Behavior of Battery Metals Lithium, Cobalt, Manganese and Lanthanum in Black Copper Smelting
Abstract
1. Introduction
1.1. Context
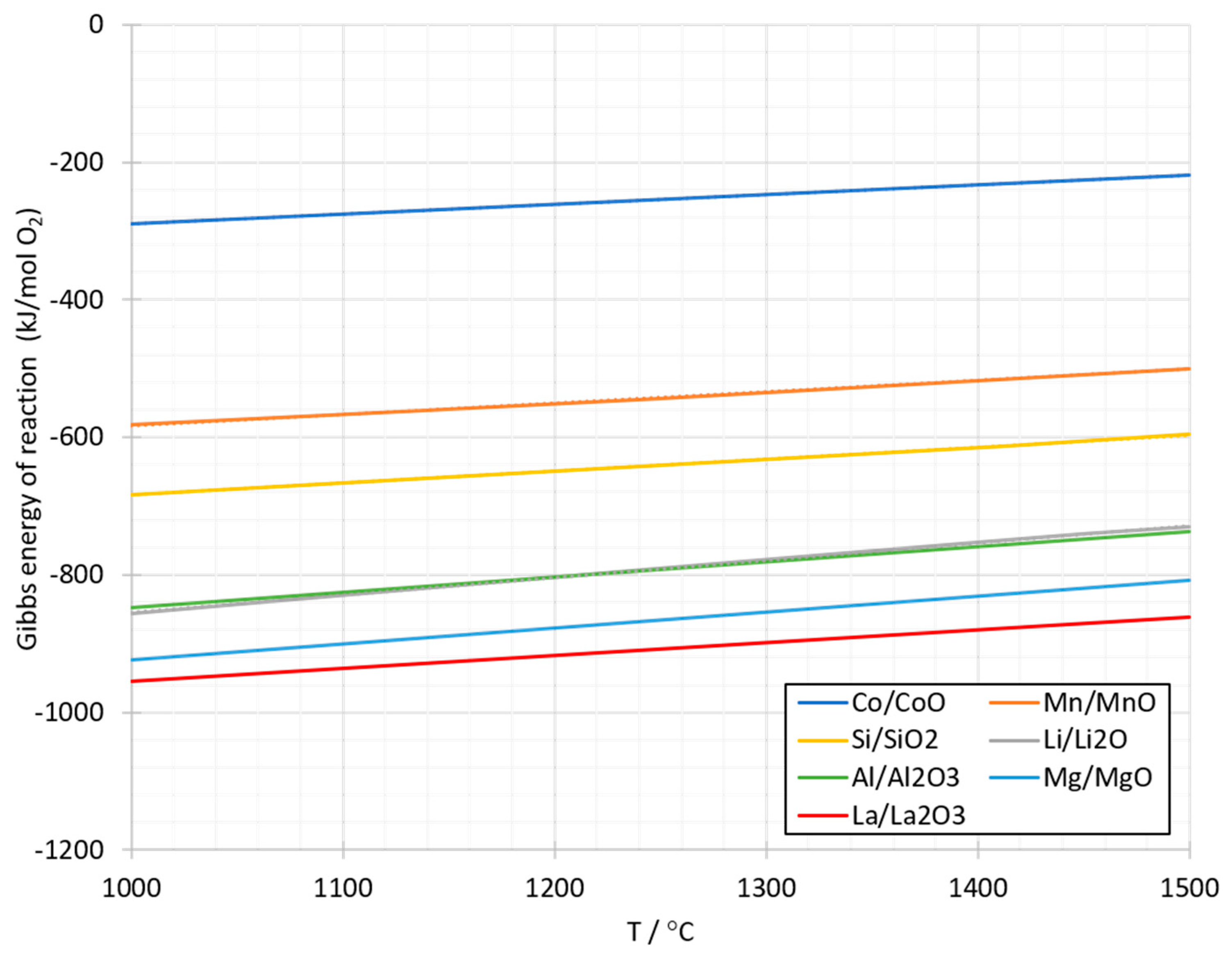
1.2. Literature
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
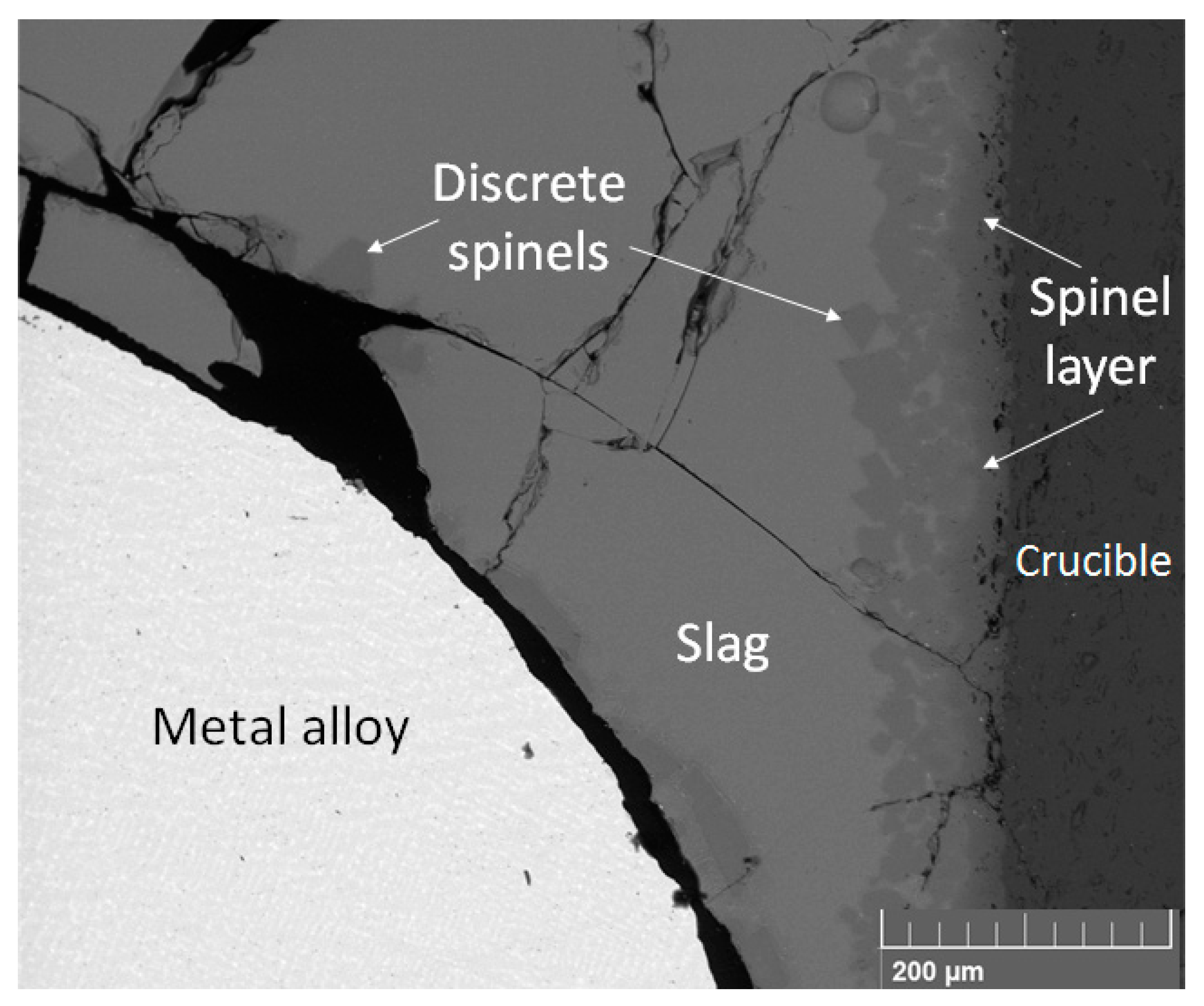
3.1. Equilibrium Sample Microstructure and Slag Composition
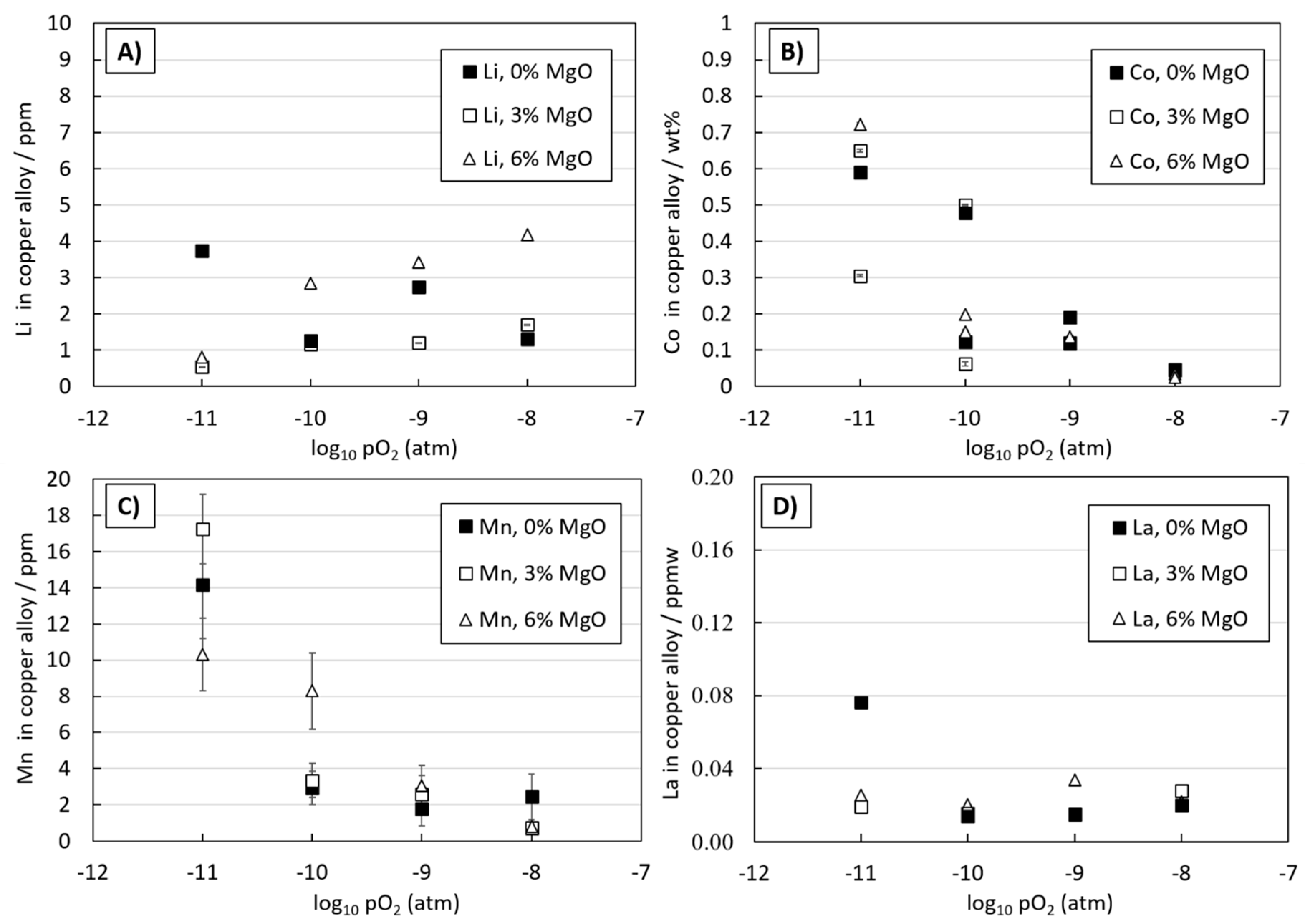
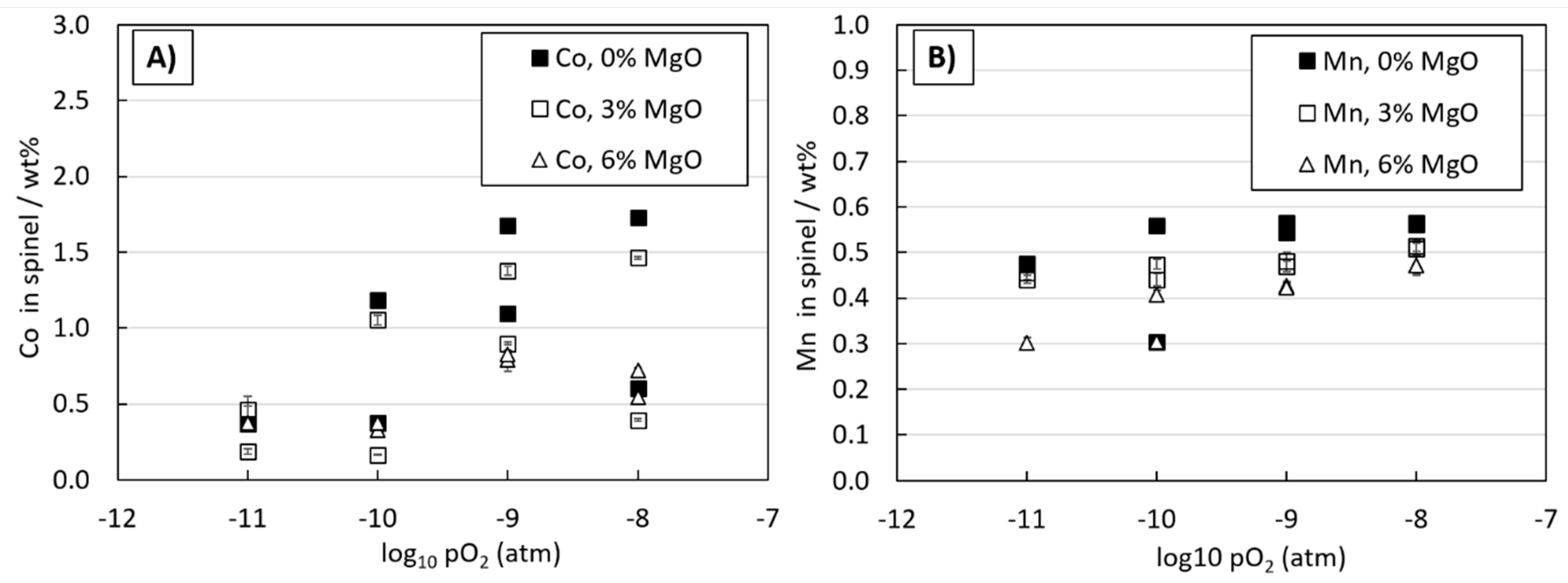
3.2. Li, Co, Mn, and La Behavior in Copper-Slag-Spinel System
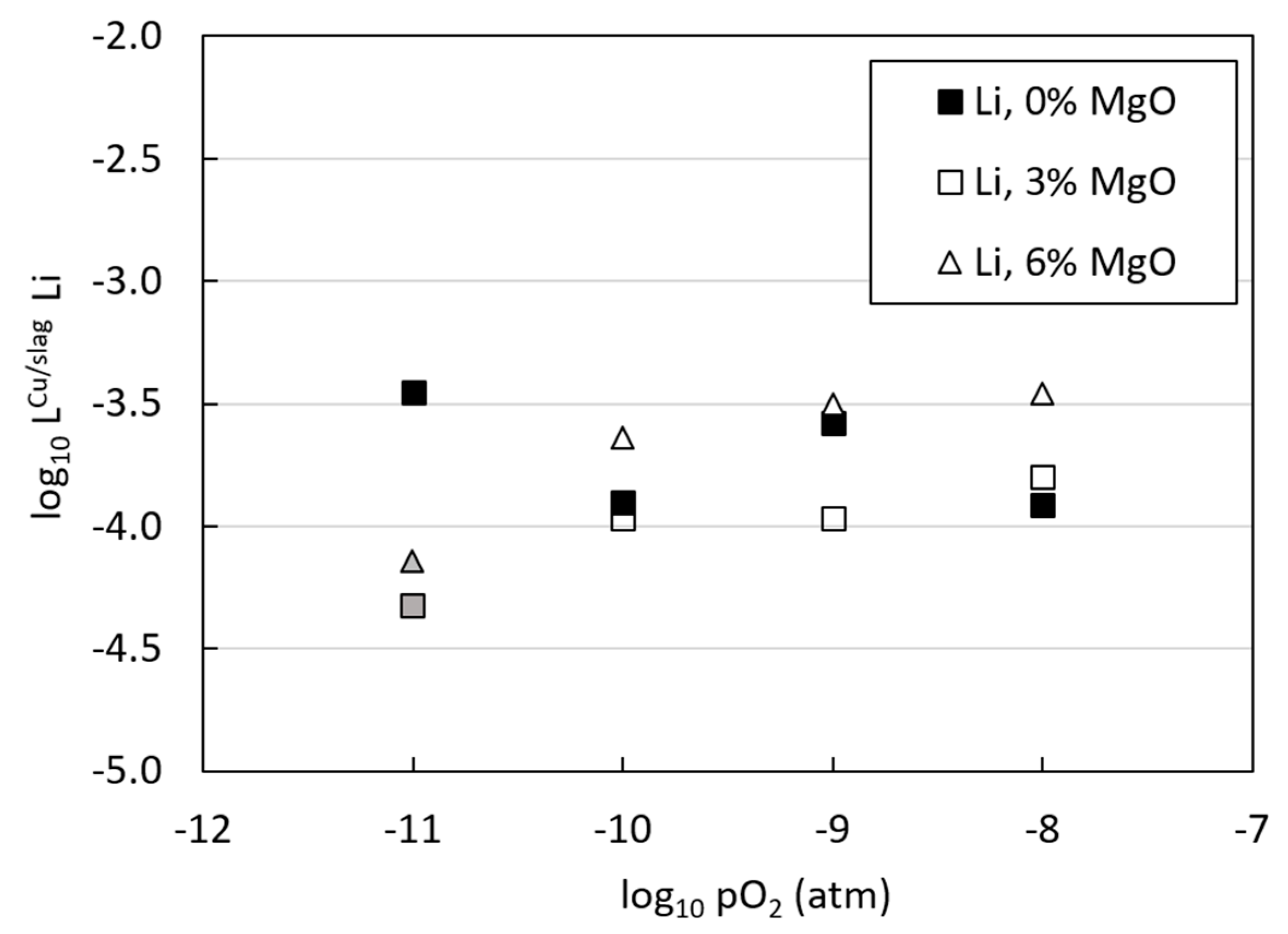
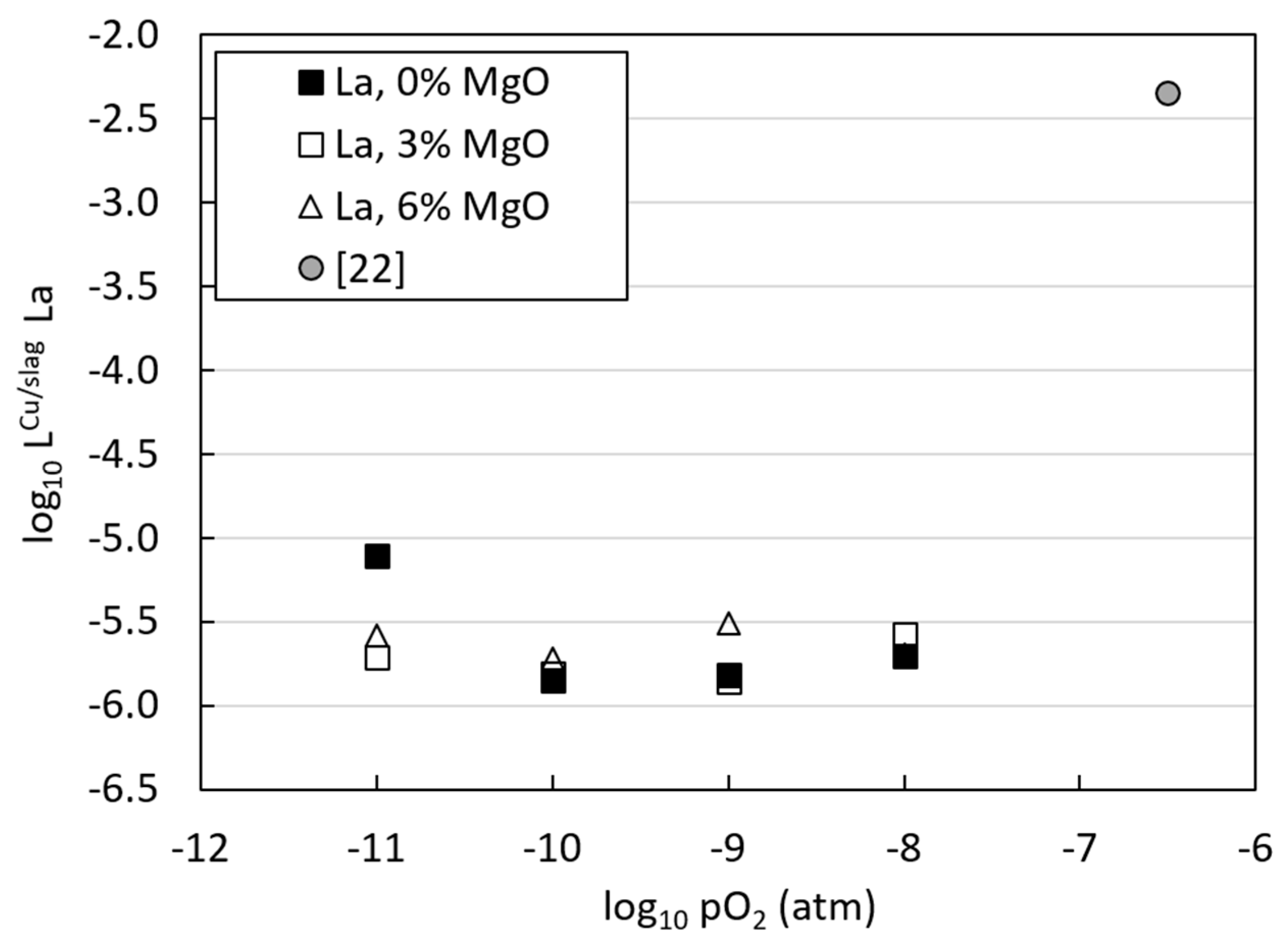
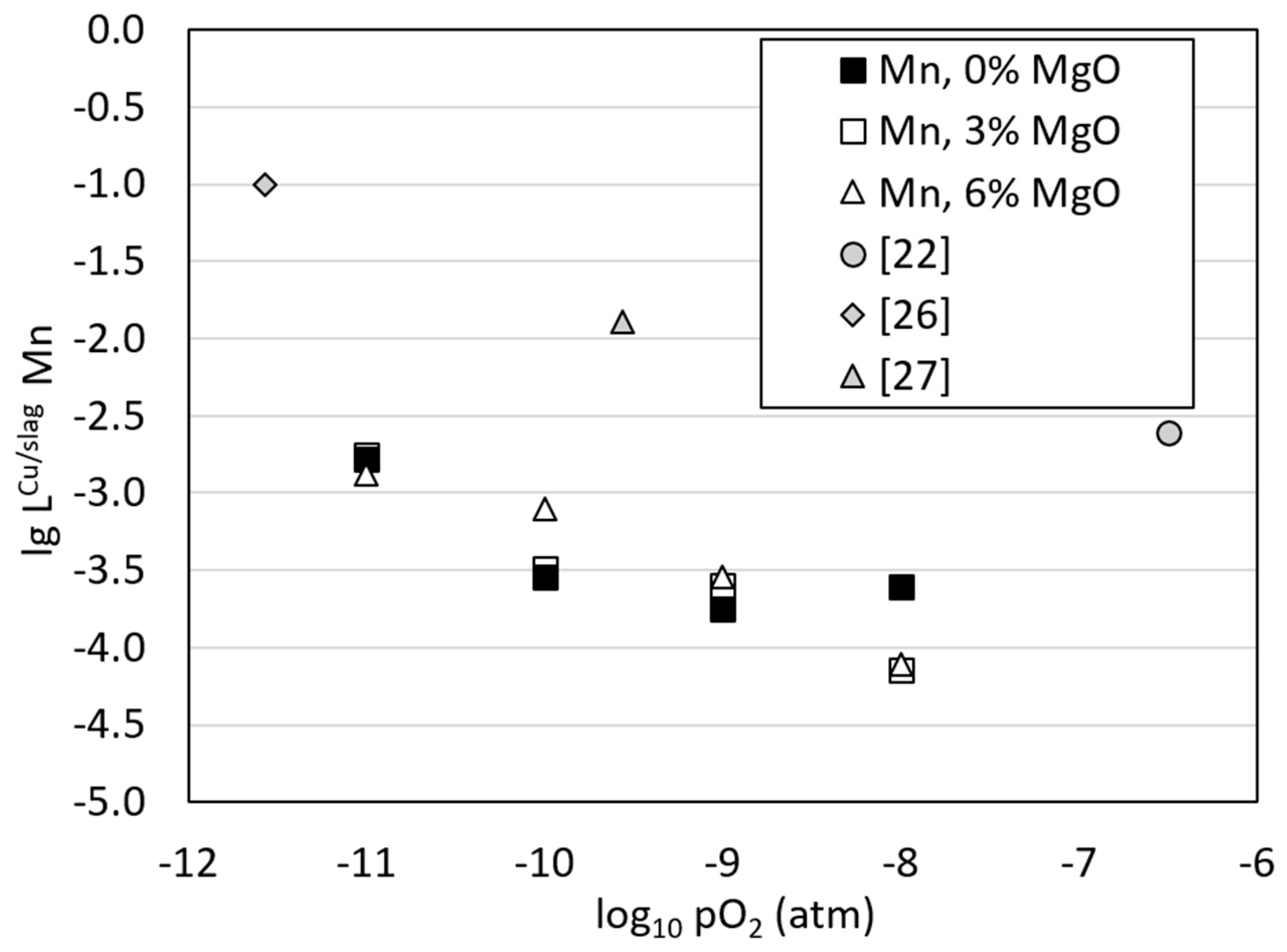
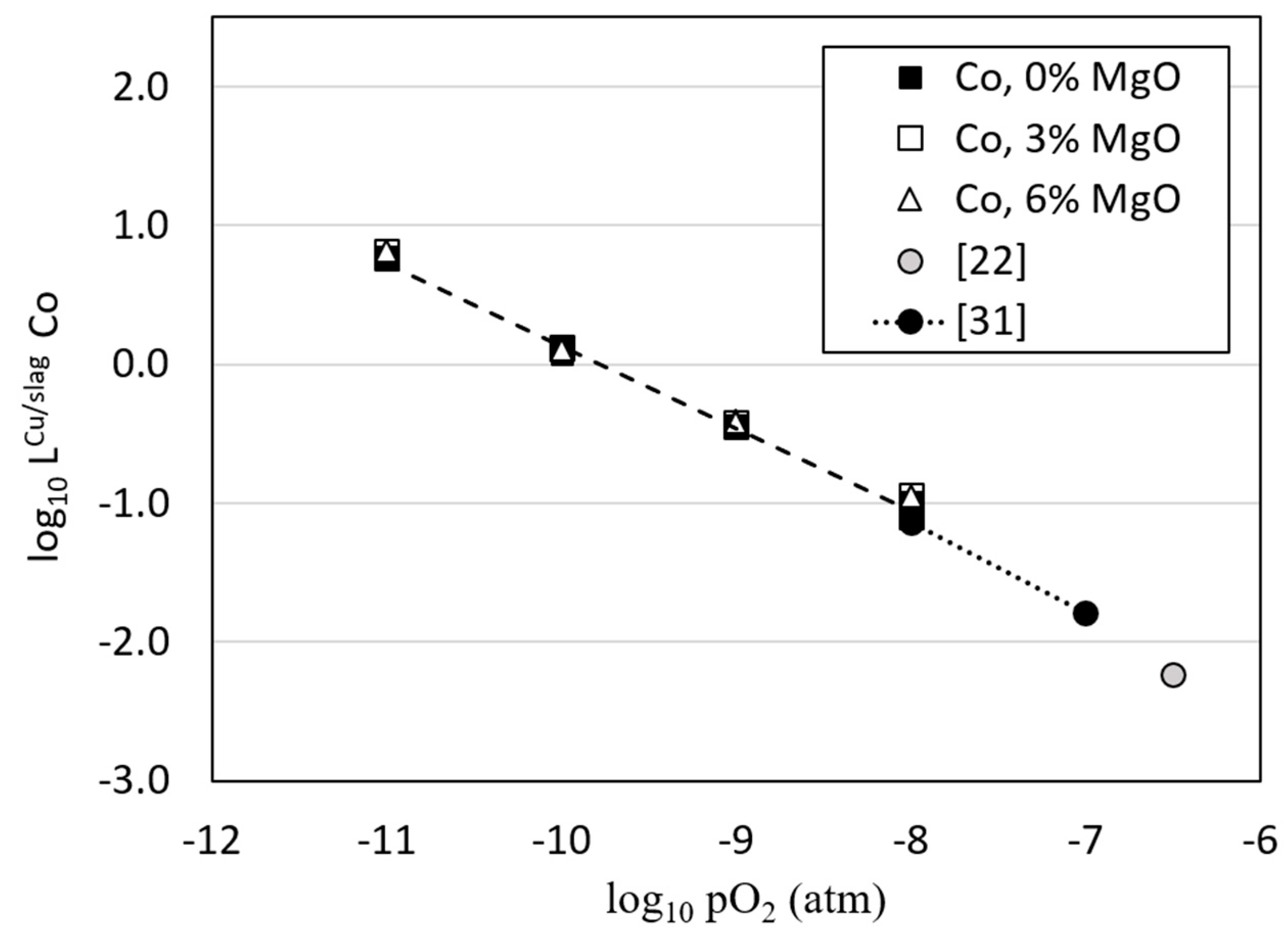
3.3. Distributions of Metals between Copper Alloy and Slag
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| log10 p(O2)/atm | MgO in slag/wt% | Phase | Average Concentration/ppmw | Standard Deviation/ppmw | ||||
| Co | Mn | La | Co | Mn | La | |||
| −8 | 0 | metal | 467.2 | <dl | <dl | 32.6 | - | - |
