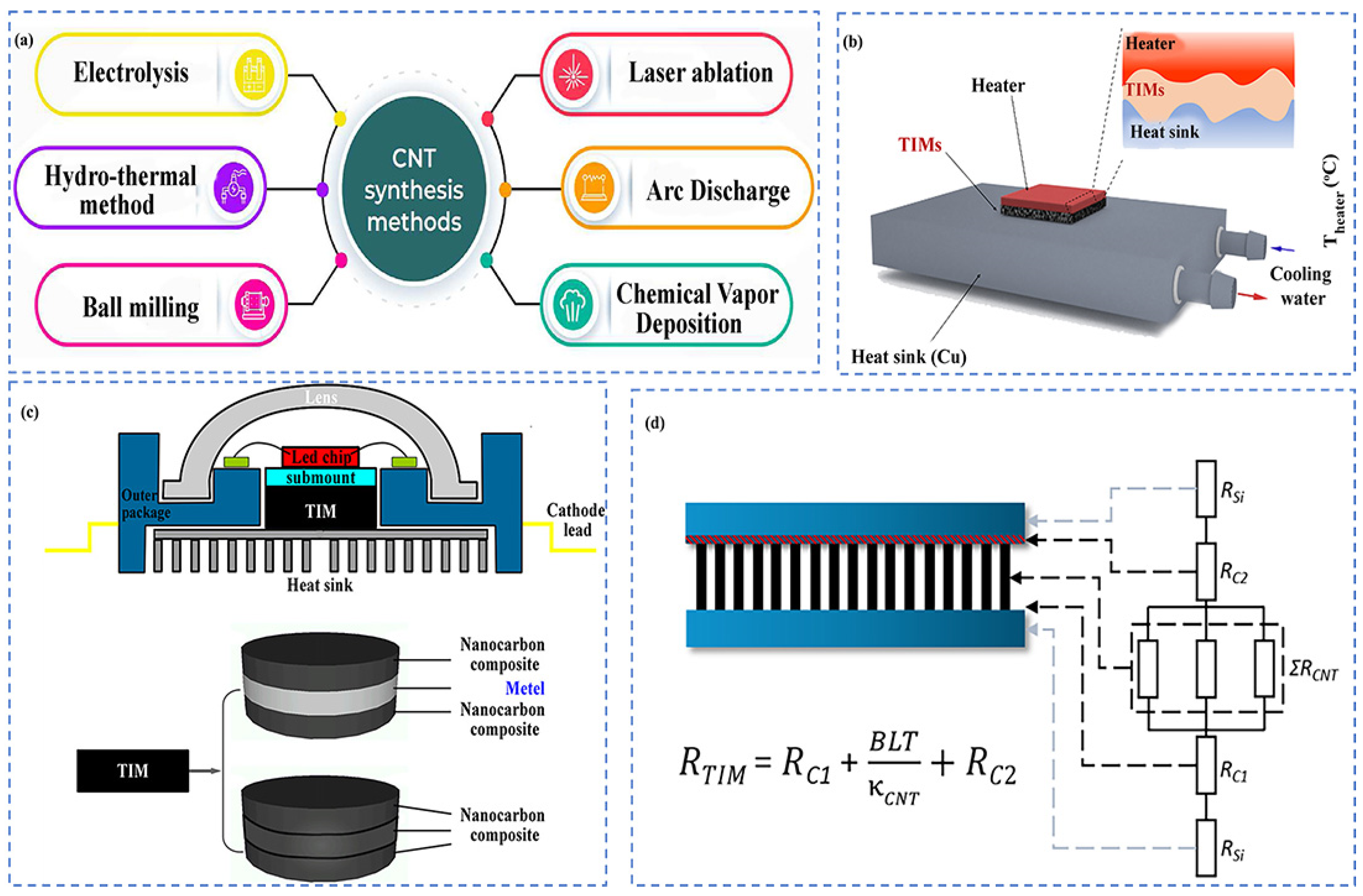
4.3.2. Carbon Nanotube (CNT)-Based TIMs
4.3.3. Composite-Based TIMs
5. Hybrid C-bMs Composites for BTMS
Integrated Thermal Management with C-bMs for ESS
6. Outlook and Research Direction
- (i)
- Sustainable and scalable manufacturing of C-bMs
- (ii)
- Multifunctional carbon-based thermal management solutions
- (iii)
- Self-healing C-bMs composites
- (iv)
- Seamless intelligent IoT C-bMs composites
- (v)
- Further exploration of uncommon C-bMs
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nikolaidis, P.; Poullikkas, A. Battery energy storage systems: A methodical enabler of reliable power. In Advanced Materials for Battery Separators; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–33. [Google Scholar]
- Bahloul, M.; Trivedi, R.; Cardo-Miota, J.; Papadimitriou, C.; Efthymiou, V.; Nouri, A.; Khadem, S. Analysis of barriers and key enablers toward citizen ESS successful integration. J. Energy Storage 2024, 86, 111166. [Google Scholar]
- Tawiah, B.; Seidu, R.K.; Asinyo, B.K.; Fei, B. A review of fiber-based supercapacitors and sensors for energy-autonomous systems. J. Power Sources 2024, 595, 234069. [Google Scholar] [CrossRef]
- Tawiah, B.; Ofori, E.A.; Chen, D.; Jia, H.; Fei, B. Sciento-qualitative study of zinc-iodine energy storage systems. J. Energy Storage 2024, 79, 110086. [Google Scholar]
- Gao, J.; Xie, Y.; Zeng, P.; Zhang, L. Strategies for Optimizing the Zn Anode/Electrolyte Interfaces Toward Stable Zn-Based Batteries. Small Methods 2023, 7, 2300855. [Google Scholar] [CrossRef]
- Duh, Y.-S.; Sun, Y.; Lin, X.; Zheng, J.; Wang, M.; Wang, Y.; Lin, X.; Jiang, X.; Zheng, Z.; Zheng, S. Characterization on thermal runaway of commercial 18650 lithium-ion batteries used in electric vehicles: A review. J. Energy Storage 2021, 41, 102888. [Google Scholar]
- Qin, P.; Jia, Z.; Wu, J.; Jin, K.; Duan, Q.; Jiang, L.; Sun, J.; Ding, J.; Shi, C.; Wang, Q. The thermal runaway analysis on LiFePO4 electrical energy storage packs with different venting areas and void volumes. Appl. Energy 2022, 313, 118767. [Google Scholar]
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems—A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Jeevarajan, J.A.; Joshi, T.; Parhizi, M.; Rauhala, T.; Juarez-Robles, D. Battery Hazards for Large Energy Storage Systems; ACS Publications: Washington, DC, USA, 2022. [Google Scholar]
- Zalosh, R.; Gandhi, P.; Barowy, A. Lithium-ion energy storage battery explosion incidents. J. Loss Prev. Process Ind. 2021, 72, 104560. [Google Scholar]
- The National Highway Traffic Safety Administration (NHTSA). Vehicle Recalls. 2023. Available online: https://www.nhtsa.gov/recalls (accessed on 25 June 2024).
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A Review of Battery Fires in Electric Vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Shi, C.; Hamann, T.; Takeuchi, S.; Alexander, G.V.; Nolan, A.M.; Limpert, M.; Fu, Z.; O’Neill, J.; Godbey, G.; Dura, J.A. 3D asymmetric bilayer garnet-hybridized high-energy-density lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2022, 15, 751–760. [Google Scholar]
- Thakur, A.K.; Ahmed, M.S.; Kang, H.; Prabakaran, R.; Said, Z.; Rahman, S.; Sathyamurthy, R.; Kim, J.; Hwang, J.Y. Critical review on internal and external battery thermal management systems for fast charging applications. Adv. Energy Mater. 2023, 13, 2202944. [Google Scholar] [CrossRef]
- Yue, Q.; He, C.; Wu, M.; Zhao, T. Advances in thermal management systems for next-generation power batteries. Int. J. Heat Mass Transf. 2021, 181, 121853. [Google Scholar] [CrossRef]
- Lohrasbi, S.; Hammer, R.; Essl, W.; Reiss, G.; Defregger, S.; Sanz, W. A comprehensive review on the core thermal management improvement concepts in power electronics. IEEE Access 2020, 8, 166880–166906. [Google Scholar] [CrossRef]
- Togun, H.; Aljibori, H.S.S.; Biswas, N.; Mohammed, H.I.; Sadeq, A.M.; Rashid, F.L.; Abdulrazzaq, T.; Zearah, S.A. A critical review on the efficient cooling strategy of batteries of electric vehicles: Advances, challenges, future perspectives. Renew. Sustain. Energy Rev. 2024, 203, 114732. [Google Scholar] [CrossRef]
- Liu, X.; Jia, H.; Li, H. Flame-retarding quasi-solid polymer electrolytes for high-safety lithium metal batteries. Energy Storage Mater. 2024, 67, 103263. [Google Scholar] [CrossRef]
- Mohammadian, S.K.; Zhang, Y. Thermal management optimization of an air-cooled Li-ion battery module using pin-fin heat sinks for hybrid electric vehicles. J. Power Sources 2015, 273, 431–439. [Google Scholar] [CrossRef]
- Widyantara, R.D.; Naufal, M.A.; Sambegoro, P.L.; Nurprasetio, I.P.; Triawan, F.; Djamari, D.W.; Nandiyanto, A.B.D.; Budiman, B.A.; Aziz, M. Low-cost air-cooling system optimization on battery pack of electric vehicle. Energies 2021, 14, 7954. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M.; Zhang, H. A review of air-cooling battery thermal management systems for electric and hybrid electric vehicles. J. Power Sources 2021, 501, 230001. [Google Scholar] [CrossRef]
- Teng, H.; Ma, Y.; Yeow, K.; Thelliez, M. An analysis of a lithium-ion battery system with indirect air cooling and warm-up. SAE Int. J. Passeng. Cars-Mech. Syst. 2011, 4, 1343–1357. [Google Scholar] [CrossRef]
- Ding, Y.; Ji, H.; Wei, M.; Liu, R. Effect of liquid cooling system structure on lithium-ion battery pack temperature fields. Int. J. Heat Mass Transf. 2022, 183, 122178. [Google Scholar] [CrossRef]
- Saw, L.; Tay, A.; Zhang, L.W. Thermal management of lithium-ion battery pack with liquid cooling. In Proceedings of the 2015 31st Thermal Measurement, Modeling & Management Symposium (SEMI-THERM), San Jose, CA, USA, 15–19 March 2015; pp. 298–302. [Google Scholar]
- Wang, Y.; Yu, Y.; Jing, Z.; Wang, C.; Zhou, G.; Zhao, W. Thermal performance of lithium-ion batteries applying forced air cooling with an improved aluminium foam heat sink design. Int. J. Heat Mass Transf. 2021, 167, 120827. [Google Scholar]
- Lee, N.; Choi, J.; Lee, J.; Shin, D.; Um, S. Prognostic analysis of thermal interface material effects on anisotropic heat transfer characteristics and state of health of a 21700 cylindrical lithium-ion battery module. J. Energy Storage 2023, 72, 108594. [Google Scholar]
- Maddila, R.V.; Rostami, S. Investigation of the Effect of Thermal Interface Materials on the Cooling of Battery Cells: A Comparative Study on the Heat Transfer Properties. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2023. [Google Scholar]
- Wang, X.; Liu, S.; Zhang, Y.; Lv, S.; Ni, H.; Deng, Y.; Yuan, Y. A review of the power battery thermal management system with different cooling, heating and coupling system. Energies 2022, 15, 1963. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase change materials application in battery thermal management system: A review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef] [PubMed]
- Jilte, R.; Afzal, A.; Panchal, S. A novel battery thermal management system using nano-enhanced phase change materials. Energy 2021, 219, 119564. [Google Scholar]
- Burchell, T.D. Carbon Materials for Advanced Technologies; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Jia, H.; Qiu, M.; Tang, C.; Liu, H.; Xu, J.; Tawiah, B.; Jiang, S.-X.; Zhang, X. Advanced flexible carbon-based current collector for zinc storage. Adv. Fiber Mater. 2022, 4, 1500–1510. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Li, P.; Yu, F. Lauric acid encapsulated in P-doped carbon matrix with reinforced heat storage performance for efficient battery cooling. J. Energy Storage 2021, 44, 103461. [Google Scholar]
- Hu, L.; An, Y.; Zhang, L.; Mai, L.; Ma, T.; An, Q.; Wang, Q. Shape-stabilized phase change material based on MOF-derived oriented carbon nanotubes for thermal management of lithium-ion battery. J. Energy Storage 2023, 72, 108520. [Google Scholar] [CrossRef]
- Adams, R.A.; Varma, A.; Pol, V.G. Carbon anodes for nonaqueous alkali metal-ion batteries and their thermal safety aspects. Adv. Energy Mater. 2019, 9, 1900550. [Google Scholar]
- Hwang, F.S.; Confrey, T.; Reidy, C.; Picovici, D.; Callaghan, D.; Culliton, D.; Nolan, C. Review of battery thermal management systems in electric vehicles. Renew. Sustain. Energy Rev. 2024, 192, 114171. [Google Scholar]
- Leoncini, G.; Mothier, R.; Michel, B.; Clausse, M. A review on challenges concerning thermal management system design for medium duty electric vehicles. Appl. Therm. Eng. 2024, 236, 121464. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, I.; Thakur, A.K.; Yu, S.; Lau, K.T.; He, S.; Dong, K.; Chen, J.; Li, X.; Ahmad, M. Advancements in battery thermal management system for fast charging/discharging applications. Energy Storage Mater. 2024, 65, 103144. [Google Scholar] [CrossRef]
- Quintiere, J.G. On a method to mitigate thermal runaway and propagation in packages of lithium ion batteries. Fire Saf. J. 2022, 130, 103573. [Google Scholar] [CrossRef]
- Niu, H.; Chen, C.; Ji, D.; Li, L.; Li, Z.; Liu, Y.; Huang, X. Thermal-Runaway Propagation over a Linear Cylindrical Battery Module. Fire Technol. 2020, 56, 2491–2507. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, L.; Xu, C.; Wu, H.; Huang, D.; Jin, K.; Xu, X. Study on Thermal Runaway Risk Prevention of Lithium-Ion Battery with Composite Phase Change Materials. Fire 2023, 6, 208. [Google Scholar] [CrossRef]
- Huang, J.; Fan, Z.; Xu, C.; Jiang, F.; Feng, X. Experimental Investigation of Thermal Runaway Characteristics of Large-Format Li(Ni0.8Co0.1Mn0.1)O2 Battery under Different Heating Powers and Areas. Batteries 2024, 10, 241. [Google Scholar] [CrossRef]
- Mallarapu, A.; Sunderlin, N.; Boovaragavan, V.; Tamashiro, M.; Peabody, C.; Pelloux-gervais, T.; Li, X.X.; Sizikov, G. Effects of Trigger Method on Fire Propagation during the Thermal Runaway Process in Li-ion Batteries. J. Electrochem. Soc. 2024, 171, 040514. [Google Scholar] [CrossRef]
