Low Voltage Charge/Discharge Behavior of Manganese Hexacyanoferrate
Abstract
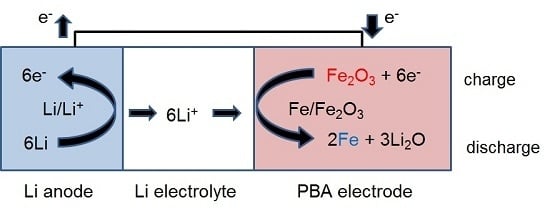
:1. Introduction
2. Results
2.1. Scanning Electron Microscopy Image
2.2. Charge/Discharge Curves and Framework Structure
2.3. Synchrotron–Radiation X-ray Absorption Spectroscopy Measurement
2.4. Synchrotron Radiation X-ray Diffraction Measurement
3. Discussion
4. Materials and Methods
4.1. Film Preparation and Characterization
4.2. X-ray Diffraction and X-ray Absorption Spectroscopy
4.3. Electrochemical Measurement
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, N.; Morikawa, T.; Kondo, J.; Takeda, Y.; Yamamoto, O.; Kinugasa, N.; Yamagishi, T. Lithium intercalation behavior into iron cyanide complex as positive electrode of lithium secondary battery. J. Power Sources 1999, 79, 215–219. [Google Scholar] [CrossRef]
- Imanishi, N.; Morikawa, T.; Kondo, J.; Yamane, R.; Takeda, Y.; Yamamoto, O.; Sakaebe, H.; Tabuchi, M. Lithium intercalation behavior of iron cyanometallates. J. Power Sources 1999, 81–82, 530–534. [Google Scholar] [CrossRef]
- Okubo, M.; Asakura, D.; Mizuno, Y.; Kim, J.-D.; Mizokawa, T.; Kudo, T.; Honnma, I. Switching redox-active sites by valence tautomerism in Prussian blue analogues AxMny[Fe(CN)6]·nH2O (A: K, Rb): Robust frameworks for reversible Li storage. J. Phys. Chem. Lett. 2010, 1, 2063–2071. [Google Scholar] [CrossRef]
- Matsuda, T.; Moritomo, Y. Thin film electrode of Prussian blue analogue for Li-ion battery. Appl. Phys. Express 2011, 4, 047101. [Google Scholar] [CrossRef]
- Takachi, M.; Matsuda, T.; Moritomo, Y. Structural, electronic, and electrochemical properties of LixCo[Fe(CN)6]0.90·2.9H2O. Jpn. J. Appl. Phys. 2013, 52, 044301. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Takachi, M.; Moritomo, Y. A sodium manganese ferrocyanide thin film for Na-ion batteries. Chem. Commun. 2013, 49, 2750–2752. [Google Scholar] [CrossRef] [PubMed]
- Takachi, M.; Matsuda, T.; Moritomo, Y. Cobalt hexacyanoferrate as cathode material for Na+ secondary battery. Appl. Phys. Express 2013, 6, 025802. [Google Scholar] [CrossRef]
- Yang, D.; Xu, J.; Liao, X.-Z.; He, Y.-S.; Liu, H.; Ma, Z.-F. Structure optimization of Prussian blue analogue cathode materials for advanced sodium ion batteries. Chem. Commum. 2014, 50, 13377–13380. [Google Scholar] [CrossRef] [PubMed]
- Moritomo, Y.; Urase, S.; Shibata, T. Enhanced battery performance in manganese hexacyanoferrate by partial substitution. Electrochim. Acta 2016, 210, 963–969. [Google Scholar] [CrossRef]
- Lee, H.W.; Wang, R.Y.; Pasta, M.; Lee, S.W.; Liu, N.; Chi, Y. Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries. Nat. Commun. 2014, 5, 5280. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, J.; Qiao, R.; Wray, L.A.; Hossain, M.A.; Chung, Y.-D.; Yang, W.; Lu, Y.; Evans, D.; Lee, J.-J.; et al. Rhombohedral Prussian white as cathode for rechargeable sodium-ion batteries. J. Am. Chem. Soc. 2015, 137, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian blue: Fe4[Fe(CN)6]3·xH2O. Inorg. Chem. 1997, 16, 2704–2710. [Google Scholar] [CrossRef]
- Herren, F.; Fischer, P.; Ludi, A.; Halg, W. Neutron diffraction study of Prussian blue, Fe4[Fe(CN)6]3·xH2O. Location of water molecules and long-range magnetic order. Inorg. Chem. 1980, 19, 956–959. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Yu, S.-H.; Lee, D.-C.; Ling, D.; Hyeon, T.; Sung, Y.-E. Metal hexacyanoferrate nanoparticles as electrode materials for lithium ion batteries. Nanosci. Nanotechnol. Lett. 2013, 5, 770–774. [Google Scholar] [CrossRef]