| slag | 5741 | 9840 | 10,171 | 34.4 | 155.2 | 137.8 | ||
| metal | 186.9 | <dl | <dl | 28.8 | - | - | ||
| slag | 1831 | 9657 | 10,135 | 33.5 | 130.6 | 87.8 | ||
| −9 | 0 | metal | 1913 | <dl | <dl | 29.1 | - | - |
| slag | 5527 | 9975 | 9850 | 58.7 | 80.2 | 187.5 | ||
| metal | 1182 | <dl | <dl | 56.2 | - | - | ||
| slag | 3426 | 9999 | 9903 | 73.2 | 184.2 | 307.5 | ||
| −10 | 0 | metal | 4786 | <dl | <dl | 101.7 | - | - |
| slag | 3719 | 10,194 | 9982 | 41.5 | 96.5 | 190.2 | ||
| metal | 1217 | <dl | <dl | 37.0 | - | - | ||
| slag | 1021 | 8185 | 9438 | 39.2 | 251.2 | 285.6 | ||
| −11 | 0 | metal | 5897 | <dl | <dl | 72.6 | - | - |
| slag | 996.5 | 8574 | 9825 | 36.7 | 115.7 | 210.9 | ||
| log10 p(O2)/atm | MgO in slag/wt% | Phase | Average Concentration/ppmw | Standard Deviation/ppmw | ||||
| Co | Mn | La | Co | Mn | Co | |||
| −8 | 3 | metal | 452.7 | <dl | <dl | 22.0 | - | - |