- Liu, G.; Ouyang, M.; Lu, L.; Jianqiu, L.; Han, X. Analysis of the heat generation of lithium-ion battery during charging and discharging considering different influencing factors. J. Therm. Anal. Calorim. 2014, 116, 1001–1010. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, X.; Tran, M.-K.; Fowler, M.; Ouyang, M.; Burke, A.F. Battery safety: Fault diagnosis from laboratory to real world. J. Power Sources 2024, 598, 234111. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, B.; Li, B.; Yan, Y. A critical review of thermal management models and solutions of lithium-ion batteries for the development of pure electric vehicles. Renew. Sustain. Energy Rev. 2016, 64, 106–128. [Google Scholar] [CrossRef]
- Wu, T.; Wang, C.; Hu, Y.; Liang, Z.; Fan, C. Research on electrochemical characteristics and heat generating properties of power battery based on multi-time scales. Energy 2023, 265, 126416. [Google Scholar] [CrossRef]
- Wilke, S.; Schweitzer, B.; Khateeb Razack, S.A.; Al-Hallaj, S. Preventing thermal runaway propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- Chai, Z.; Li, J.; Liu, Z.; Liu, Z.; Jin, X. Comprehensive Modeling and Safety Protection Strategy for Thermal Runway Propagation in Lithium-Ion Battery Modules under Multi-Factor Influences. Batteries 2024, 10, 31. [Google Scholar] [CrossRef]
- Dai, X.; Ping, P.; Kong, D.; Gao, X.; Zhang, Y.; Wang, G.; Peng, R. Heat transfer enhanced inorganic phase change material compositing carbon nanotubes for battery thermal management and thermal runaway propagation mitigation. J. Energy Chem. 2024, 89, 226–238. [Google Scholar] [CrossRef]
- Duston, C.; Seghi, S.; Watts, R.; Carney, B. Strength Enhancement and Application Development of Carbon Foam for Thermal Management Systems; Work Sponsored under SBIR Contract; Defense Technical Information Center: Fort Belvoir, VA, USA, 2004; Available online: https://apps.dtic.mil/sti/citations/ADA457609 (accessed on 27 January 2025).
- Joo, S.-J.; Yu, M.-H.; Kim, W.S.; Kim, H.-S. Damage detection and self-healing of carbon fiber polypropylene (CFPP)/carbon nanotube (CNT) nano-composite via addressable conducting network. Compos. Sci. Technol. 2018, 167, 62–70. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Zhan, W.; Chen, Y.; Chen, M. Flame retardant composite phase change materials with MXene for lithium-ion battery thermal management systems. J. Energy Storage 2024, 86, 111293. [Google Scholar]
- Gulsoy, B.; Vincent, T.; Sansom, J.; Marco, J. In-situ temperature monitoring of a lithium-ion battery using an embedded thermocouple for smart battery applications. J. Energy Storage 2022, 54, 105260. [Google Scholar] [CrossRef]
- Zhong, L.; Guo, L.; Wang, J.; Song, Q.; Li, H.; Li, Y. Excellent heat transfer and mechanical properties of graphite material with rolled-up graphene layers. Carbon 2023, 208, 123–130. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Carbon-based functional nanomaterials: Preparation, properties and applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X. Thermal conductivity of carbon-based nanomaterials: Deep understanding of the structural effects. Green Carbon 2023, 1, 47–57. [Google Scholar] [CrossRef]
- Slepičková Kasálková, N.; Slepička, P.; Švorčík, V. Carbon Nanostructures, Nanolayers, and Their Composites. Nanomaterials 2021, 11, 2368. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef]
- Jarosinski, L.; Rybak, A.; Gaska, K.; Kmita, G.; Porebska, R.; Kapusta, C. Enhanced thermal conductivity of graphene nanoplatelets epoxy composites. Mater. Sci. Pol. 2017, 35, 382–389. [Google Scholar] [CrossRef]
- Karimi, D.; Behi, H.; Van Mierlo, J.; Berecibar, M. An Experimental Study on Thermal Performance of Graphite-Based Phase-Change Materials for High-Power Batteries. Energies 2022, 15, 2515. [Google Scholar] [CrossRef]
- Teboho Clement, M.; Mokgaotsa Jonas, M.; Jeremia Shale, S.; Setumo Victor, M.; Dickson Mubera, A. Thermal conductivity of graphite-based polymer composites. In Impact of Thermal Conductivity on Energy Technologies; Aamir, S., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 11. [Google Scholar]
- Zhang, L.; Deng, K.K.; Nie, K.B.; Wang, C.J.; Xu, C.; Shi, Q.X.; Liu, Y.; Wang, J. Thermal conductivity and mechanical properties of graphite/Mg composite with a super-nano CaCO(3) interfacial layer. iScience 2023, 26, 106505. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Liu, L.; Liang, J.; Liu, W. Polymer/expanded graphite-based flexible phase change material with high thermal conductivity for battery thermal management. J. Clean. Prod. 2022, 331, 130014. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, J.; Fu, Y.; Cao, M.; Liu, M. Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Wang, Z.; Du, C.; Qi, R.; Wang, Y. Experimental study on thermal management of lithium-ion battery with graphite powder based composite phase change materials covering the whole climatic range. Appl. Therm. Eng. 2022, 216, 119072. [Google Scholar] [CrossRef]
- Talluri, T.; Angani, A.; Shin, K.; Hwang, M.-H.; Cha, H.-R. A novel design of lithium-polymer pouch battery pack with passive thermal management for electric vehicles. Energy 2024, 304, 132205. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, S.; Tang, K.; Xu, Y.; Tang, Y.; Ma, Y.; Li, T. A biomimetic melting-evaporation cooling bilayer for efficient thermal management of ultrafast-cycling batteries. Energy Storage Mater. 2024, 71, 103602. [Google Scholar] [CrossRef]
- Yan, J.; Li, K.; Chen, H.; Wang, Q.; Sun, J. Experimental study on the application of phase change material in the dynamic cycling of battery pack system. Energy Convers. Manag. 2016, 128, 12–19. [Google Scholar] [CrossRef]
- Wang, S.; Xing, Y.; Hao, Z.; Yin, J.; Hou, X.; Wang, Z. Experimental study on the thermal performance of PCMs based heat sink using higher alcohol/graphite foam. Appl. Therm. Eng. 2021, 198, 117452. [Google Scholar] [CrossRef]
- Wu, W.; Wu, W.; Wang, S. Thermal optimization of composite PCM based large-format lithium-ion battery modules under extreme operating conditions. Energy Convers. Manag. 2017, 153, 22–33. [Google Scholar] [CrossRef]
- Zhao, X.; Li, C.; Bai, K.; Xie, B.; Chen, J.; Liu, Q. Multiple structure graphite stabilized stearic acid as composite phase change materials for thermal energy storage. Int. J. Min. Sci. Technol. 2022, 32, 1419–1428. [Google Scholar] [CrossRef]
- Menaa, F.; Fatemeh, Y.; Vashist, S.K.; Iqbal, H.; Sharts, O.N.; Menaa, B. Graphene, an Interesting Nanocarbon Allotrope for Biosensing Applications: Advances, Insights, and Prospects. Biomed. Eng. Comput. Biol. 2021, 12, 1179597220983821. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; khan, A.U. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Yusaf, T.; Mahamude, A.S.; Farhana, K.; Harun, W.S.; Kadirgama, K.; Ramasamy, D.; Kamarulzaman, M.K.; Subramonian, S.; Hall, S.; Dhahad, H.A. A Comprehensive Review on Graphene Nanoparticles: Preparation, Properties, and Applications. Sustainability 2022, 14, 12336. [Google Scholar] [CrossRef]
- Du, Y.; Wang, M.; Ye, X.; Liu, B.; Han, L.; Jafri, S.H.; Liu, W.; Zheng, X.; Ning, Y.; Li, H. Advances in the Field of Graphene-Based Composites for Energy–Storage Applications. Crystals 2023, 13, 912. [Google Scholar] [CrossRef]
- Zhu, Q.; Ong, P.J.; Goh, S.H.A.; Yeo, R.J.; Wang, S.; Liu, Z.; Loh, X.J. Recent advances in graphene-based phase change composites for thermal energy storage and management. Nano Mater. Sci. 2024, 6, 115–138. [Google Scholar] [CrossRef]
- Wu, S.; Cao, S.; Xie, H.; Wu, Z.; He, X. Enhanced thermal performance of 3D hybrid graphene aerogel encapsulating paraffin for battery thermal management. Int. Commun. Heat Mass Transf. 2024, 156, 107618. [Google Scholar] [CrossRef]
- Jung, E.; Kong, D.; Kang, M.; Park, J.; Kim, J.-H.; Jeong, J.; In, J.B.; Oh, K.-Y.; Lee, H. Enhanced immersion cooling using laser-induced graphene for Li-ion battery thermal management. Int. Commun. Heat Mass Transf. 2024, 155, 107558. [Google Scholar] [CrossRef]
- Liu, Y.; Thiringer, T.; Wang, N.; Fu, Y.; Lu, H.; Liu, J. Graphene based thermal management system for battery cooling in electric vehicles. In Proceedings of the 2020 IEEE 8th Electronics System-Integration Technology Conference (ESTC), Tonsberg, Norway, 15–18 September 2020; pp. 1–4. [Google Scholar]
- Wang, J.-X.; Salmean, C.; Li, J.-X.; Lei, C.; Li, J.; Zhong, M.; Qi, B.; Mao, Y. A nano-sheet graphene-based enhanced thermal radiation composite for passive heat dissipation from vehicle batteries. Nano Mater. Sci. 2024, 6, 443–455. [Google Scholar] [CrossRef]
- Goli, P.; Legedza, S.; Dhar, A.; Salgado, R.; Renteria, J.; Balandin, A.A. Graphene-enhanced hybrid phase change materials for thermal management of Li-ion batteries. J. Power Sources 2014, 248, 37–43. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, R.K.; Khalid, M.; Goyal, R.; Sarı, A.; Tyagi, V.V. Evaluation of carbon based-supporting materials for developing form-stable organic phase change materials for thermal energy storage: A review. Sol. Energy Mater. Sol. Cells 2022, 246, 111896. [Google Scholar] [CrossRef]
- Xing, M.; Yu, J.; Wang, R. Experimental study on the thermal conductivity enhancement of water based nanofluids using different types of carbon nanotubes. Int. J. Heat Mass Transf. 2015, 88, 609–616. [Google Scholar] [CrossRef]
- Razeeb, K.M.; Dalton, E.; Cross, G.L.W.; Robinson, A.J. Present and future thermal interface materials for electronic devices. Int. Mater. Rev. 2018, 63, 1–21. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Jin, H.; Wang, J.; Li, Q. Carbon Nanotube-Derived Materials for Smart Thermal Management. Adv. Sustain. Syst. 2025, 9, 2400757. [Google Scholar]
- Liu, P.; Chen, X.; Li, Y.; Cheng, P.; Tang, Z.; Lv, J.; Aftab, W.; Wang, G. Aerogels meet phase change materials: Fundamentals, advances, and beyond. ACS Nano 2022, 16, 15586–15626. [Google Scholar] [CrossRef]
- Zou, D.; Ma, X.; Liu, X.; Zheng, P.; Hu, Y. Thermal performance enhancement of composite phase change materials (PCM) using graphene and carbon nanotubes as additives for the potential application in lithium-ion power battery. Int. J. Heat Mass Transf. 2018, 120, 33–41. [Google Scholar] [CrossRef]
