Further Cost Reduction of Battery Manufacturing
Abstract
:1. Introduction
2. Problem Definition
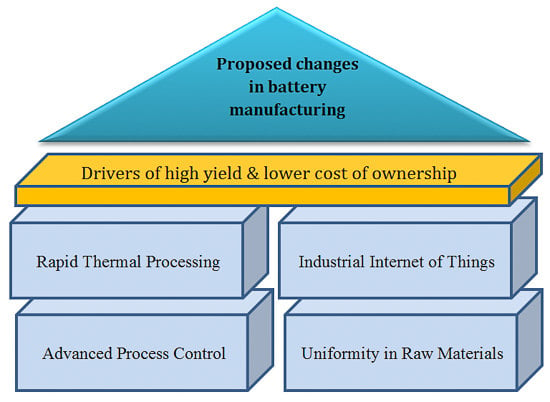
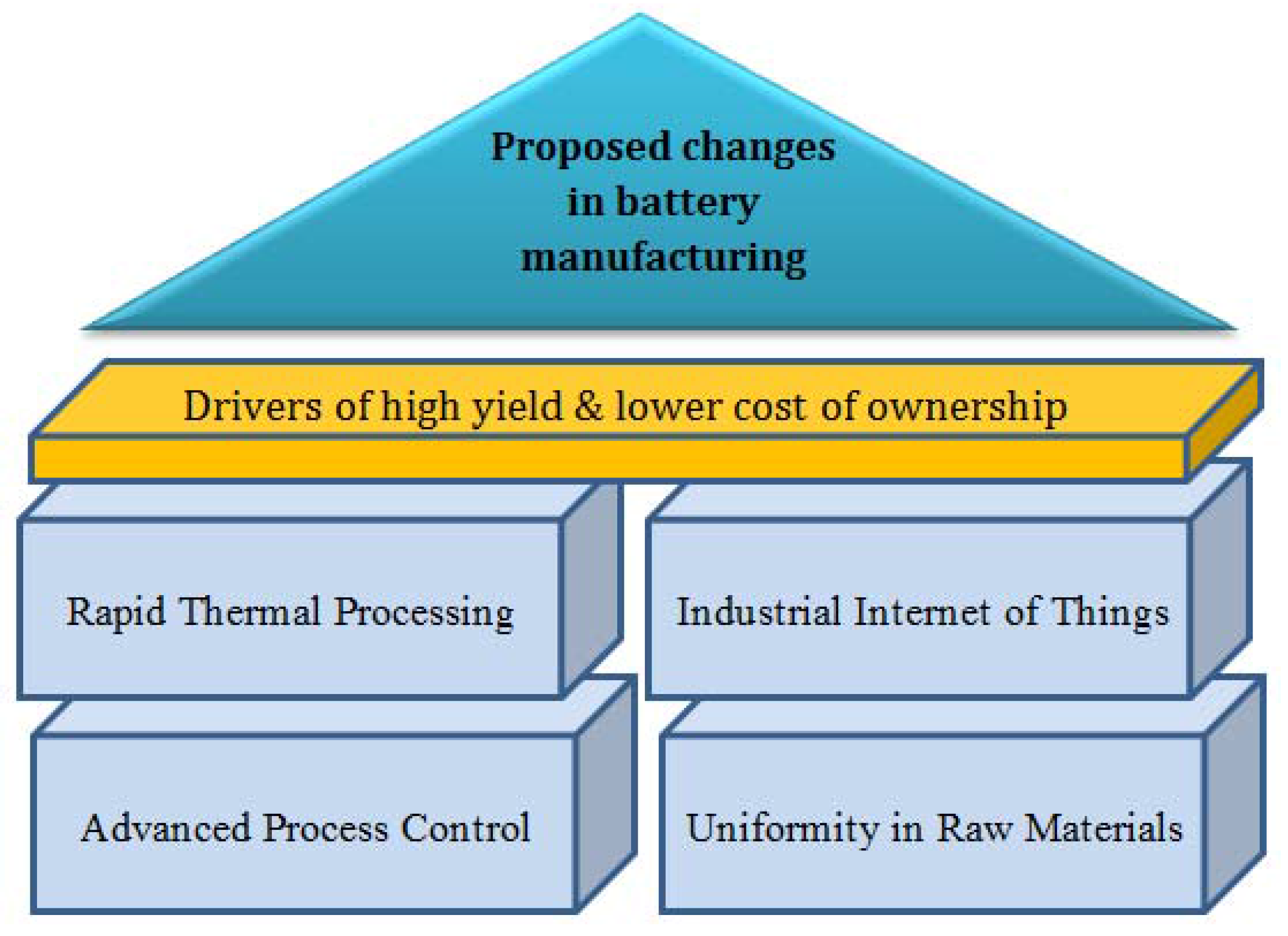
3. Proposed Changes in Lithium-Ion Battery Manufacturing to Address Process Variability
3.1. Advanced Process Control
3.2. Modifying Process Mechanism
3.3. Synthesis of the Raw Materials with Uniformity, Supply Chain and Industrial Internet of Things
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ehrlich, G.M. Lithium-Ion Batteries. In Handbook of Batteries, 3rd ed.; Linden, D., Reddy, T.B., Eds.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Sasaki, T.; Ukyo, Y.; Novak, P. Memory effect in a lithium-ion battery. Nat. Mater. 2013, 12, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Nita, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- What’s the Best Battery? Available online: http://batteryuniversity.com/learn/archive/whats_the_best_battery (accessed on 20 January 2017).