| slag | 5699 | 10,079 | 10,382 | 173.7 | 108.3 | 217.3 | ||
| metal | 154.2 | <dl | <dl | 29.7 | - | - | ||
| slag | 1349 | 10,168 | 10,487 | 34.4 | 180.2 | 76.8 | ||
| −9 | 3 | metal | 1902 | <dl | <dl | 40.8 | - | - |
| slag | 4942 | 10,163 | 10470 | 36.4 | 172.0 | 235.4 | ||
| metal | 1200 | <dl | <dl | 22.5 | - | - | ||
| slag | 3332 | 10,155 | 10,041 | 69.6 | 165.9 | 285.3 | ||
| −10 | 3 | metal | 4993 | <dl | <dl | 58.1 | - | - |
| slag | 3722 | 10,330 | 9949 | 69.4 | 79.0 | 187.0 | ||
| metal | 628.0 | <dl | <dl | 38.0 | - | - | ||
| slag | 479.5 | 9294 | 9425 | 72.3 | 231.6 | 275.8 | ||
| −11 | 3 | metal | 3052 | <dl | <dl | 43.4 | - | - |
| slag | 468.9 | 9717 | 9742 | 46.3 | 142.7 | 136.7 | ||
| metal | 6491 | <dl | <dl | 316.3 | - | - | ||
| slag | 1117 | 9677 | 9708 | 47.9 | 250.1 | 223.0 | ||
| log10 p(O2)/atm | MgO in slag/wt% | Phase | Average Concentration/ppmw | Standard Deviation/ppmw | ||||