- Wang, Y.; Bailey, J.; Zhu, Y.; Zhang, Y.; Boetcher, S.K.S.; Li, Y.; Wu, C. Application of carbon nanotube prepared from waste plastic to phase change materials: The potential for battery thermal management. Waste Manag. 2022, 154, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J. Sebacic acid/CNT sponge phase change material with excellent thermal conductivity and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2018, 179, 217–222. [Google Scholar] [CrossRef]
- Sahoo, R.; Chaudhuri, P.; Nayak, A.K. Introduction to different types of 2D carbon and nanodiamond. In Diamane; IOP Publishing: Bristol, UK, 2024; pp. 1-1–1-29. [Google Scholar]
- Shang, B.; Yang, G.; Zhang, B. Phase change nanocapsules incorporated with nanodiamonds for efficient photothermal energy conversion and storage. Appl. Energy 2024, 360, 122806. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, M.; Li, H.; Du, Y.; Bu, Q.; Cao, L.; Zong, C. Mussel inspired modification of nanodiamonds for thermally conductive, and electrically insulating rubber composites. Diam. Relat. Mater. 2022, 130, 109457. [Google Scholar] [CrossRef]
- Nan, B.; Wu, K.; Qu, Z.; Xiao, L.; Xu, C.; Shi, J.; Lu, M. A multifunctional thermal management paper based on functionalized graphene oxide nanosheets decorated with nanodiamond. Carbon 2020, 161, 132–145. [Google Scholar] [CrossRef]
- Wei, Z.; Gong, P.; Kong, X.; Li, M.; Cheng, J.; Zhou, H.; Li, D.; Ye, Y.; Lu, X.; Yu, J.; et al. Enhanced Thermal Conductivity of Nanodiamond Nanosheets/Polymer Nanofiber Composite Films by Uniaxial and Coaxial Electrospinning: Implications for Thermal Management of Nanodevices. ACS Appl. Nano Mater. 2023, 6, 8358–8366. [Google Scholar] [CrossRef]
- Gong, P.; Li, L.; Fu, G.-e.; Shu, S.; Li, M.; Wang, Y.; Qin, Y.; Kong, X.; Chen, H.; Jiao, C.; et al. Highly flexible cellulose nanofiber/single-crystal nanodiamond flake heat spreader films for heat dissipation. J. Mater. Chem. C 2022, 10, 12070–12079. [Google Scholar] [CrossRef]
- Kang, J.; Wei, Z.; Li, J. Graphyne and Its Family: Recent Theoretical Advances. ACS Appl. Mater. Interfaces 2019, 11, 2692–2706. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, N.; Yu, R.; Zhang, Y.; Zhang, J.; Li, Y.; Wang, D. Unique structural advances of graphdiyne for energy applications. EnergyChem 2020, 2, 100041. [Google Scholar] [CrossRef]
- Dang, X.; Zhao, H. Graphdiyne: A promising 2D all-carbon nanomaterial for sensing and biosensing. TrAC Trends Anal. Chem. 2021, 137, 116194. [Google Scholar] [CrossRef]
- Ivanovskii, A.L. Graphynes and graphdyines. Prog. Solid State Chem. 2013, 41, 1–19. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Y.; Qi, L.; Xue, Y.; Li, Y. 2D graphdiyne: An emerging carbon material. Chem. Soc. Rev. 2022, 51, 2681–2709. [Google Scholar] [CrossRef]
- Peng, Q.; Dearden, A.K.; Crean, J.; Han, L.; Liu, S.; Wen, X.; De, S. New materials graphyne, graphdiyne, graphone, and graphane: Review of properties, synthesis, and application in nanotechnology. Nanotechnol. Sci. Appl. 2014, 7, 1–29. [Google Scholar] [CrossRef]
- Yang, F.; Xin, Y.; Zhu, X.; Tang, A.; Yu, L.; Han, D.; Jia, J.; Lu, Y.; Zhang, Z. Hard Template-Assisted Trans-Crystallization Synthesis of Hierarchically Porous Cu-SSZ-13 with Enhanced NH3-SCR Performance. Catalysts 2023, 13, 1217. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S.; Tian, N.; Yan, F.; You, C.; Yang, Y. Carbon foams: 3D porous carbon materials holding immense potential. J. Mater. Chem. A 2020, 8, 23699–23723. [Google Scholar] [CrossRef]
- Jana, P.; Palomo del Barrio, E.; Fierro, V.; Medjahdi, G.; Celzard, A. Design of carbon foams for seasonal solar thermal energy storage. Carbon 2016, 109, 771–787. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Yan, X.; Dou, B.; Zhang, D.; Cui, G.; Yang, Q. Composite phase change materials with carbon foam and fibre combination for efficient battery thermal management: Dual modulation roles of interfacial heat transfer. J. Mater. Res. Technol. 2023, 23, 551–563. [Google Scholar]
- Zhao, L. Purification of Engineered Graphite for Advanced Application. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2022. [Google Scholar]
- Malik, M.; Dincer, I.; Rosen, M.A. Review on use of phase change materials in battery thermal management for electric and hybrid electric vehicles. Int. J. Energy Res. 2016, 40, 1011–1031. [Google Scholar] [CrossRef]
- Fu, Y.; Hansson, J.; Liu, Y.; Chen, S.; Zehri, A.; Samani, M.K.; Wang, N.; Ni, Y.; Zhang, Y.; Zhang, Z.-B. 2D Materials Graphene related materials for thermal management Graphene related materials for thermal management. 2D Materials 2019. [Google Scholar] [CrossRef]
- Gspann, T.S.; Juckes, S.M.; Niven, J.F.; Johnson, M.B.; Elliott, J.A.; White, M.A.; Windle, A.H. High thermal conductivities of carbon nanotube films and micro-fibres and their dependence on morphology. Carbon 2017, 114, 160–168. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, S.; Cai, W.; Zhang, Y.; Zhang, X.-A. A review of carbon-based thermal interface materials: Mechanism, thermal measurements and thermal properties. Mater. Des. 2021, 209, 109936. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Kondrin, M.V. Chapter Six—High-pressure, high-temperature synthesis and doping of nanodiamonds. In Semiconductors and Semimetals; Nebel, C.E., Aharonovich, I., Mizuochi, N., Hatano, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 103, pp. 161–199. [Google Scholar]
- Zhang, Y.; Choi, J.R.; Park, S.-J. Thermal conductivity and thermo-physical properties of nanodiamond-attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. Part A Appl. Sci. Manuf. 2017, 101, 227–236. [Google Scholar] [CrossRef]
- Jana, P.; Fierro, V.; Pizzi, A.P.; Celzard, A. Thermal conductivity improvement of composite carbon foams based on tannin-based disordered carbon matrix and graphite fillers. Mater. Des. 2015, 83, 635–643. [Google Scholar] [CrossRef]
- Nada, S.A.; Alshaer, W.G. Comprehensive parametric study of using carbon foam structures saturated with PCMs in thermal management of electronic systems. Energy Convers. Manag. 2015, 105, 93–102. [Google Scholar] [CrossRef]
- Park, S.-J.; Heo, G.-Y. Precursors and Manufacturing of Carbon Fibers; Springer Series in Materials Science; Springer: Dordrecht, The Netherland, 2015; Volume 210, pp. 31–66. [Google Scholar] [CrossRef]
- Ali, Z.; Gao, Y.; Tang, B.; Wu, X.; Wang, Y.; Li, M.; Xiao, H.; Li, L.; Jiang, N.; Yu, J. Preparation, Properties and Mechanisms of Carbon Fiber/Polymer Composites for Thermal Management Applications. Polymers 2021, 13, 169. [Google Scholar] [CrossRef]
- Maiti, S.; Islam, M.R.; Uddin, M.A.; Afroj, S.; Eichhorn, S.J.; Karim, N. Sustainable Fiber-Reinforced Composites: A Review. Adv. Sustain. Syst. 2022, 6, 2200258. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Verdejo, R.; Bernal, M.M.; Romasanta, L.J.; Lopez-Manchado, M.A. Graphene filled polymer nanocomposites. J. Mater. Chem. 2011, 21, 3301–3310. [Google Scholar] [CrossRef]
- Verma, D.; Goh, K.L. Chapter 11—Functionalized Graphene-Based Nanocomposites for Energy Applications. In Functionalized Graphene Nanocomposites and Their Derivatives; Jawaid, M., Bouhfid, R., Kacem Qaiss, A.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–243. [Google Scholar]
- Al Faruque, M.A.; Syduzzaman, M.; Sarkar, J.; Bilisik, K.; Naebe, M. A Review on the Production Methods and Applications of Graphene-Based Materials. Nanomaterials 2021, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Han, J.; Wang, M.; Chen, X.; Jia, S. Electrophoretic deposition of graphene-based materials: A review of materials and their applications. J. Mater. 2018, 4, 108–120. [Google Scholar] [CrossRef]
- González, Z.; Pérez-Mas, A.M.; Blanco, C.; Granda, M.; Santamaría, R. Influence of the electrophoretic deposition parameters on the formation of suspended graphene-based films. Mater. Des. 2018, 160, 58–64. [Google Scholar] [CrossRef]
- Kumanek, B.; Pusz, S.; Trzebicka, B. Review: Tailoring the properties of macroporous carbon foams. J. Mater. Sci. 2014, 49, 1–17. [Google Scholar] [CrossRef]
- Wang, R.; Yu, J.; Islam, F.; Tahmasebi, A.; Lee, S.; Chen, Y. State-of-the-Art Research and Applications of Carbon Foam Composite Materials as Electrodes for High-Capacity Lithium Batteries. Energy Fuels 2020, 34, 7935–7954. [Google Scholar] [CrossRef]
- Bussiba, A.; Gilad, I.; Lugassi, S.; David, S.; Bortman, J.; Yosibash, Z. Mechanical Response and Fracture of Pultruded Carbon Fiber/Epoxy in Various Modes of Loading. Crystals 2022, 12, 850. [Google Scholar] [CrossRef]
- Volk, M.; Yuksel, O.; Baran, I.; Hattel, J.H.; Spangenberg, J.; Sandberg, M. Cost-efficient, automated, and sustainable composite profile manufacture: A review of the state of the art, innovations, and future of pultrusion technologies. Compos. Part B Eng. 2022, 246, 110135. [Google Scholar] [CrossRef]
- Vedernikov, A.; Safonov, A.; Tucci, F.; Carlone, P.; Akhatov, I. Pultruded materials and structures: A review. J. Compos. Mater. 2020, 54, 002199832092289. [Google Scholar] [CrossRef]
- Stiller, J.H.M.; Roder, K.; Löpitz, D.; Knobloch, M.; Nestler, D.; Drossel, W.-G.; Kroll, L. Combining Pultrusion with Carbonization: Process Analysis and Material Properties of CFRP and C/C. Ceramics 2023, 6, 330–341. [Google Scholar] [CrossRef]
- Shrivastava, A. 5—Plastics Processing. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: New York, NY, USA, 2018; pp. 143–177. [Google Scholar]
- Riedel, U. 10.18—Biocomposites: Long Natural Fiber-Reinforced Biopolymers. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 295–315. [Google Scholar]