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Shahan, Z. Tesla’s Battery Prices Falling Faster than Everyone Else’s. Available online: https://cleantechnica.com/2016/06/08/teslas-batteries-cheaper-everyone-elses-knew-cool-ev-battery-charts (accessed on 20 January 2016).
- Perez, R.; Zweibel, K.; Hoff, T.E. Solar Power Generation in the US: Too Expensive, or A Bargain? Available online: http://www.asrc.cestm.albany.edu/perez/2011/solval.pdf (accessed on 20 January 2017).
- Why Moore’s Law Does Not Apply to Clean Technologies. Greentech Media. Available online: http://www.greentechmedia.com/articles/read/why-moores-law-doesnt-apply-to-clean-technologies (accessed on 20 January 2017).
- Singh, R.; Shenai, K. DC Microgrids and the Virtues of Local Electricity. IEEE Spectrum. 6 February 2014. Available online: http://spectrum.ieee.org/green-tech/buildings/dc-microgrids-and-the-virtues-of-local-electricity (accessed on 20 January 2017).
- Singh, R.; Asif, A.A.; Venayagamoorthy, G.K. Transformative role of photovoltaics in phasing out alternating current based grid by local dc power networks for sustainable global economic growth. In Proceedings of the 43rd IEEE PV Specialist Conference, Portland, OR, USA, 5–10 June 2016. [Google Scholar]
- Asif, A.A.; Singh, R.; Venayagamoorthy, G.K. Ultra-low cost and solar storm secured local DC electricity to address climate change challenges for all economies. In Proceedings of the IEEE-CU Power System Conference, Clemson, SC, USA, 8–11 March 2016. [Google Scholar] [CrossRef]
- Lacey, S. How Distributed Battery Storage Will Surpass Grid-Scale Storage in the US by 2020. Available online: https://www.greentechmedia.com/articles/read/how-distributed-battery-storage-will-surpass-grid-scale-storage-in-the-us-b (accessed on 20 January 2017).
- Fialka, J. World’s Largest Storage Battery Will Power Los Angeles. Available online: https://www.scientificamerican.com/article/world-s-largest-storage-battery-will-power-los-angeles/ (accessed on 20 January 2017).
- Singh, R.; Alapatt, G.F.; Lakhtakia, A. Making solar cells a reality in every home: Opportunities and challenges for photovoltaic device design. IEEE J. Electron Devices Soc. 2013, 1, 129–144. [Google Scholar] [CrossRef]
- Advanced and Post Lithium-Ion Batteries 2016–2026: Technologies, Markets, Forecasts. Available online: http://www.prnewswire.com/news-releases/advanced-and-post-lithium-ion-batteries-2016-2026-technologies-markets-forecasts-300182252.html (accessed on 20 January 2017).
- Transparency Market Research. Lithium-Ion Battery Market Is Projected to Reach US $77.42 bn in 2024; Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2016–2024. Available online: http://www.marketwatch.com/story/lithium-ion-battery-market-is-projected-to-reach-us-7742-bn-in-2024-global-industry-analysis-size-share-growth-trends-and-forecast-2016---2024-tmr-2016-09-19 (accessed on 20 January 2017).
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Hu, Y.S. Batteries: Getting solid. Nat. Energy 2016, 1, 16042. [Google Scholar] [CrossRef]
- Gradne, L. Solid-State and Polymer Batteries 2017–2027: Technology, Markets, Forecasts. Available online: http://www.idtechex.com/research/reports/solid-state-and-polymer-batteries-2017-2027-technology-markets-forecasts-000498.asp (accessed on 20 January 2017).