| Co | Mn | La | Co | Mn | Co | |||
| −8 | 6 | metal | 345.2 | <dl | <dl | 21.0 | - | - |
| slag | 3185 | 10,243 | 10,757 | 49.8 | 119.9 | 129.6 | ||
| metal | 251.4 | <dl | <dl | 47.9 | - | - | ||
| slag | 2234 | 10,437 | 11,378 | 86.8 | 138.1 | 141.6 | ||
| −9 | 6 | metal | 1341 | <dl | <dl | 42.2 | - | - |
| slag | 3440 | 10,519 | 10,799 | 40.0 | 162.7 | 257.4 | ||
| metal | 1362 | <dl | <dl | 36.3 | - | - | ||
| slag | 3585 | 10,151 | 10,549 | 55.5 | 210.9 | 256.8 | ||
| −10 | 6 | metal | 1515 | <dl | <dl | 54.3 | - | - |
| slag | 1127 | 10,555 | 10,537 | 30.7 | 194.3 | 199.1 | ||
| metal | 1983 | <dl | <dl | 49.0 | - | - | ||
| slag | 1567 | 7286 | 9170 | 40.9 | 151.3 | 356.1 | ||
| −11 | 6 | metal | 7224 | <dl | <dl | 55.3 | - | - |
| slag | 1108 | 7749 | 9777 | 40.1 | 53.7 | 106.2 | ||
| log10 p(O2)/atm | MgO in slag/wt% | Phase | Average Concentration/ppm | Standard Deviation/ppm | |||
| Li | Mn | La ** | Li ** | Mn | |||