- Acquah, C.; Datskov, I.; Mawardi, A.; Zhang, F.; Achenie, L.; Pitchumani, R.; Santos, E. Optimization under uncertainty of a composite fabrication process using a deterministic one-stage approach. Comput. Chem. Eng. 2006, 30, 947–960. [Google Scholar] [CrossRef]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Developments in polyester composite materials—An in-depth review on natural fibres and nano fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Boon, Y.D.; Joshi, S.C.; Bhudolia, S.K. Review: Filament Winding and Automated Fiber Placement with In Situ Consolidation for Fiber Reinforced Thermoplastic Polymer Composites. Polymers 2021, 13, 1951. [Google Scholar] [CrossRef]
- Srebrenkoska, S.; Kochoski, F.; Srebrenkoska, V.; Risteska, S.; Kotynia, R. Effect of Process Parameters on Thermal and Mechanical Properties of Filament Wound Polymer-Based Composite Pipes. Polymers 2023, 15, 2829. [Google Scholar] [CrossRef]
- Mertiny, P.; Ellyin, F.; Hothan, A. An experimental investigation on the effect of multi-angle filament winding on the strength of tubular composite structures. Compos. Sci. Technol. 2004, 64, 1–9. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A. Flexural and impact behaviour of carbon/basalt fibers hybrid laminates. J. Compos. Mater. 2013, 48, 1121–1130. [Google Scholar] [CrossRef]
- Risteska, S.; Samakoski, B.; Stefanovska, M. Properties of composite trapezoidal parts manufactured with help of filament winding technology using Taguchi method. Int. J. Eng. Res. Technol. 2014, 3, 250–255. [Google Scholar]
- Boccaccini, A.R.; Cho, J.; Subhani, T.; Kaya, C.; Kaya, F. Electrophoretic deposition of carbon nanotube–ceramic nanocomposites. J. Eur. Ceram. Soc. 2010, 30, 1115–1129. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Shahalizade, T.; Riahifar, R.; Yaghmaee, M.S.; Raissi, B.; Gholam, S.; Aghaei, A.; Rahimisheikh, S.; Ghazvini, A.S. Electrophoretic deposition as a fabrication method for Li-ion battery electrodes and separators—A review. J. Power Sources 2022, 535, 231448. [Google Scholar] [CrossRef]
- Sa’adati, H.; Raissi, B.; Riahifar, R.; Yaghmaee, M.S. How preparation of suspensions affects the electrophoretic deposition phenomenon. J. Eur. Ceram. Soc. 2016, 36, 299–305. [Google Scholar] [CrossRef]
- Lalau, C.C.; Low, C.T.J. Electrophoretic Deposition for Lithium-Ion Battery Electrode Manufacture. Batter. Supercaps 2019, 2, 551–559. [Google Scholar] [CrossRef]
- Chakrabarti, B.K.; Gençten, M.; Bree, G.; Dao, A.H.; Mandler, D.; Low, C.T.J. Modern practices in electrophoretic deposition to manufacture energy storage electrodes. Int. J. Energy Res. 2022, 46, 13205–13250. [Google Scholar] [CrossRef]
- Arinova, A.; Kalimuldina, G.; Nurpeissova, A.; Bakenov, Z. Electrophoretic Deposition of Poly(ethylene oxide) Gel-Polymer Electrolyte for 3D NiO/Ni Foam Anode Based Lithium-Ion Batteries. J. Electrochem. Soc. 2023, 170, 100501. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q.; Shang, J.-K.; Xu, J.; Li, Y.-X. Advances in the synthesis of mesoporous carbon nitride materials. Acta Phys. Chim. Sin. 2016, 32, 1913–1928. [Google Scholar]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated mesoporous carbons: Synthesis and applications. Carbon 2016, 107, 448–473. [Google Scholar] [CrossRef]
- Wang, C.; Yan, B.; Zheng, J.; Feng, L.; Chen, Z.; Zhang, Q.; Liao, T.; Chen, J.; Jiang, S.; Du, C.; et al. Recent progress in template-assisted synthesis of porous carbons for supercapacitors. Adv. Powder Mater. 2022, 1, 100018. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis Strategies of Porous Carbon for Supercapacitor Applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, R.; Bhatia, G.; Verma, G.L. Development of mesophase pitch derived high thermal conductivity graphite foam using a template method. Carbon 2011, 49, 3622–3630. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Wei, Y.; Yan, W.; Feng, J. Preparation of Templated Materials and Their Application to Typical Pollutants in Wastewater: A Review. Front. Chem. 2022, 10, 882876. [Google Scholar] [CrossRef]
- Keyhani-Asl, A.; Perera, N.; Lahr, J.; Hasan, R. Porous media and foam application in battery thermal management systems: A comprehensive review focused on its impact, numerical modeling, and experimental preparation. J. Energy Storage 2024, 93, 112306. [Google Scholar] [CrossRef]
- Pavlenko, V.; Khosravi, H.S.; Żółtowska, S.; Haruna, A.B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.I.; Abbas, Q.; et al. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.; Zhou, Y.; Qiao, H.; Gurung, A.; Naderi, R.; Elbohy, H.; Smirnova, A.L.; Lu, H.; Chen, S.; et al. Binder Free Hierarchical Mesoporous Carbon Foam for High Performance Lithium Ion Battery. Sci. Rep. 2017, 7, 1440. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Liu, L.; Wang, Y. Template-directed fabrication of 3D graphene-based composite and their electrochemical energy-related applications. Sci. Bull. 2016, 61, 443–450. [Google Scholar]
- Sequino, L.; Sebastianelli, G.; Vaglieco, B.M. Carbon and Graphene Coatings for the Thermal Management of Sustainable LMP Batteries for Automotive Applications. Materials 2022, 15, 7744. [Google Scholar] [CrossRef]
- Song, N.; Cao, D.; Luo, X.; Wang, Q.; Ding, P.; Shi, L. Highly thermally conductive polypropylene/graphene composites for thermal management. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105912. [Google Scholar] [CrossRef]
- Ke, F.; Song, F.; Zhang, H.; Xu, J.; Wang, H.; Chen, Y. Layer-by-layer assembly for all-graphene coated conductive fibers toward superior temperature sensitivity and humidity independence. Compos. Part B Eng. 2020, 200, 108253. [Google Scholar] [CrossRef]
- Nan, B.; Zhan, Y.; Xu, C.-A. A review on the thermal conductivity properties of polymer/nanodiamond nanocomposites. Polym. Plast. Technol. Mater. 2023, 62, 486–509. [Google Scholar]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar]
- Khan, J.; Momin, S.A.; Mariatti, M. A review on advanced carbon-based thermal interface materials for electronic devices. Carbon 2020, 168, 65–112. [Google Scholar] [CrossRef]
- Dhumal, A.R.; Kulkarni, A.P.; Ambhore, N.H. A comprehensive review on thermal management of electronic devices. J. Eng. Appl. Sci. 2023, 70, 140. [Google Scholar] [CrossRef]
- Bianco, V.; De Rosa, M.; Vafai, K. Phase-change materials for thermal management of electronic devices. Appl. Therm. Eng. 2022, 214, 118839. [Google Scholar] [CrossRef]
- Samykano, M. Role of phase change materials in thermal energy storage: Potential, recent progress and technical challenges. Sustain. Energy Technol. Assess. 2022, 52, 102234. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Li, D.; Zuo, X.; Yang, H. Multifunctional composite phase change materials: Preparation, enhanced properties and applications. Compos. Part A Appl. Sci. Manuf. 2024, 185, 108331. [Google Scholar] [CrossRef]
- Tien Nguyen, G.; Minh Tam, L.; Thi Nhung, T. Using Carbonized Cotton Fabric Waste to Prepare Poly(ethylene glycol) Composite Phase Change Materials with Improved Thermal Conductivity and Solar-to-Thermal Conversion. ACS Omega 2024, 9, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wan, Y.; Gao, Y.; Dong, C.; Chen, X. Polypyrrole-coated expanded graphite-based phase change materials for photothermal energy storage. Mater. Today Nano 2023, 23, 100376. [Google Scholar] [CrossRef]
- Nartowska, E.; Styś-Maniara, M.; Kozłowski, T. The Potential Environmental and Social Influence of the Inorganic Salt Hydrates Used as a Phase Change Material for Thermal Energy Storage in Solar Installations. Int. J. Environ. Res. Public Health 2023, 20, 1331. [Google Scholar] [CrossRef]
- Rashid, F.L.; Al-Obaidi, M.A.; Dulaimi, A.; Bernardo, L.F.; Redha, Z.A.; Hoshi, H.A.; Mahood, H.B.; Hashim, A. Recent Advances on The Applications of Phase Change Materials in Cold Thermal Energy Storage: A Critical Review. J. Compos. Sci. 2023, 7, 338. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A. A review on phase change materials (PCMs) for thermal energy storage implementations. Mater. Today Proc. 2022, 58, 1360–1367. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.K.; Ansu, A.K.; Goyal, R.; Sarı, A.; Tyagi, V.V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Ramadan, M.; Atiqure Rahman, S.M.; Abdelkareem, M.A. Recent progress on Carbon-based nanomaterial for phase change materials: Prospects and challenges. Therm. Sci. Eng. Prog. 2021, 23, 100920. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Jia, L.; Zhu, X.; Qin, G.; Chen, Y. Preparation and characterization of Na2HPO4·12H2O@polymethyl methacrylate nanocapsule for efficient thermal energy storage. J. Energy Storage 2022, 53, 105133. [Google Scholar] [CrossRef]
- Choo, Y.M.; Wei, W. Salt hydrates as phase change materials for photovoltaics thermal management. Energy Sci. Eng. 2022, 10, 1630–1642. [Google Scholar] [CrossRef]
- Adeel Hassan, H.M.; Lund, I. Inorganic PCMs applications in passive cooling of buildings—A review. J. Phys. Conf. Ser. 2021, 2116, 012103. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Dinker, A.; Agarwal, M.; Agarwal, G.D. Heat storage materials, geometry and applications: A review. J. Energy Inst. 2017, 90, 1–11. [Google Scholar] [CrossRef]
- Afdhol, M.K.; Abdurrahman, M.; Hidayat, F.; Chong, F.K.; Mohd Zaid, H.F. Review of Solvents Based on Biomass for Mitigation of Wax Paraffin in Indonesian Oilfield. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Amir Reza, V. Paraffin as Phase Change Material. In Paraffin; Fathi Samir, S., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 5. [Google Scholar]
- Magendran, S.S.; Khan, F.S.A.; Mubarak, N.M.; Vaka, M.; Walvekar, R.; Khalid, M.; Abdullah, E.C.; Nizamuddin, S.; Karri, R.R. Synthesis of organic phase change materials (PCM) for energy storage applications: A review. Nano-Struct. Nano-Objects 2019, 20, 100399. [Google Scholar] [CrossRef]
- Gandhi, M.; Kumar, A.; Elangovan, R.; Meena, C.S.; Kulkarni, K.S.; Kumar, A.; Bhanot, G.; Kapoor, N.R. A Review on Shape-Stabilized Phase Change Materials for Latent Energy Storage in Buildings. Sustainability 2020, 12, 9481. [Google Scholar] [CrossRef]