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Bjorefors, F.; Endstrom, K.; Gustafsson, T. Improved Performance of the silicon anode for li-ion batteries: Understanding the surface modification mechanism of fluoroethylene carbonate as an effective electrolyte additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Hassoun, J.; Park, J.B.; Lee, Y.J.; Sun, Y.K.; Passerini, S.; Scrosati, B. The lithium/air battery: Still an emerging system or a practical reality? Adv. Mater. 2015, 27, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Atacama, S.P. An Increasingly Precious Metal. The Economist. 14 January 2016. Available online: http://www.economist.com/news/business/21688386-amid-surge-demand-rechargeable-batteries-companies-are-scrambling-supplies (accessed on 21 January 2017).
- Lambert, F. Tesla Is Now Claiming 35% Battery Cost Reduction at ‘Gigafactory 1’—Hinting at Breakthrough Cost below $125/kWh. Available online: https://electrek.co/2017/02/18/tesla-battery-cost-gigafactory-model-3/ (accessed on 24 April 2017).
- Tesla Powerwall. Available online: https://www.tesla.com/powerwall (accessed on 21 January 2017).
- Mims, C. In Battery Revolution, a Clean Leap Forward. Wall Street J. 15 March 2015. Available online: http://www.wsj.com/articles/in-battery-revolution-a-clean-leap-forward-1426461806 (accessed on 21 January 2017).
- Singh, R. Why silicon is and will remain the dominant photovoltaic material. J. Nanophotonics 2009, 3, 032503. [Google Scholar] [CrossRef]
- There Enough Lithium to Maintain the Growth of the Lithium-Ion Battery Market? Greentech Media. Available online: http://www.greentechmedia.com/articles/read/Is-There-Enough-Lithium-to-Maintain-the-Growth-of-the-Lithium-Ion-Battery-M (accessed on 21 January 2017).
- Deign, J. An Australian Company Says Its New Extraction Process Could Bring Unlimited Lithium Supplies. Greentech Media. 1 June 2015. Available online: https://www.greentechmedia.com/articles/read/new-process-promises-unlimited-lithium-supplies (accessed on 21 January 2017).
- What Price Lithium, the Metal of the Future? Available online: http://fortune.com/2016/06/06/lithium-price-tesla-metal-future/ (accessed on 21 January 2017).
- Bohlsen, Lithium Miner News for the Month of January 2017. Available online: http://seekingalpha.com/article/4040100-lithium-miner-news-month-january-2017 (accessed on 21 January 2017).
- Cappel, R.; Sullivan, C.P. Yield and Cost Challenges at 16 nm and Beyond. Available online: http://electroiq.com/blog/2016/02/yield-and-cost-challenges-at-16nm-and-beyond/ (accessed on 21 January 2017).
- Kundu, S.; Sreedhar, A. Nanoscale CMOS VLSI Circuits: Design for Manufacturability; McGraw-Hill, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Samsung Starts Industry’s First Mass Production of System-on-Chip with 10-Nanometer FinFET Technology. Available online: https://news.samsung.com/global/samsung-starts-industrys-first-mass-production-of-system-on-chip-with-10-nanometer-finfet-technology (accessed on 21 January 2017).