- Nie, P.; Shen, L.; Luo, H.; Ding, B.; Xu, G.; Wang, J. Prussian blue analogues: A new class of anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 5852–5857. [Google Scholar] [CrossRef]
- Xiong, P.; Zeng, G.; Zeng, L.; Wei, M. Prussian blue analogues Mn[Fe(CN)6]0.6667·nH2O cubes as an anode material for lithium-ion batteries. Dalton Trans. 2015, 44, 16746–16751. [Google Scholar] [CrossRef] [PubMed]
- Piernas-Muñoz, M.J.; Castillo-Martínez, E.; Roddatis, V.; Armand, M.; Rojo, T. K1-xFe2+x/3(CN)6·yH2O, Prussian blue as a displacement anode for lithium ion batteries. J. Power Sources 2014, 271, 489–496. [Google Scholar]
- Sun, X.; Ji, X.-Y.; Zhou, Y.-T.; Shao, Y.; Zang, Y.; Wen, Z.-Y.; Chen, C.-H. A new gridding cyanoferrate anode material for lithium and sodium ion batteries: Ti0.75Fe0.25[Fe(CN)6]0.96·1.9H2O with excellent electrochemical properties. J. Power Sources 2016, 314, 35–38. [Google Scholar] [CrossRef]
- Moritomo, Y.; Kurihara, Y.; Matsuda, T.; Kim, J. Structural phase diagram of Mn-Fe cyanide against cation concentration. J. Phys. Soc. Jpn. 2011, 80, 103601. [Google Scholar] [CrossRef]
- Moritomo, Y.; Matsuda, T.; Kurihara, Y.; Kim, J. Cubic-rhombohedral structural phase transition in Na1.32Mn[Fe(CN)6]0.83·3.6H2O. J. Phys. Soc. Jpn. 2011, 80, 074608. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Bianconi, A.; Dell’Ariccia, M.; Durham, P.J.; Pendry, J.B. Multiple-scattering resonances and structural effects in the X-ray-absorption near-edge spectra of Fe II and Fe III hexacyanide complexes. Phys. Rev. B 1982, 26, 6502–6508. [Google Scholar] [CrossRef]
- Hayashi, H.; Abe, H. X-ray spectroscopic analysis of liesegang patterns in Mn-Fe-based Prussian blue analogs. J. Anal. At. Spectrom. 2016, 31, 1658–1672. [Google Scholar] [CrossRef]
- Li, H.; Balaya, P.; Maier, J. Li-storage via heterogeneous reaction in selected binary metal fluorides and oxides. J. Electrochem. Soc. 2004, 151, A1878–A1885. [Google Scholar] [CrossRef]
- Larcher, D.; Masquelier, C.; Bonnin, D.; Chabre, Y.; Masson, V.; Leriche, J.-B.; Tarascon, J.-M. Effect of particle size on lithium intercalation into α-Fe2O3. J. Electrochem. Soc. 2003, 150, A133–A139. [Google Scholar] [CrossRef]
- Nishibori, E.; Takata, M.; Kato, K.; Sakata, M.; Kubota, Y.; Aoyagi, S.; Kuroiwa, Y.; Yamakawa, M.; Ikeda, N. The large Debye-Scherrer camera installed at SPring-8 Bl02B2 for charge density studies. J. Phys. Chem. Solids 2001, 62, 2095–2098. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, T.; Takachi, M.; Moritomo, Y. Low Voltage Charge/Discharge Behavior of Manganese Hexacyanoferrate. Batteries 2017, 3, 7. https://doi.org/10.3390/batteries3010007
Shibata T, Takachi M, Moritomo Y. Low Voltage Charge/Discharge Behavior of Manganese Hexacyanoferrate. Batteries. 2017; 3(1):7. https://doi.org/10.3390/batteries3010007
Chicago/Turabian StyleShibata, Takayuki, Masamitsu Takachi, and Yutaka Moritomo. 2017. "Low Voltage Charge/Discharge Behavior of Manganese Hexacyanoferrate" Batteries 3, no. 1: 7. https://doi.org/10.3390/batteries3010007
APA StyleShibata, T., Takachi, M., & Moritomo, Y. (2017). Low Voltage Charge/Discharge Behavior of Manganese Hexacyanoferrate. Batteries, 3(1), 7. https://doi.org/10.3390/batteries3010007