- Radouane, N. A Comprehensive Review of Composite Phase Change Materials (cPCMs) for Thermal Management Applications, Including Manufacturing Processes, Performance, and Applications. Energies 2022, 15, 8271. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Ding, Y. Preparation and properties of composite phase change material based on solar heat storage system. J. Energy Storage 2021, 40, 102805. [Google Scholar] [CrossRef]
- Song, J.; He, H.; Wang, Y.; Shao, L.; Wang, Q.; Wei, Q.; Cai, Y. Shape-stabilized phase change composites supported by biomass loofah sponge-derived microtubular carbon scaffold toward thermal energy storage and electric-to-thermal conversion. J. Energy Storage 2022, 56, 105891. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Kumar, N.; Hirschey, J.; LaClair, T.J.; Gluesenkamp, K.R.; Graham, S. Review of stability and thermal conductivity enhancements for salt hydrates. J. Energy Storage 2019, 24, 100794. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.; Zainal, Z.; Rusli, R. Encapsulation techniques for organic phase change materials as ther-mal energy storage medium: A review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Ma, S.; Sun, Y.; Li, C.; Li, R.; Li, C. Form-Stable phase change composites with high thermal conductivity and enthalpy enabled by Graphene/Carbon nanotubes aerogel skeleton for thermal energy storage. Appl. Therm. Eng. 2024, 255, 123954. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Li, J.; Li, T.; Cao, M.; Qi, W.; Wu, Z.; Li, Y.; Li, B. Dual-encapsulated phase change composites with hierarchical MXene-graphene monoliths in graphene foam for high-efficiency thermal management and electromagnetic interference shielding. Compos. Part B Eng. 2023, 266, 110998. [Google Scholar] [CrossRef]
- Fu, X.; Pan, H.; Xu, L.; Wang, M.; Dou, M.; Zhang, Y.; Liu, Z.; Huang, X.; Teng, Y.; Hu, L.; et al. Flexible phase change film based on lignin-derived porous carbon/Eicosane for wearable thermal management and microwave absorption. Int. J. Biol. Macromol. 2024, 275, 133630. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation methods for phase change materials—A critical review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Li, D.; Tang, Y.; Zuo, X.; Zhang, X.; Zhao, X.; Zhang, Y.; Yang, H. Functionally constructed magnetic-dielectric mineral microspheres for efficient thermal energy storage and microwave absorption. J. Colloid Interface Sci. 2023, 650, 764–774. [Google Scholar] [CrossRef]
- Mahdavian, F.; Allahbakhsh, A.; Rodrigue, D.; Bahramian, A.R. Polyethylene glycol-impregnated carbon quantum dots-phenolic phase change composites for highly efficient thermal energy storage. Carbon 2024, 219, 118840. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.-Q.; Jia, H.; Xie, L.-J.; Tao, Z.-C.; Yang, X.; Liu, D.; Sun, G.-H.; Guo, Q.-G.; Lu, C.-X.; Chen, C.-M. Ultra-high temperature graphitization of three-dimensional large-sized graphene aerogel for the encapsulation of phase change materials. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106391. [Google Scholar] [CrossRef]
- Tao, Y.; Pescarmona, P.P. Nanostructured Oxides Synthesised via scCO2-Assisted Sol-Gel Methods and Their Application in Catalysis. Catalysts 2018, 8, 212. [Google Scholar] [CrossRef]
- Kumaresan, V.; Velraj, R.; Das, S.K. The effect of carbon nanotubes in enhancing the thermal transport properties of PCM during solidification. Heat Mass Transf. 2012, 48, 1345–1355. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Li, L.; Gu, M.; Li, Y.; Cheng, X. Modulation and optimisation of the properties of n-decanoic acid-tetradecanol phase change materials by nanocomposite carbon materials prepared by atomic layer deposition methods. Mater. Today Commun. 2024, 39, 108650. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, B.; Wang, S.; Xue, B.; Liu, S.; An, M.; Yang, Z.; Xu, G. Effect of GO on the Structure and Properties of PEG/Biochar Phase Change Composites. Polymers 2023, 15, 963. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, R.; Zuo, H.; Zuo, Q.; Luo, X.; Chen, Y.; Wu, S.; Chen, W. N-doped EG@MOFs derived porous carbon composite phase change materials for thermal optimization of Li-ion batteries at low temperature. Energy 2024, 286, 129637. [Google Scholar] [CrossRef]
- Sheng, N.; Rao, Z.; Zhu, C.; Habazaki, H. Honeycomb carbon fibers strengthened composite phase change materials for superior thermal energy storage. Appl. Therm. Eng. 2020, 164, 114493. [Google Scholar] [CrossRef]
- Zou, D.; Liu, X.; He, R.; Zhu, S.; Bao, J.; Guo, J.; Hu, Z.; Wang, B. Preparation of a novel composite phase change material (PCM) and its locally enhanced heat transfer for power battery module. Energy Convers. Manag. 2019, 180, 1196–1202. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W.; Du, R.; Wang, S.; Han, H.; Jing, Y.; Wu, S.; Wang, R.; Li, T. Carbon-based phase change composites with directional high thermal conductivity for interface thermal management. Chem. Eng. J. 2024, 496, 154305. [Google Scholar] [CrossRef]
- Rajeswari, K.; Suganthi, K.; Thiruvenkatam, S.; Devaraj, S.; Rajan, K. Graphene oxide–adipic acid nanocomposites for thermal energy storage: Assessment of thermophysical properties and energy storage performance. J. Energy Storage 2024, 77, 109949. [Google Scholar]
- Wang, Y.; Tang, B.; Zhang, S. Single-Walled Carbon Nanotube/Phase Change Material Composites: Sunlight-Driven, Reversible, Form-Stable Phase Transitions for Solar Thermal Energy Storage. Adv. Funct. Mater. 2013, 23, 4354–4360. [Google Scholar] [CrossRef]
- Pathak, S.K.; Kumar, R.; Goel, V.; Pandey, A.K.; Tyagi, V.V. Recent advancements in thermal performance of nano-fluids charged heat pipes used for thermal management applications: A comprehensive review. Appl. Therm. Eng. 2022, 216, 119023. [Google Scholar] [CrossRef]
- Sikora, K. Use of Waste Heat Using Heat Pipes. Acta Mech. Slovaca 2023, 27, 54–59. [Google Scholar] [CrossRef]
- Bernagozzi, M.; Georgoulas, A.; Miché, N.; Marengo, M. Heat pipes in battery thermal management systems for electric vehicles: A critical review. Appl. Therm. Eng. 2023, 219, 119495. [Google Scholar] [CrossRef]
- Pagliarini, L.; Iwata, N.; Bozzoli, F. Pulsating heat pipes: Critical review on different experimental techniques. Exp. Therm. Fluid Sci. 2023, 148, 110980. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X.; Ji, X.; Yang, X.; Wei, J. Performance improvement of loop heat pipe by micro-pin-fins/powders composite surface. Int. J. Heat Mass Transf. 2023, 208, 124093. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Fu, Y.; Wang, N.; Mencarelli, D.; Pierantoni, L.; Lu, H.; Liu, J. A lightweight and high thermal performance graphene heat pipe. Nano Sel. 2021, 2, 364–372. [Google Scholar] [CrossRef]
- Zohuri, B. Heat Pipe Design and Technology: Modern Applications for Practical Thermal Management; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Vadakkan, U.; Chrysler, G.M.; Maveety, J.; Tirumala, M. A Novel Carbon Nano Tube based Wick Structure for Heat Pipes/Vapor Chambers. In Proceedings of the Twenty-Third Annual IEEE Semiconductor Thermal Measurement and Management Symposium, San Jose, CA, USA, 18–22 March 2007; pp. 102–104. [Google Scholar]
- Rassamakin, B.; Khairnasov, S.; Zaripov, V.; Rassamakin, A.; Alforova, O. Aluminum heat pipes applied in solar collectors. Sol. Energy 2013, 94, 145–154. [Google Scholar] [CrossRef]
- Wang, Q.; Han, X.H.; Sommers, A.; T’Joen, C.; Jacobi, A. A review on application of carbonaceous materials and carbon matrix composites for heat exchangers and heat sinks. Int. J. Refrig. 2012, 35, 7–26. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, S.; Zhang, H.; Yao, W.; Liu, C. Excellent heat dissipation properties of the super-aligned carbon nanotube films. RSC Adv. 2016, 6, 61686–61694. [Google Scholar] [CrossRef]
- Gan, J.S.; Hung, Y.M. Remarkable Thermal Performance Enhancement of Micro Heat Pipes with Graphene-Nanoplatelet Nano-Wicks. Nanomaterials 2023, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Tan, Y.; Zhang, Z.; Liu, W.; Liu, Z. Experimental investigation on the heat transfer characteristics of loop heat pipe with carbon spheres modified nickel wick. Appl. Therm. Eng. 2024, 255, 123956. [Google Scholar] [CrossRef]
- Chan, C.W.; Siqueiros, E.; Ling-Chin, J.; Royapoor, M.; Roskilly, A.P. Heat utilisation technologies: A critical review of heat pipes. Renew. Sustain. Energy Rev. 2015, 50, 615–627. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wang, J.; Luan, T.; Chen, H.; Xue, H. Experimental study on the characteristics of loop heat pipe with modified carbon fiber felt wick. Appl. Therm. Eng. 2023, 234, 121239. [Google Scholar] [CrossRef]
- Sreekumar, E.N.; Senthil Saravanan, M.S. A review on Aluminium based thermal interface materials for heat transfer application. Mater. Today Proc. 2023, 72, 3036–3039. [Google Scholar] [CrossRef]
- Chung, S.-H.; Kim, H.; Jeong, S.W. Improved thermal conductivity of carbon-based thermal interface materials by high-magnetic-field alignment. Carbon 2018, 140, 24–29. [Google Scholar] [CrossRef]
- Naghibi, S.; Kargar, F.; Wright, D.; Huang, C.Y.T.; Mohammadzadeh, A.; Barani, Z.; Salgado, R.; Balandin, A.A. Noncuring Graphene Thermal Interface Materials for Advanced Electronics. Adv. Electron. Mater. 2020, 6, 1901303. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J. Vertically aligned graphene for thermal interface materials. Small Struct. 2020, 1, 2000034. [Google Scholar]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Botlhoko, O.; Setshedi, K.; Scriba, M.; Masukume, M.; Sinha Ray, S. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180–5204. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Murugesan, M.; Martinson, K.; Nikos, A.; Zhou, T.; Zhang, H.; Almhem, L.; Liu, J. High Performance Graphene Enhanced Thermal Interface Technology for Electronics Cooling Applications. IEEE Electron. Packag. Soc. 2022. Available online: https://eps.ieee.org/publications/enews/december-2022/956-high-performance-graphene-enhanced-thermal-interface-technology-for-electronics-cooling-applications.html (accessed on 27 January 2025).