- Bhhuiyan, M.; Poddar, S.; Misra, D.; Tapily, K.; Clark, R.D.; Consiglio, S.; Wajda, C.S.; Nakamura, G.; Leusink, G.J. Impact of cyclic plasma treatment on oxygen vacancy defects in TiN/HfZrO/SiON/Si gate stacks. Appl. Phy. Lett. 2015, 106, 193508. [Google Scholar] [CrossRef]
- Bhhuiyan, M.; Misra, D. Multilayered ALD HfAlOx and HfO2 for high-quality gate stacks. IEEE Trans. Device Mater. Reliab. 2015, 2, 229–235. [Google Scholar] [CrossRef]
- Smith, K.; Neubauer, J.; Wood, E.; Jun, A.; Pesaran, M. Models for Battery Reliability and Lifetime Applications in Design and Health Management; Battery Congress: Ann Arbor, MI, USA, 2013. [Google Scholar]
- Singh, R.; Colombo, L.; Schuegraf, K.; Doering, R.; Diebold, A. Guide to State-of-the-Art Electronic Devices in Semiconductor Manufacturing, 1st ed.; Burghartz, J.N., Ed.; Wiley-IEEE Press: New Jersey, NJ, USA, 2013; pp. 121–132. [Google Scholar]
- Shin, D.; Poncino, M.; Maci, E.; Chang, N. Statistical model of cell-to-cell variation in Li-ion batteries for system-level design. In Proceedings of the 2013 IEEE International Symposium on Low Power Electronics and Design (ISLPED), Beijing, China, 4–6 September 2013. [Google Scholar]
- Santhanagopalan, S.; Guo, Q.; Ramdass, P.; White, R.E. Review of models for predicting the cycling performance of lithium ion batteries. J. Power Sources 2006, 156, 620–628. [Google Scholar] [CrossRef]
- Stroe, D.; Swierczynski, M.; Stroe, A.; Kær, S.K. Generalized characterization methodology for performance modelling of lithium-ion batteries. Batteries 2016, 2, 37. [Google Scholar] [CrossRef]
- Zhang, J.; Ci, S.; Sharif, H.; Alahmadm, M. Modeling discharge behavior of multicell battery. IEEE Trans. Energy Convers. 2010, 25, 1133–1141. [Google Scholar] [CrossRef]
- Christen, R.; Rizzo, G.; Gadola, A.; Stock, M. Test method for thermal characterization of Li-ion cells and verification of cooling concepts. Batteries 2017, 3, 3. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; White, R.E. Quantifying cell-to-cell variations in lithium ion batteries. Int. J. Electrochem. 2012, 2012, 3295838. [Google Scholar] [CrossRef]
- Wessof, E. SunPower Holds World Record for Most Efficient Rooftop Solar Panel again. Available online: https://www.greentechmedia.com/articles/read/SunPower-Again-Holds-Record-For-Worlds-Most-Efficient-Rooftop-Solar-Panel (accessed on 12 March 2017).
- Asif, A.A.; Singh, R.; Alapatt, G.F. Technical and economic assessment of perovskite solar cells for large scale manufacturing. J. Renew. Sustain. Energy 2015, 7, 043120. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyanga, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Stuart, T.A.; Zhu, W. Modularized battery management for large lithium ion cells. J. Power Sources 2011, 196, 458–464. [Google Scholar] [CrossRef]
- Canney, W.M. Advanced Process Control Powers Developments in Operations Management. Oil Gas J. 2004, 102, 50–53. [Google Scholar]
- Undey, C.; Ertunç, S.; Mistretta, T.; Looze, T. Applied advanced process analytics in biopharmaceutical manufacturing: Challenges and prospects in real-time monitoring and control. J. Process Control 2010, 20, 1009–1018. [Google Scholar] [CrossRef]
- Harks, P.P.R.M.L.; Mulder, F.M.; Notten, P.H.L. In situ methods for li-ion battery research: A review of recent developments. J. Power Sources 2015, 288, 92–105. [Google Scholar] [CrossRef]
- Bauer, M.; Craig, I.K. Economic Assessment of Advanced Process Control—A Survey and Framework. J. Process Control 2008, 18, 2–18. [Google Scholar] [CrossRef]
- Thin Film Battery. Available online: http://www.ulvac.com/applications-products/app.cfm?cid=58&scid=156 (accessed on 23 January 2017).
- Romanenko, K.; Jin, L.Y.; Madsen, L.; Pringle, J. Anisotropic MRI Contrast Reveals Enhanced Ionic Transport in Plastic Crystals. J. Am. Chem. Soc. 2014, 136, 15638–15645. [Google Scholar] [CrossRef] [PubMed]
- Salameh, Z.; Casacca, M.; Lynch, W.A. A mathematical model for lead-acid batteries. IEEE Trans. Energy Convers. 1992, 7, 93–98. [Google Scholar] [CrossRef]
- Young, K.; Wang, C.; Wang, L.Y.; Strunz, K. Electric Vehicle Battery Technologies. In Electric Vehicle Integration, 1st ed.; Garcia-Valle, R., Lopes, J.P., Eds.; Springer: New York, NY, USA, 2013; pp. 15–56. [Google Scholar]
- Sikha, G.; White, R.E. Analytical expression for the impedance response for a lithium-ion cell. J. Electrochem. Soc. 2008, 155, A893–A902. [Google Scholar] [CrossRef]
- Singh, R. Rapid isothermal processing. J. Appl. Phys. 1988, 63, R59–R114. [Google Scholar] [CrossRef]
- Ratakonda, D.; Singh, R.; Vedula, L.; Rohatgi, A.; Mejia, J.; Narayan, S. Rapid thermal processing of screen printed ohmic contacts. J. Electrochem. Soc. 1997, 144, 3237–3242. [Google Scholar] [CrossRef]
- Xue, Z.; Hu, L.; Amine, K.; Zhang, Z. High-speed fabrication of lithium-ion battery electrodes by UV-curing. Energy Technol. 2015, 3, 469–475. [Google Scholar] [CrossRef]
- Knotts, J.; Morano, J. Next-generation wafer-level processing through customized materials. Chip Scale Rev. 2015, 19, 55–56. [Google Scholar]
- Kang, J.; Conlisk, A.T.; Rizzoni, G. Integration of capacity fading in an electrochemical model of Li-ion batteries. J. Solid State Electrochem. 2014, 18, 2425–2434. [Google Scholar] [CrossRef]
- Kanno, R.; Murayama, M. Lithium ionic conductor Thio-LISICON: The Li2S, GeS2, P2S5 System. J. Electrochem. Soc. 2001, 148, A742–A746. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Zhang, L.; Renzhi, M.; Osada, M.; Sasaki, T. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater. 2006, 18, 2226–2229. [Google Scholar] [CrossRef]
- The Internet of Things: Mapping the Value beyond the Hype. Available online: http://www.mckinsey.com/~/media/McKinsey/Business%20Functions/McKinsey%20Digital/Our%20Insights/The%20Internet%20of%20Things%20The%20value%20of%20digitizing%20the%20physical%20world/Unlocking_the_potential_of_the_Internet_of_Things_Executive_summary.ashx (accessed on 25 January 2017).
