1. Introduction
The rapid integration of renewable energy generation in the current electricity network can help in relieving climate change, ensuring future energy security and providing some economic benefits [
1]. However, the electrical energy generated by renewable energy sources (RESs) is criticized for being variable or intermittent, which can affect the stability and reliability of the electricity network. These issues can be overcome by deploying highly efficient energy storage systems in RES-based electricity network [
2]. Batteries are the key elements of energy storage system. Among all the battery technologies, the vanadium redox flow battery (VRB), invented by Skyllas-Kazacos et al. [
3], is the most promising technology for the RES-based electricity networks due to its scale-integration capability [
4]. The VRB has a deep discharging capability, very long cycle life, and high energy efficiency, such that it provides more reliable operations compared with other battery technologies [
5]. The chemical reactions occurring in the cell stack during charging can be presented as:
The power flow control system plays an important role in battery operation especially if the input power is time-varying. Several battery charging methods such as constant-current (CC), constant-voltage (CV) and constant-current followed by constant-voltage (CC-CV) have been reported in literature [
6]. The existing control approaches fail to capture the maximum available power from RESs. Moreover, the tracking of constant charging current is a challenge if the available input power is insufficient. If the power from RESs is sufficient, the conventional charging scheme can track the charging current accurately but often fails to protect the VRB from overcharging. The CC-CV charging technique shows a better performance in protecting VRBs from overcharging compared with the CC and CV methods [
7]. This CC-CV charging approach protects batteries from overcharging by pre-setting a voltage limit. This voltage limit is generally determined based on the state-of-charge (SOC) of the battery system. However, gassing side-reactions, namely oxygen and hydrogen evolutions, can take place before the preset limit depending on the variation of charging current.
As the voltage limiting method cannot ensure the safe operation of VRB, the limiting current constraint-based control technique could be a better alternative. Moreover, the charging scheme also needs to deal with the time-varying nature of the input power for capturing the maximum amount of free energy from RESs. Several research activities have been performed for determining the optimal charging [
8,
9] and limiting current [
10] based on the battery operating efficiency. These charging methods did not consider the variable available power. A limiting current constraint-based advanced charging scheme is proposed in [
11], which considers the variable available input power condition. However, this advanced charging scheme losses its optimality due to the constant electrolyte flow rate of the VRB system.
The electrolyte flow rate is an important factor in the VRB operations. Several flow-rate optimization studies such as constant flow rates in different pre-defined battery terminal voltage level [
12], model-based flow-rate optimization [
13], optimized flow rate based on parametric study [
14], optimized flow rate considering pump energy loss [
15], pulsating flow rate for the minimal pump energy consumption [
16], and variable flow rate based on the thermal model [
17] have been reported in the literature. The electrolyte flow rate depends on the system SOC and the charging current. Furthermore, the constraints of pressure-drop in the VRB hydraulic system (main pipes, channels, and porous electrodes) and pump power consumption rating is also an important factor in determining the optimal electrolyte flow rate. These constraints of pump energy consumption can be calculated from a pre-defined look-up table and the concentration distribution can be estimated from the mass balance model of the VRB system.
This paper proposes an optimal charging scheme of a VRB system, which calculates the optimal charging and limiting current and dynamically optimizes the electrolyte flow rate to capture the maximum power from RESs, ensure proper safe operation of VRB and minimize the power loss in the energy storage system. The proposed control technique efficiently charges the energy storage system through a bidirectional isolated dc-dc converter [
18]. A single phase-shifted PI controller is used to operate the dc-dc converter as reported in [
19]. The rest of the paper is organized as follows: the nonlinear VRB model considering the concentration distribution of vanadium ions, mass balance, stack voltage, and the hydraulic system is presented in
Section 2.
Section 3 describes the isolated dc-dc converter topology and its control technique. The calculation of charging and limiting current, flow-rate optimization and the proposed control algorithm are demonstrated in
Section 4. The proposed optimal charging control scheme is investigated with a MATLAB/Simulink simulation, and the results and comparison studies are discussed in
Section 5 and
Section 6, respectively. Finally, the conclusions are drawn in
Section 7.