| −8 | 0 | metal | 1.31(1)* | 2.44(2) | 0.020(1) | - | 1.27 |
| slag | 10,704 | - | - | 220.10 | - | ||
| −9 | 0 | metal | 2.75(2) | 1.77(6) | 0.015(3) | - | 0.93 |
| slag | 10,466 | - | - | 210.24 | - | ||
| −10 | 0 | metal | 1.27(1) | 2.939(6) | 0.014(2) | - | 0.93 |
| slag | 10,170.67 | - | - | 139.63 | - | ||
| −11 | 0 | metal | 3.74(2) | 14.14(6) | 0.08(7) | - | 2.97 |
| slag | 10,591.24 | - | - | 153.48 | - | ||
| log10 p(O2)/atm | MgO in slag/wt% | Phase | Average Concentration/ppm | Standard Deviation/ppm | |||
| Li | Mn | La | Li | Mn | |||
| −8 | 3 | metal | 1.70(1) | 0.73(4) | 0.028(3) | 0.21 | |
| slag | 10,699.18 | - | - | 274.3 | - | ||
| −9 | 3 | metal | 1.21(2) | 2.56(7) | 0.015(4) | 1.05 | |
| slag | 11,258.77 | - | - | 241.4 | - | ||
| −10 | 3 | metal | 1.17(2) | 3.35(8) | 0.016(2) | 0.96 | |
| slag | 10,807.25 | - | - | 257.7 | - | ||
| −11 | 3 | metal | <dl | 17.25(8) | 0.019(6) | - | 1.94 |