- Xu, S.; Wang, S.; Chen, Z.; Sun, Y.; Gao, Z.; Zhang, H.; Zhang, J. Electric-Field-Assisted Growth of Vertical Graphene Arrays and the Application in Thermal Interface Materials. Adv. Funct. Mater. 2020, 30, 2003302. [Google Scholar] [CrossRef]
- Gao, J.; Yan, Q.; Lv, L.; Tan, X.; Ying, J.; Yang, K.; Yu, J.; Du, S.; Wei, Q.; Xiang, R.; et al. Lightweight thermal interface materials based on hierarchically structured graphene paper with superior through-plane thermal conductivity. Chem. Eng. J. 2021, 419, 129609. [Google Scholar] [CrossRef]
- Ma, J.; Du, W.; Chen, Z.; Wang, W.; Zhang, L. Preparation of Graphene-Based Hydrogel Thermal Interface Materials with Excellent Heat Dissipation and Mechanical Properties. Macromol. Mater. Eng. 2023, 308, 2200332. [Google Scholar] [CrossRef]
- Zhang, X.; Yeung, K.K.; Gao, Z.; Li, J.; Sun, H.; Xu, H.; Zhang, K.; Zhang, M.; Chen, Z.; Yuen, M.M.F.; et al. Exceptional thermal interface properties of a three-dimensional graphene foam. Carbon 2014, 66, 201–209. [Google Scholar] [CrossRef]
- Yun, J.; Lee, J.; Kim, J.; Lee, J.; Choi, W. Hexagonal boron nitride nanosheets/graphene nanoplatelets/cellulose nanofibers-based multifunctional thermal interface materials enabling electromagnetic interference shielding and electrical insulation. Carbon 2024, 228, 119397. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, K.; Liu, X.; Li, K.; Lu, J.; Cai, S.; Liu, Y.; Xu, Z.; Gao, C. Environment-adaptive, anti-fatigue thermal interface graphene foam. Carbon 2023, 212, 118142. [Google Scholar] [CrossRef]
- Lv, P.; Tan, X.-W.; Yu, K.-H.; Zheng, R.-L.; Zheng, J.-J.; Wei, W. Super-elastic graphene/carbon nanotube aerogel: A novel thermal interface material with highly thermal transport properties. Carbon 2016, 99, 222–228. [Google Scholar] [CrossRef]
- Lu, J.; Ming, X.; Cao, M.; Liu, Y.; Wang, B.; Shi, H.; Hao, Y.; Zhang, P.; Li, K.; Wang, L.; et al. Scalable Compliant Graphene Fiber-Based Thermal Interface Material with Metal-Level Thermal Conductivity via Dual-Field Synergistic Alignment Engineering. ACS Nano 2024, 18, 18560–18571. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Qin, M.; Zhang, H.; Yue, J.; Peng, L.; Liu, G.; Feng, Y.; Feng, W. Patterned liquid metal embedded in brush-shaped polymers for dynamic thermal management. Mater. Horiz. 2024, 11, 531–544. [Google Scholar] [CrossRef]
- Ali, A.; Rahimian Koloor, S.S.; Alshehri, A.H.; Arockiarajan, A. Carbon nanotube characteristics and enhancement effects on the mechanical features of polymer-based materials and structures—A review. J. Mater. Res. Technol. 2023, 24, 6495–6521. [Google Scholar] [CrossRef]
- Zhan, H.; Chen, Y.W.; Shi, Q.; Zhang, Y.; Mo, R.; Wang, J. Highly Aligned and Densified Carbon Nanotube Films with Superior Thermal Conductivity and Mechanical Strength. Carbon 2021, 186, 205–214. [Google Scholar] [CrossRef]
- Nylander, A.; Hansson, J.; Nilsson, T.; Ye, L.; Fu, Y.; Liu, J. Degradation of Carbon Nanotube Array Thermal Interface Materials through Thermal Aging: Effects of Bonding, Array Height, and Catalyst Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 30992–31000. [Google Scholar] [CrossRef]
- Bowland, C.C.; Wang, Y.; Naskar, A.K. Development of nanoparticle embedded sizing for enhanced structural health monitoring of carbon fiber composites. Proc. SPIE 2017, 101690L. [Google Scholar] [CrossRef]
- Zhang, K.; Chai, Y.; Yuen, M.M.F.; Xiao, D.G.W.; Chan, P.C.H. Carbon nanotube thermal interface material for high-brightness light-emitting-diode cooling. Nanotechnology 2008, 19, 215706. [Google Scholar] [CrossRef]
- Guo, H.; Liu, J.; Wang, Q.; Liu, M.; Du, C.; Li, B.; Feng, L. High thermal conductive poly(vinylidene fluoride)-based composites with well-dispersed carbon nanotubes/graphene three-dimensional network structure via reduced interfacial thermal resistance. Compos. Sci. Technol. 2019, 181, 107713. [Google Scholar] [CrossRef]
- Yu, H.; Peng, L.; Chen, C.; Qin, M.; Feng, W. Regulatable Orthotropic 3D Hybrid Continuous Carbon Networks for Efficient Bi-Directional Thermal Conduction. Nano-Micro Lett. 2024, 16, 198. [Google Scholar] [CrossRef]
- Bahru, R.; Mohamed, A. Effect of Carbon Nanotubes as Thermal Interface Materials on Thermal Conductivity Using Electrophoretic Deposition. J. Phys. Sci. 2019, 30, 149–158. [Google Scholar] [CrossRef]
- Zhang, G.; Song, M.; Li, Z.; Zhao, P.; Gu, Z.; Wang, H.; Xu, Y.; Wang, M. A novel heat dissipation material for high-brightness light-emitting-diode devices. Mater. Chem. Phys. 2013, 139, 741–746. [Google Scholar] [CrossRef]
- Xing, Y.; Cao, W.; Li, W.; Chen, H.; Wang, M.; Wei, H.; Hu, D.; Chen, M.; Li, Q. Carbon Nanotube/Cu Nanowires/Epoxy Composite Mats with Improved Thermal and Electrical Conductivity. J. Nanosci. Nanotechnol. 2015, 15, 3265–3270. [Google Scholar] [CrossRef]
- Bahru, R.; Mohamed, M.A. Enhancement of thermal interface material properties using carbon nanotubes through simple electrophoretic deposition method. Int. J. Energy Res. 2020, 44, 4944–4960. [Google Scholar] [CrossRef]
- Kalnaus, S.; Asp, L.E.; Li, J.; Veith, G.M.; Nanda, J.; Daniel, C.; Chen, X.C.; Westover, A.; Dudney, N.J. Multifunctional approaches for safe structural batteries. J. Energy Storage 2021, 40, 102747. [Google Scholar] [CrossRef]
- Kaul, P.B.; Bifano, M.F.P.; Prakash, V. Multifunctional carbon nanotube–epoxy composites for thermal energy management. J. Compos. Mater. 2013, 47, 77–95. [Google Scholar] [CrossRef]
- Baltopoulos, A.; Polydorides, N.; Pambaguian, L.; Vavouliotis, A.; Kostopoulos, V. Exploiting carbon nanotube networks for damage assessment of fiber reinforced composites. Compos. Part B Eng. 2015, 76, 149–158. [Google Scholar] [CrossRef]
- Li, Y.; Diao, X.; Li, P.; Liu, P.; Gao, Y.; Zhao, Z.; Chen, X.; Wang, G. Advanced multifunctional Co/N co-doped carbon foam-based phase change materials for wearable thermal management. Chem. Eng. J. 2024, 485, 149858. [Google Scholar] [CrossRef]
- He, H.; Dong, M.; Wang, Q.; Zhang, J.; Feng, Q.; Wei, Q.; Cai, Y. A multifunctional carbon-base phase change composite inspired by “fruit growth”. Carbon 2023, 205, 499–509. [Google Scholar] [CrossRef]
- Shivram, S.; Harish, R. Impact of dual nano-enhanced phase change materials on mitigating thermal runaway in lithium-ion battery cell. Case Stud. Therm. Eng. 2024, 60, 104667. [Google Scholar] [CrossRef]
- Rana, S.; Zahid, H.; Kumar, R.; Bharj, R.S.; Rathore, P.K.S.; Ali, H.M. Lithium-ion battery thermal management system using MWCNT-based nanofluid flowing through parallel distributed channels: An experimental investigation. J. Energy Storage 2024, 81, 110372. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Z.; Yin, X.; Ling, G. Avoiding thermal runaway propagation of lithium-ion battery modules by using hybrid phase change material and liquid cooling. Appl. Therm. Eng. 2021, 184, 116380. [Google Scholar] [CrossRef]