2. VRB Model
The schematic diagram of the VRB stack is presented in
Figure 1. This paper assumes that all the vanadium species are fully balanced in the VRB stack. The concentration distribution of all four vanadium ions can be accurately determined from the system SOC. The VRB offers a special advantage that the open circuit potential of inlet and outlet of VRB stack can be measured during real-time operation [
20]. The SOC of the battery cell (
) and tank (
) can be determined from the outlet
and inlet
open circuit potential as:
and
where,
is the formal potential,
n is the number of electrons transferred in the redox reaction,
F and
R are the Faraday and gas constant, and
T is temperature.
The concentration distribution of all four vanadium ions in the VRB system (cell and tank) becomes:
where, the concentration distribution of all vanadium ions in VRB cell and tank can be determined based on the total vanadium concentration (
) and SOC as:
and
2.1. VRB Stack Voltage
The total potential across a VRB cell
can be described as:
where,
i is the current density, (
) is the ohmic resistivity, and
is the over-potential in each VRB cell. Many research articles published to date use the geometric surface area
of the porous electrode for determining the current density
. This geometrical surface area-based current density is only valid for the compact electrodes. In the case of the practical porous electrode, the actual electrochemically active surface area can be significantly larger than the geometrical surface area. However, the determination of this actual surface area of the porous electrode can be difficult. Therefore, in this paper, we adopt a scaling factor
to represent the effect of the actual electrochemical surface area of the porous electrodes, as in [
21]. Considering this actual electrode surface area, the current density of the VRB system can be calculated as follows:
where,
is the charging current. The value of
k varies with the cell/stack design and the active electrode areas.
This cell over-potential
is the summation of concentration (
) and activation over-potential (
). The
is usually small and can be neglected when the surface area of porous electrode is large. On the other hand, the expression of concentration over-potential is:
where,
and
are the bulk and surface concentrations, respectively.
The concentrations of
and
ions in the electrode surface (
,
) depend on the bulk concentrations (
,
), and the current density
as:
and
where,
and
are the mass transfer coefficients in the negative (
or
ions) and positive (
or
ions) half-cell, respectively. The mass transfer coefficient for vanadium species
i, adopted from [
21], can be calculated as follows:
where,
is the diffusion coefficient of the vanadium species
i, in the electrode surface,
is the electrode fiber diameter,
and
are the electrolyte density and viscosity, respectively.
is the fluid velocity of the electrolyte, which can be calculated from the volumetric electrolyte flow rate (
) in the cell and the available electrode cross-sectional area (
) by assuming the isotropic behavior of the porous electrode, as follows:
Therefore, the stack voltage of the complete VRB system can be presented as:
2.2. Hydraulic System
The total pressure-drop of the VRB hydraulic system consists the pressure drops in the main piping system, channel and the porous electrodes as reported in [
22]. Therefore the total pressure-drop (
) in VRB system can be presented as:
where
is the dynamic viscosity of the electrolyte and
is the permeability of the porous electrode. This
can be described by Kozeny-Carman equation as:
where,
is the fiber diameter,
K is the Kozeny-Carman constant and
is the porosity of the porous electrode.
Hence, the total power consumed by the two pumps (
) of the VRB system can be presented as:
where,
is the pump efficiency. This paper considers a typical variable speed pump efficiency profile as presented in
Figure 2.
3. Design of Dual-Active-Bridge (DAB) Isolated dc-dc Converter
Energy storage system requires power electronic converters for sharing the electrical power between the battery storage devices and electricity networks. The dual-active-bridge (DAB) dc-dc converter offers high power transfer capabilities with galvanic isolation and works in a wide operating range [
19]. These characteristics enhance the uses of DAB dc-dc converter in the RES-based energy storage systems. The schematic diagram of the bidirectional dc-dc converter is presented in
Figure 3, which has two identical bridge converters. These two bridge converters are denoted as bridge 1 and bridge 2, which are isolated with a 20-kHz high-frequency transformer. Two different switching devices such as IGBT for bridge-1 and MOSFET for bridge 2 are used due to the operating voltage requirement of these two bridges. The soft-switching characteristics are also achieved by inserting a snubber circuit parallel with each semiconductor power switches.