| slag | 11,512.41 | - | - | 185.0 | - | ||
| log10 p(O2)/atm | MgO in slag/wt% | Phase | Average Concentration/ppm | Standard Deviation/ppm | |||
| Li | Mn | La | Li | Mn | |||
| −8 | 6 | metal | 4.18(2) | 0.80(4) | 0.022(3) | 0.08 | |
| slag | 12,035 | - | - | 209.5 | - | ||
| −9 | 6 | metal | 3.43(4) | 3.04(8) | 0.034(5) | 1.13 | |
| slag | 10,866 | - | - | 238.9 | - | ||
| −10 | 6 | metal | 2.85(4) | 8.28(8) | 0.020(5) | 2.11 | |
| slag | 12,462 | - | - | 217.1 | - | ||
| −11 | 6 | metal | <dl | 10.32(8) | 0.026(4) | - | 2.01 |
| slag | 11,242 | - | - | 293.9 | - | ||
Appendix B
References
- Pinegar, H.; Smitch, Y.R. Recycling of End-of Life Lithium Ion Batteries, Part 1: Commercial Processes. J. Sustain. Met. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Business Finland 201s9. Available online: https://www.businessfinland.fi/en/for-finnish-customers/services/programs/batteries-from-finland/ (accessed on 21 August 2019).