- Weng, J.; He, Y.; Ouyang, D.; Yang, X.; Chen, M.; Cui, S.; Zhang, G.; Yuen, R.K.K.; Wang, J. Honeycomb-inspired design of a thermal management module and its mitigation effect on thermal runaway propagation. Appl. Therm. Eng. 2021, 195, 117147. [Google Scholar] [CrossRef]
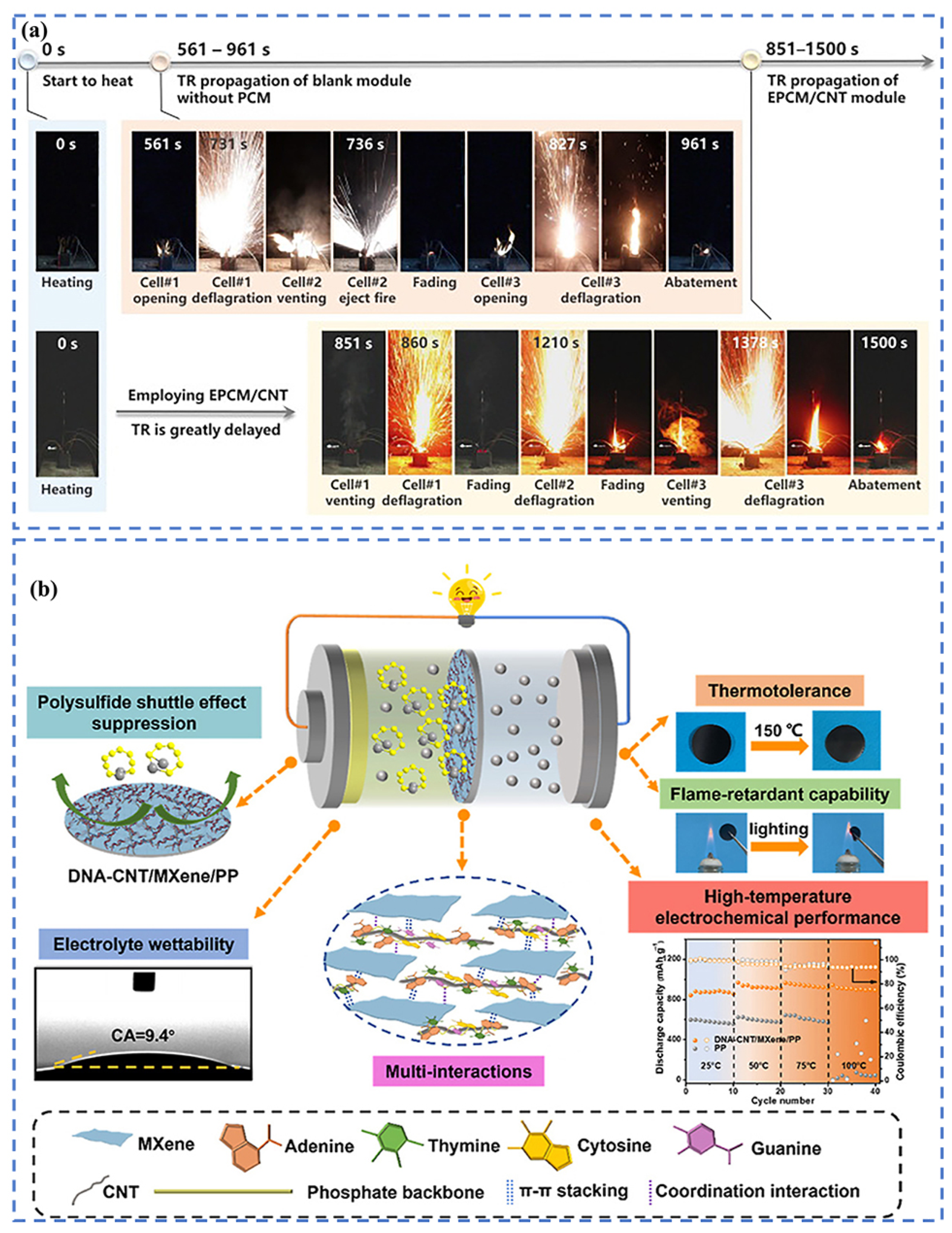
- Li, Y.; Li, M.; Zhu, Y.-C.; Song, S.; Li, S.-N.; Aarons, J.; Tang, L.-C.; Bae, J. Polysulfide-inhibiting, thermotolerant and nonflammable separators enabled by DNA co-assembled CNT/MXene networks for stable high-safety Li–S batteries. Compos. Part B Eng. 2023, 251, 110465. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, J.; Chen, Y.; Ouyang, D.; Chen, M. Application of Polyethylene Glycol-Based Flame-Retardant Phase Change Materials in the Thermal Management of Lithium-Ion Batteries. Polymers 2023, 15, 4450. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Huang, A.-C.; Tang, Y.; Yang, Y.-P.; Liu, Y.-C.; Li, Z.-P.; Zhou, H.-L.; Huang, C.-F.; Xing, Z.-X.; Shu, C.-M.; et al. Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery. Polymers 2021, 13, 1675. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Tang, Z.; Lv, J.; Cheng, P.; Diao, X.; Jiang, Y.; Chen, X.; Wang, G. Integrating thermal energy storage and microwave absorption in phase change material-encapsulated core-sheath MoS2@CNTs. J. Energy Chem. 2023, 84, 41–49. [Google Scholar] [CrossRef]
- Chen, L.; Zou, R.; Xia, W.; Liu, Z.; Shang, Y.; Zhu, J.; Wang, Y.; Lin, J.; Xia, D.; Cao, A. Electro- and Photodriven Phase Change Composites Based on Wax-Infiltrated Carbon Nanotube Sponges. ACS Nano 2012, 6, 10884–10892. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Shi, L.; Yan, Y. Thermophysical characteristics and enhancement analysis of carbon-additives phase change mono and hybrid materials for thermal management of electronic devices. J. Energy Storage 2021, 34, 102231. [Google Scholar] [CrossRef]
- Bontempi, E.; Sorrentino, G.P.; Zanoletti, A.; Alessandri, I.; Depero, L.E.; Caneschi, A. Sustainable Materials and their Contribution to the Sustainable Development Goals (SDGs): A Critical Review Based on an Italian Example. Molecules 2021, 26, 1407. [Google Scholar] [CrossRef]
- Lan, G.; Yang, J.; Ye, R.; Boyjoo, Y.; Liang, J.; Liu, X.; Li, Y.; Liu, J.; Qian, K. Sustainable Carbon Materials toward Emerging Applications. Small Methods 2021, 5, 2001250. [Google Scholar] [CrossRef]
- Ince, J.C.; Peerzada, M.; Mathews, L.D.; Pai, A.R.; Al-qatatsheh, A.; Abbasi, S.; Yin, Y.; Hameed, N.; Duffy, A.R.; Lau, A.K.; et al. Overview of emerging hybrid and composite materials for space applications. Adv. Compos. Hybrid Mater. 2023, 6, 130. [Google Scholar] [CrossRef]
- Chen, W.; Wan, M.; Liu, Q.; Xiong, X.; Yu, F.; Huang, Y. Heteroatom-Doped Carbon Materials: Synthesis, Mechanism, and Application for Sodium-Ion Batteries. Small Methods 2019, 3, 1800323. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.-J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef]
- Narayan, R.; Laberty-Robert, C.; Pelta, J.; Tarascon, J.-M.; Dominko, R. Self-healing: An emerging technology for next-generation smart batteries. Adv. Energy Mater. 2022, 12, 2102652. [Google Scholar]
- Bandodkar, A.J.; López, C.S.; Vinu Mohan, A.M.; Yin, L.; Kumar, R.; Wang, J. All-printed magnetically self-healing electrochemical devices. Sci. Adv. 2016, 2, e1601465. [Google Scholar] [PubMed]
- Wu, Y.; Huang, L.; Huang, X.; Guo, X.; Liu, D.; Zheng, D.; Zhang, X.; Ren, R.; Qu, D.; Chen, J. A room-temperature liquid metal-based self-healing anode for lithium-ion batteries with an ultra-long cycle life. Energy Environ. Sci. 2017, 10, 1854–1861. [Google Scholar]
- Zhao, Y.; Zhang, Y.; Sun, H.; Dong, X.; Cao, J.; Wang, L.; Xu, Y.; Ren, J.; Hwang, Y.; Son, I.H. A self-healing aqueous lithium-ion battery. Angew. Chem. Int. Ed. 2016, 55, 14384–14388. [Google Scholar]
- Fortier, A.; Tsao, M.; Williard, N.D.; Xing, Y.; Pecht, M.G. Preliminary study on integration of fiber optic Bragg grating sensors in li-ion batteries and in situ strain and temperature monitoring of battery cells. Energies 2017, 10, 838. [Google Scholar] [CrossRef]



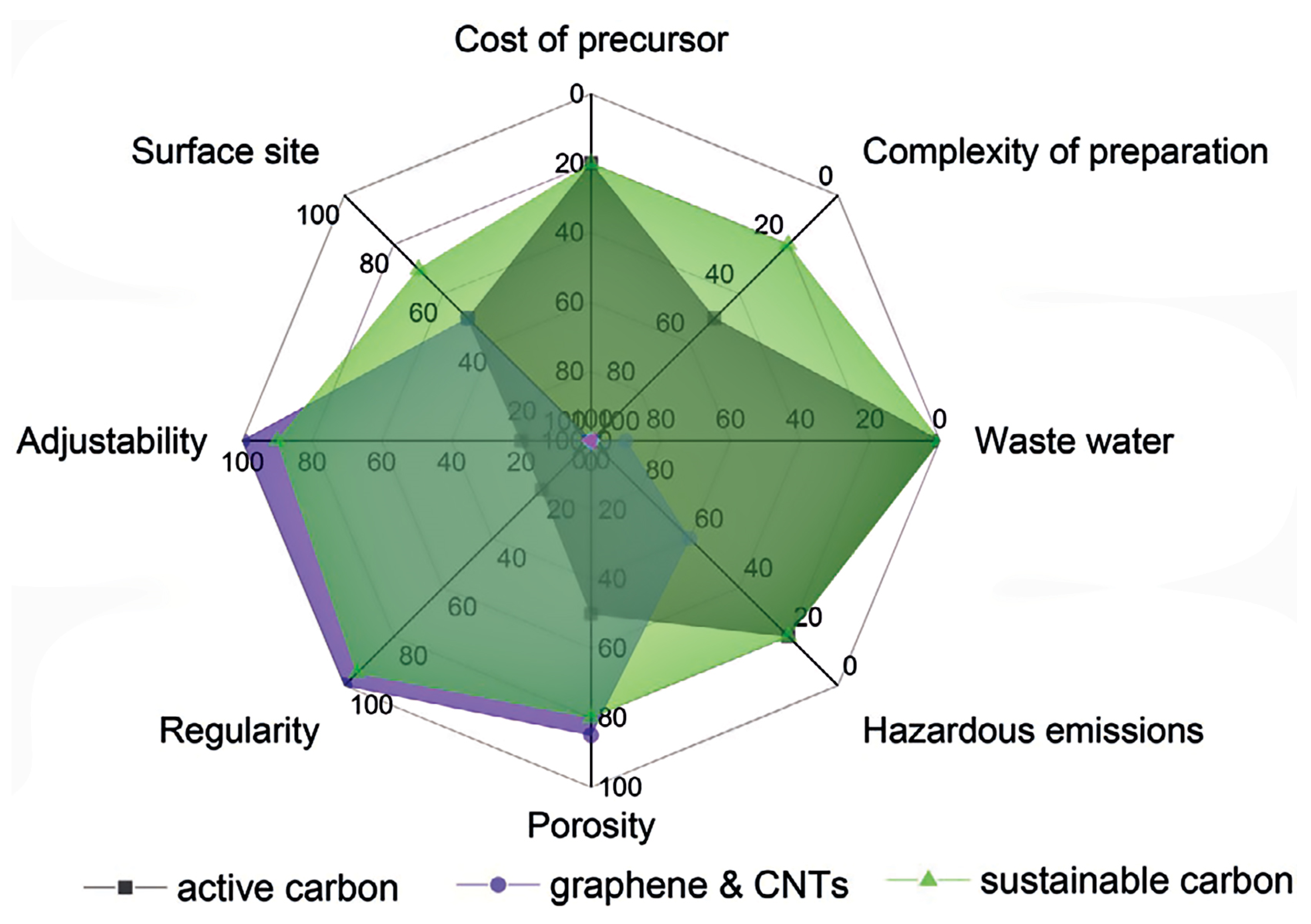
| Carbon Material Type | Genesis Synthesis Approach | Structure | Thermal Properties | Advantages for Thermal Management | Challenges for Thermal Management | Ref. |
|---|---|---|---|---|---|---|
| Graphite | Natural or synthetic graphite Purification and processing of ore graphite | Layered structure with strong in-plane bonding | Anisotropic thermal properties | Well-established manufacturing processes. High thermal stability Cost-effective | Difficulty in achieving uniform and high thermal conductivity in composites Potential thermal expansion mismatch with other materials | [110,111] |
| Graphene | Mechanical exfoliation of graphite Chemical vapour deposition (CVD) Reduction of graphene oxide | 2D honeycomb lattice structure | Extremely high in-plane thermal conductivity (up to 5000 W/m·K) | Flexible and lightweight Large surface area-to-volume ratio Ability to tailor thermal properties through doping and functionalisation | Limitations in large-scale manufacturing and integration Potential thermal interface resistance | [112] |
| Carbon nanotube | Chemical vapour deposition (CVD) Arc discharge Laser ablation | 1D cylindrical structure | High thermal conductivity along the tube axis (up to 3500 W/m·K) | Anisotropic thermal properties High aspect ratio and surface area Compatibility with various matrix materials | Potential for selective heat transfer paths Difficulties in uniform dispersion and alignment within composites Potential thermal interface resistance | [113,114] |
| Nanodiamonds | Detonation synthesis High-pressure, high-temperature (HPHT) synthesis | Spherical nanostructure with diamond cubic lattice | Exceptionally high thermal conductivity (up to 2000 W/m·K) | Thermal stability at high temperatures Small size and high surface area Ability to form stable dispersions and composites | Limited large-scale production capabilities Challenges in uniform dispersion and integration | [115,116] |
| Graphdiyne | Chemical vapour deposition (CVD) Hydrothermal synthesis | Hybrid structure combining graphene and diamond | Extremely high thermal conductivity (up to 2000 W/m·K) | Exceptional mechanical strength Tailorable thermal and electrical properties Potential for multifunctional applications | Emerging material with limited research and development Challenges in scalable synthesis and integration | [105] |