The amount of power transfer (
P) through this bidirectional dc-dc converter is controlled by the phase-shift
between the two half-bridges as [
23]:
where,
and
are the voltage of the high and low voltage bus respectively,
N is transformer turns ration,
is switching frequency, and
L is the leakage inductance. Using the above relation of (
18), the current at the bridge 2 (
) can be controlled by the phase-shift as:
5. Results and Discussion
A MATLAB/Simulink model of VRB system with a power rating of 2.0 kW is used to verify the proposed optimal charging control scheme under the variable power condition. The detail dynamic model of the process is presented in the
Appendix A. The model assumes the battery cell stack acts as a continuous stir tank reactor (CSTR).
Table 1 and
Table 2 show all the parameters, which are used in design and simulation.
This paper considers the power loss associated with the VRB hydraulic system. This hydraulic power loss consists the power consumption by two pumps and the pressure losses across the piping system, flow frame, and porous electrodes. In this case, study, we consider the same cell/stack/electrode design (with the same active electrode area) as in [
21] and adopt the value of the scaling factor
from [
21].
Table 3 shows the parameters, which are considered for the hydraulic system and the
Figure 5 presents the pressure-drop
and total power consumption
by the electrical pumps of VRB.
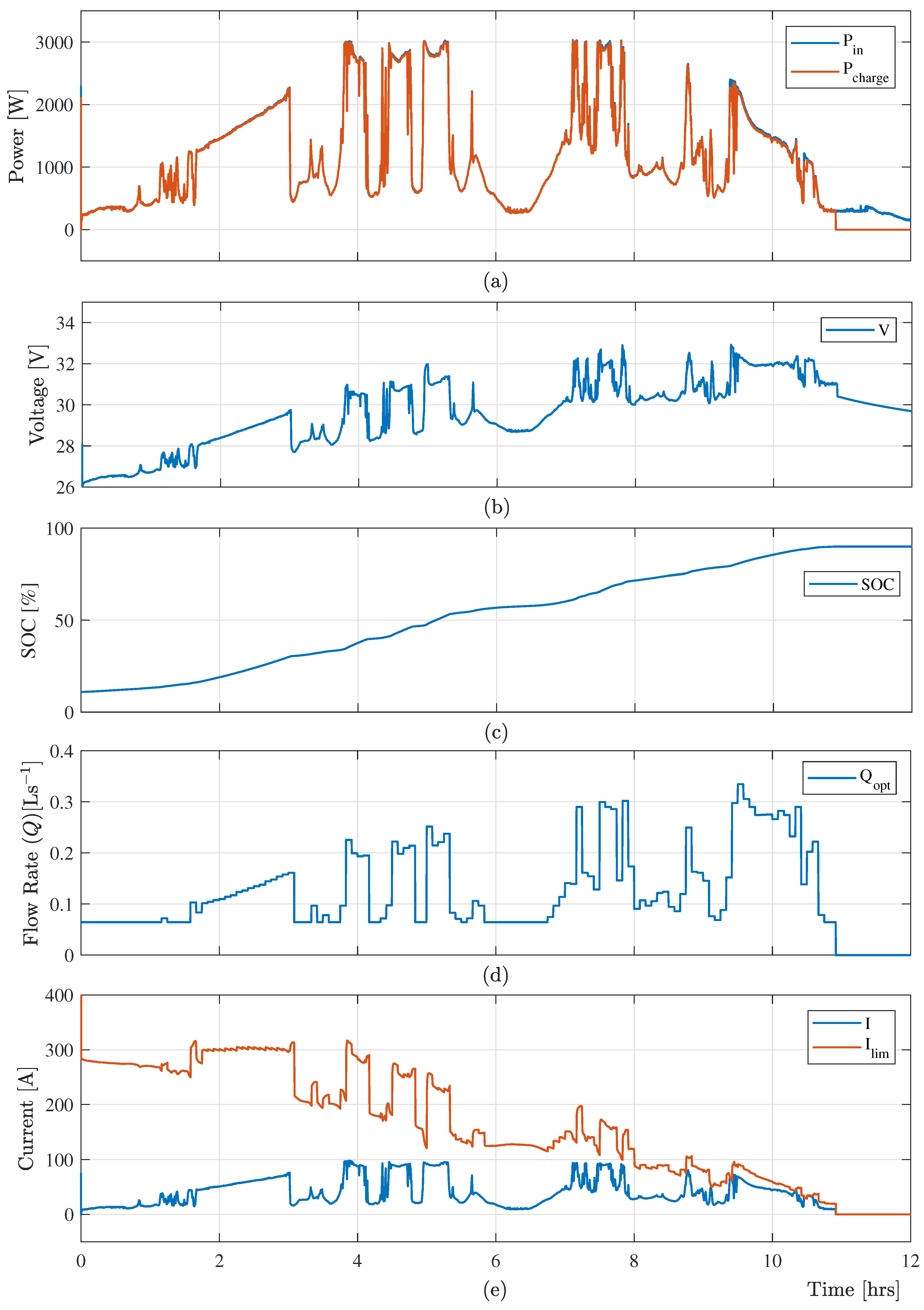
The real-time experimental data from a 3.0 kW (peak) photovoltaic (PV) system is considered in this paper as a variable input power. The power consumption
by the VRB system and the available variable input power
are presented in
Figure 6a. This
Figure 6a confirms that the proposed control algorithm provides a significant amount of charging power when the input power is low. Moreover, the proposed controller maintains minimum electrolyte flow under the low power to reduce the power loss in the VRB system as presented in
Figure 6d.
The optimal charging control scheme determines the current limit considering the available time-varying input power from RESs, current SOC, and electrolyte flow rate. This limiting current constraint protects the VRB system from gassing side-reactions and overcharging when the input power is high.
Figure 6b,e shows the battery voltage
, charging current
and limiting current
, which shows that the terminal voltage of the VRB system and the charging current are within their limit.
Using the charging current, the proposed optimal charging control algorithm effectively determine the optimized electrolyte flow rate (
) at a specified time-step according to the optimal cost function (