- Turbide, T. Manufacturing Embraces the Industrial Internet of Things. Available online: http://searchmanufacturingerp.techtarget.com/opinion/Manufacturing-embraces-the-Industrial-Internet-of-Things (accessed on 2 February 2017).
- Xu, L.D.; He, W.; Li, S. Internet of things in industries: A survey. IEEE Trans. Ind. Inform. 2014, 10, 2233–2243. [Google Scholar] [CrossRef]
- Gartner. Five Ways the Internet of Things Will Benefit the Supply Chain. Available online: http://www.gartner.com/smarterwithgartner/five-ways-the-internet-of-things-will-benefit-the-supply-chain-2/ (accessed on 3 February 2017).
- Morley, M. The Brilliant Disruptive Potential of IoT. Available online: http://www.itproportal.com/2015/03/28/the-brilliant-disruptive-potential-iot (accessed on 3 February 2017).
- Bi, Z.; Xu, L.D.; Wang, C. Internet of things for enterprise systems of modern manufacturing. IEEE Trans. Ind. Inform. 2014, 10, 1537–1546. [Google Scholar]
- Zhang, L.; Luo, Y.; Tao, F.; Li, B.H.; Ren, L.; Zhang, X.; Guo, H.; Cheng, Y.; Hu, A.; Liu, Y. Cloud Manufacturing: A New Manufacturing Paradigm. Enterp. Inform. Syst. 2014, 8, 167–187. [Google Scholar] [CrossRef]
- Hartmann, B.; King, W.P.; Narayanan, S. Digital Manufacturing: The Revolution Will Be Virtualized. Available online: http://www.mckinsey.com/business-functions/operations/our-insights/digital-manufacturing-the-revolution-will-be-virtualized (accessed on 3 November 2016).
- Fehrenbacher, K. Tesla Tried to Buy a Lithium Startup for $325 Million. Available online: http://fortune.com/2016/06/08/tesla-lithium-startup-simbol (accessed on 3 February 2017).
- Nikačević, N.M.; Huesman, A.E.M.; Hof, P.M.V.; Stankiewicz, A.I. Opportunities and challenges for process control in process intensification. Chem. Eng. Process. Process Intensif. 2012, 52, 1–15. [Google Scholar] [CrossRef]
- Sohn, S.Y.; Moon, H.U. Cost of ownership model for inspection of multiple quality attributes. IEEE Trans. Semicond. Manuf. 2003, 16, 565–571. [Google Scholar] [CrossRef]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, A.A.; Singh, R. Further Cost Reduction of Battery Manufacturing. Batteries 2017, 3, 17. https://doi.org/10.3390/batteries3020017
Asif AA, Singh R. Further Cost Reduction of Battery Manufacturing. Batteries. 2017; 3(2):17. https://doi.org/10.3390/batteries3020017
Chicago/Turabian StyleAsif, Amir A., and Rajendra Singh. 2017. "Further Cost Reduction of Battery Manufacturing" Batteries 3, no. 2: 17. https://doi.org/10.3390/batteries3020017
APA StyleAsif, A. A., & Singh, R. (2017). Further Cost Reduction of Battery Manufacturing. Batteries, 3(2), 17. https://doi.org/10.3390/batteries3020017