| Carbon foams | Pyrolysis of organic precursors Template-assisted synthesis | Porous and lightweight structure | Thermal Conductivity: Typically, in the range of 0.1 to 1 W/m·K, depending on the porosity, pore structure, and density of the carbon foam. | Effective thermal insulation and heat dissipation Tuneable thermal and mechanical properties High surface area and permeability Potential for thermal management in high-power applications | Limited thermal conductivity compared to other carbon materials Challenges in achieving desired pore structure and density | [117,118] |
| Carbon fibres | Organic precursors (e.g., polyacrylonitrile, rayon, pitch) Oxidation, carbonisation, and graphitisation processes | Continuous, high-aspect-ratio filaments with predominantly graphitic structure | High thermal conductivity along the fibre axis (up to 1000 W/m·K) | Anisotropic thermal behaviour (high conductivity in the fibre direction, lower in the transverse direction) Ability to create thermally conductive pathways within composites Compatibility with various matrix materials for integration Potential for tailoring thermal and mechanical properties | Achieving uniform dispersion and alignment within composites Potential thermal interface resistance between fibres and matrix | [119,120] |
| Fabrication Method | Carbon Material | Polymer Matrix | Thermal Performance | Mechanical Performance | Specific Use in BTMSs | Ref. |
|---|---|---|---|---|---|---|
| Pyrolysis of Organic Precursors | Carbon Foam | Phenolic Resin, Epoxy | High thermal insulation. Low thermal conductivity (0.1–0.5 W/m·K) | Lightweight Compressive strength: 0.5–5 MPa | Thermal barriers between cells/modules | [109] |
| Template-Assisted Synthesis | Carbon Foam | Polymer, Ceramic | High thermal insulation. Low thermal conductivity (0.1–0.5 W/m·K) | Tailorable mechanical properties. Compressive strength: 0.5–5 MPa | Battery pack enclosures. Thermal barriers between cells/modules | [158] |
| Infiltration Techniques | Carbon Foam | Polymer, Ceramic | High thermal insulation. Low thermal conductivity (0.1–0.5 W/m·K) | Improved mechanical properties compared to standalone foam. Compressive strength: 1–10 MPa | Battery pack enclosures. Thermal barriers between cells/modules. Thermal interface materials | [52] |
| Solution Mixing | Graphene, Graphene Oxid | Epoxy, Polymer | Moderate thermal insulation. Thermal conductivity: 0.1–1 W/m·K | Improved mechanical properties compared to neat polymer. Tensile strength: 50–100 MPa | Thermal insulation coatings/liners for battery enclosures. Thermal barriers between cells/modules | [159] |
| Melt Blending | Graphene, Graphene Nanoplatelets | Thermoplastic Polymer | Moderate thermal insulation. Thermal conductivity: 0.1–1 W/m·K | Improved mechanical properties compared to neat polymer. Tensile strength: 50–100 MPa | Thermal insulation coatings/liners for battery enclosures. Thermal barriers between cells/modules | [160] |
| Layer-by-Layer Assembly | Graphene | Polymer | High thermal insulation. Low thermal conductivity (0.1–0.5 W/m·K) | Tailorable mechanical properties. Tensile strength: 50–100 MPa | Thermal barriers between cells/modules | [161] |
| Solution Mixing | Nanodiamonds | Polymer, Ceramic | High thermal insulation. Low thermal conductivity (0.1–0.5 W/m·K) | Improved mechanical properties compared to neat matrix. Tensile strength: 50–100 MPa | Thermal insulation coatings/liners for battery enclosures. Thermal barriers between cells/modules. Thermal interface materials | [98] |
| Melt Blending | Nanodiamonds | Metal, Polymer | High thermal insulation. Low thermal conductivity (0.1–0.5 W/m·K) | Improved mechanical properties compared to neat matrix. Tensile strength: 50–100 MPa | Thermal insulation coatings/liners for battery enclosures. Thermal barriers between cells/module. Thermal interface materials | [162] |
| In situ Growth | Nanodiamonds | Polymer, Ceramic | High thermal insulation. Low thermal conductivity (0.1–0.5 W/m·K) | Improved mechanical properties compared to neat matrix. Tensile strength: 50–100 MPa | Thermal insulation coatings/liners for battery enclosures. Thermal barriers between cells/modules. Thermal interface materials | [163] |
| Carbon nanotubes | Epoxy, PMMA, or polyimide | Significantly enhanced thermal conductivity | Improved tensile strength and modulus | Thermal interface materials, heat spreaders in battery packs | [164] |
| Material Composition | Thermal Conductivity (W/m·K) | Interface Resistance (K/W) | TIMs Application | Main Findings | Ref. |
|---|---|---|---|---|---|
| GNPs/BNNSs/CNFs | 25.5 | - | TIMs | GNP layers provide in-plane thermally conductive paths and EMI shielding capability and reinforce the mechanical strength | [237] |
| Carbon-based graphene foam roll (GFR-TIM) | ~17.42 | 4.96 mm2 | CPU cooling | GFRs are made by hydroplastic foaming (HPF) and interface strengthening methods. Impregnation with GO enables its superior structural integrity and stability after 10,000 cycles as compared to most traditional TIMs. Due to its exceptionally high porosity (>93%), enabling its lower interface thermal resistance | [238] |
| Graphene/CNT hybrid aerogel | 88.5 | 13.6 mm2 | High-power electronics | Fabrication of graphene/carbon nanotube aerogel TIMs overcomes poor thermal transport problems due to proper entanglement of bonds. This achieves super-elastic properties, improved conductivity, and a low thermal interface resistance | [239] |
| Graphene fibre | 82.4 | 7.4 mm2 | High-power electronics | The graphene fibre (GF)-based TIM achieves thermal conductivity comparable to metals with enhanced structural and mechanical properties, making it durable and reliable in overcoming the long-standing bottleneck of mechanical–thermal mismatch in TIM design | [240] |
| LM–VGA/BPDMS | 7.11 | 14.1 mm2 | High-performance electronics and sensors | The fabrication of elastic TIMs made of vertically oriented vertical aerogel with a liquid metal network achieves a directional pathway for heat dissipation with thermal resistance. | [241] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawiah, B.; Ofori, E.A.; Chen, D.; Ming, Y.; Hou, Y.; Jia, H.; Fei, B. Carbon-Based Thermal Management Solutions and Innovations for Improved Battery Safety: A Review. Batteries 2025, 11, 144. https://doi.org/10.3390/batteries11040144
Tawiah B, Ofori EA, Chen D, Ming Y, Hou Y, Jia H, Fei B. Carbon-Based Thermal Management Solutions and Innovations for Improved Battery Safety: A Review. Batteries. 2025; 11(4):144. https://doi.org/10.3390/batteries11040144
Chicago/Turabian StyleTawiah, Benjamin, Emmanuel A. Ofori, Daming Chen, Yang Ming, Yongdan Hou, Hao Jia, and Bin Fei. 2025. "Carbon-Based Thermal Management Solutions and Innovations for Improved Battery Safety: A Review" Batteries 11, no. 4: 144. https://doi.org/10.3390/batteries11040144
APA StyleTawiah, B., Ofori, E. A., Chen, D., Ming, Y., Hou, Y., Jia, H., & Fei, B. (2025). Carbon-Based Thermal Management Solutions and Innovations for Improved Battery Safety: A Review. Batteries, 11(4), 144. https://doi.org/10.3390/batteries11040144