24).
Figure 6d shows the optimal flow, which ensures the maximum power consumed by the VRB system. Finally, the electrolyte flow rate and the charging current are switched off when the system SOC reached 90% as presented in
Figure 6c.
6. Comparison Studies
A comparative study of VRB charging system with different flow rate has been performed in this section to verify the effectiveness of the proposed optimal charging scheme.
Figure 7 the shows variable input power from RES
and charging power
, and measured charging
and limiting
with the minimum electrolyte flow rate (
). As the lower electrolyte flow minimizes the limiting current, the charging scheme associated with the minimum electrolyte flow rate
losses significant amount of free energy from the RESs.
Figure 7 confirms the loss of free energy from RESs, which limits the effectiveness of the minimum flow-rate-based charging controller. On the other hand, the maximum flow-rate-based VRB charging scheme also fails to charge the battery optimally as it increases the power losses in the hydraulic system.
Figure 8 shows that the maximum electrolyte flow-rate-based control method increases the limiting current, which ensures the maximum capturing of the free energy from RESs. This method causes huge power losses in the hydraulic system, which finally reduces the power harvesting of the VRB-based energy storage system from the RESs.
The limitations associated with the different constant flow-rate-based charging method can be overcome with the proposed optimal charging scheme, which applies the optimal variable electrolyte flow rate with a specific sampling time (
). The simulations results presented in
Figure 6a,e confirm that the proposed optimal charging algorithm ensures maximum capturing of free energy from RESs by changing the flow rate optimally. The optimal capturing of variable input power can be further verified with a variation of
change with different flow-rate condition as shown in
Figure 9.
Figure 9 shows that the VRB system takes 10.6 h minimum time to charge up to
with the optimal flow rate while other constant charging methods cannot charge fully within this time frame. Moreover,
Table 4 shows that at the minimum flow rate (
), only
of the available energy is used for charging due to the high concentration over-voltage at high currents and SOCs. At the maximum flow rate, only
of the available energy is used for charging since more energy is consumed by the pumps (
in this case). On the other hand, the optimal flow-rate control algorithm uses
of free energy, which is much higher compared with these constant flow-rate-based control algorithms.