- Liu, L.; Park, J.; Lin, X.; Sastry, A.M.; Lu, W. A thermal-electrochemical model that gives spatial-dependent growth of solid electrolyte interphase in a Li-ion battery. J. Power Sources 2014, 268, 482–490. [Google Scholar] [CrossRef]
- Lin, X.; Park, J.; Liu, L.; Lee, Y.; Sastry, A.M.; Lua, W. A Comprehensive Capacity Fade Model and Analysisfor Li-Ion Batteries. J. Electrochem. Soc. 2013, 160, A1701–A1710. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L. Optimal Design of Li-Ion Batteries through Multi-PhysicsModeling and Multi-Objective Optimization. J. Electrochem. Soc. 2017, 164, E3254–E3264. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Mansur, M.B. Recycling Batteries. Available online: https://doi.org/10.1016/B978-0-08-102158-3.00014-8 (accessed on 28 February 2020).
- Veloso, L.R.S.; Rodrigues, L.E.O.C.; Ferreira, D.A.; Magalhães, F.S.; Mansur, M.B. Development of a hydrometallurgical route for the recovery of zinc and manganese from spent alkaline batteries. J. Power Sources 2005, 152, 295–302. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Sverdrup, H.U.; Ragnarsdottir, K.V.; Koca, D. An assessment of metal supply sustainability as an input to policy: Security of supply extraction rates, stocks-in-use, recycling, and risk of scarcity. J Clean. Prod. 2017, 140, 359–372. [Google Scholar] [CrossRef]
- Shedd, K. Mineral Commodity Summaries of Cobalt. U.S. Geological Survey. 2019. Available online: https://prd-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/s3fs-public/atoms/files/mcs-2019-cobal_0.pdf (accessed on 21 August 2019).
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef]
- Shedd, K. Mineral Commodity Summaries of Lithium. U.S. Geological Survey. 2019. Available online: https://prd-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/atoms/files/mcs-2019-lithi.pdf (accessed on 21 August 2019).
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research —Global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Reck, B.K.; Graedel, T.E. Challenges in metal recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef]
- Helbig, C.; Bradshaw, A.M.; Wietschel, L.; Thorenz, A.; Tuma, A. Supply risks associated with lithium-ion battery materials. J. Clean. Prod. 2017, 172, 274–286. [Google Scholar] [CrossRef]
- Shuva, M.A.H.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.; Reuter, M.A. Thermodynamics data of valuable elements relevant to e-waste processing through primary and secondary copper production: A review. J. Clean. Prod. 2016, 131, 795–809. [Google Scholar] [CrossRef]
- EU Report on Critical Raw Materials and the Circular Economy 2018. Commission Staff Working Document. Available online: http://ec.europa.eu/docsroom/documents/27348 (accessed on 12 June 2018).
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Cheret, D.; Santen, S. Battery Recycling. U.S. Patent 7,169,206 B2, 30 January 2007. [Google Scholar]
- Tirronen, T.; Sukhomlinov, D.; O’Brien, H.; Taskinen, P.; Lundström, M. Distributions of lithium-ion and nickel-metal hydride battery elements in copper converting. J. Clean. Prod. 2017, 168, 399–409. [Google Scholar] [CrossRef]
- Kim, H.G.; Sohn, H.Y. Effects of CaO, Al2O3, and MgO additions on the copper solubility, ferric/ferrous ratio, and minor-element behavior of iron-silicate slags. Metall. Mater. Trans. B 1998, 29, 583–590. [Google Scholar] [CrossRef]
- Takeda, Y.; Ishiwata, S.; Yazawa, A. Distribution equilibria of minor elements between liquid copper and calcium ferrite slag. Trans. Jpn. Inst. Met. 1983, 24, 518–528. [Google Scholar] [CrossRef]
- Liu, X.; Grimsey, E.J. The effect of silica, alumina, calcia and magnesia on the activity coefficient of cobalt oxide in iron silicate slags. In Proceedings of the 5th International Conference on Molten Slags, Fluxes and Salts ’97, Sydney, Australia, 5–8 January 1997; pp. 709–718. [Google Scholar]
- Jung, S.M.; Kim, S.H.; Rhee, C.H.; Min, D.J. Thermodynamic Containing FeO Study on MnO Behavior in MgO-saturated Slag. ISIJ Int. 1993, 33, 1049–1054. [Google Scholar] [CrossRef][Green Version]
- Eom, C.H.; Lee, S.H.; Park, J.G.; Park, J.H.; Min, D.J. Thermodynamic Behavior of Manganese Oxide in Lime-based Manganese Smelting Slags. ISIJ Int. 2015, 56, 37–43. [Google Scholar] [CrossRef]
- Haccuria, E.; Hayes, P.C.; Jak, E. Phase equilibria studies of the “MnO”–Al2O3–SiO2 system in equilibrium with metallic alloy. Part 1: Development of the technique and determination of liquidus isotherms between 1 423 K and 1 523 K. Int. J. Mater. Res. 2014, 105, 941–952. [Google Scholar] [CrossRef]
- Haccuria, E.; Hayes, P.C.; Jak, E. Phase equilibria studies of the “MnO”–Al2O3–SiO2 system in equilibrium with metallic alloy. Part 2: Phase equilibria. Int. J. Mater. Res. 2015, 106, 225–236. [Google Scholar] [CrossRef]
- Guoxing, R.; Songwen, X.; Meiqiu, X.; Bing, P.; Youqi, F.; Fenggang, W.; Xing, X. Recovery of valuable metals from spent lithium-ion batteries by smelting reduction process based on MnO-SiO2-Al2O3 slag system. In Advances in Molten Slags, Fluxes, and Salts: Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts (MOLTEN16); Reddy, R.G., Chaubal, P., Pistorius, P.C., Pal, U., Eds.; TMS (The Minerals, Metals & Materials Society): Pittsburgh, PA, USA, 2016. [Google Scholar]
- Sukhomlinov, D.; Klemettinen, L.; Avarmaa, K.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. Distribution of Ni, Co, Precious, and Platinum Group Metals in Copper Making Process. Metall. Mater. Trans. B 2019, 50, 1752–1765. [Google Scholar] [CrossRef]
- Sun, T.; Kennedy, M.W.; Yurramendi, L.; Aldana, J.L.; Del Rio, C.; Arnout, S.; Tranell, G.; Aune, R.E. Pyrometallurgical Treatment of Apatite Concentrate with the Objective of Rare Earth Element Extraction: Part I. J. Sustain. Metall. 2017, 3, 829–845. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- Klemettinen, L.; Aromaa, R.; Dańczak, A.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. Distribution Kinetics of Rare Earth Elements in Copper Smelting. Sustainability 2020, 12, 208. [Google Scholar] [CrossRef]
- Piskunen, P.; Avarmaa, K.; O’Bren, H.; Klemettinen, L.; Johto, H.; Taskinen, P. Precious Metal Distributions in Direct Nickel Matte Smelting with Low-Cu Mattes. Metall. Mater. Trans. B 2017, 49, 98–112. [Google Scholar] [CrossRef]
- Klemettinen, L.; Avarmaa, K.; Taskinen, P. Slag Chemistry of High-Alumina Iron Silicate Slags at 1300 °C in WEEE Smelting. J. Sustain. Metall. 2017, 3, 772–781. [Google Scholar] [CrossRef]
- Klemettinen, L.; Avarmaa, K.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. Behavior of Tin and Antimony in Secondary Copper Smelting Process. Minerals 2019, 9, 39. [Google Scholar] [CrossRef]
- Davies, R.H.; Dinsdale, A.T.; Gisby, J.A.; Robinson, J.A.J.; Martin, S.M. MTDATA—Thermodynamics and Phase Equilibrium Software from the National Physical Laboratory. CALPHAD 2002, 26, 229–271. [Google Scholar] [CrossRef]
- Bastin, G.F.; Heijligers, H.J.M. Quantitative Electron Probe Microanalysis of Ultralight Elements (Boron-Oxygen). Scanning 1990, 12, 225–236. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. Basic expression of “PAP” computation for quantitative EPMA. In Proceedings of the 11th International Congress on X-ray Optics and Microanalysis (ICXOM), London, ON, Canada, 4–8 August 1986; Brown, J.D., Packwood, R.H., Eds.; University of Western Ontario: London, ON, Canada, 1986; pp. 249–256. [Google Scholar]
- UQAC Pressed Sulphide Pellet Reference Material. Available online: https://sulfideslasericpms.wordpress.com/rm-available/ (accessed on 21 August 2019).
- Wilson, S.A.; Ridley, W.I.; Koenig, A.E. Development of sulfide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. Atom. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610-617 Glasses Following ISO Guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Avarmaa, K.; Yliaho, S.; Taskinen, P. Recoveries of rare elements Ga, Ge, in and Sn from waste electric and electronic equipment through secondary copper smelting. Waste Manag. 2018, 71, 400–410. [Google Scholar] [CrossRef] [PubMed]

| Chemical | Purity (wt%) | Form | Supplier |
|---|---|---|---|
| Cu | 99.9% | powder | Alfa Aesar, Kandel, Germany |
| Co | 99.99% | sponge | Koch-Light Laboratories Ltd, Colnbrook, UK |
| Ni | 99.996% | powder, APS 3‒7 micron | Alfa Aesar, Kandel, Germany |
| Sn | 99.85% | powder, 100 mesh | Alfa Aesar, Kandel, Germany |
| La2O3 | 99.9% | powder | Alfa Aesar, Kandel, Germany |
| MnO | 99.99% | powder/chunks | Alfa Aesar, Kandel, Germany |
| Li2CO3 | 99.998% | powder | Alfa Aesar, Kandel, Germany |
| MgO | 99.95 | powder, 325 mesh | Alfa Aesar, Kandel, Germany |
| Al2O3 | 99.99% | powder | Sigma Aldrich, St. Louis, MO, USA |
| SiO2 | 99.995% | powder, 40 mesh | Alfa Aesar, Kandel, Germany |
| Fe2O3 | 99.999% | powder | Alfa Aesar, Kandel, Germany |
| log10 p(O2)/atm | νCO2 (mL/min) | νCO (mL/min) | CO2/CO |
|---|---|---|---|
| −11.0 | 56.0 | 244.0 | 0.23 |
| −10.0 | 125.6 | 174.5 | 0.72 |
| −9.0 | 208.5 | 91.5 | 2.28 |
| −8.0 | 263.4 | 36.6 | 7.20 |
| EPMA Detection Limit (ppmw) | ||||||||||||||||
| Phase | O | Si | Al | Mg | Fe | Ni | Cu | Co | Mn | La | Sn | |||||
| Cu-alloy | 451 | 149 | 133 | 175 | 77 | 268 | 348 | 80 | 84 | 247 | 153 | |||||
| Slag | 1088 | 203 | 203 | 91 | 199 | 94 | 278 | 105 | 196 | 278 | 146 | |||||
| Spinel | 1082 | 81 | 216 | 91 | 204 | 111 | 113 | 118 | 194 | 199 | 145 | |||||
| LA-ICP-MS Detection Limit (ppmw) | ||||||||||||||||
| Phase | Si | Ni | Co | Mn | Li | La | ||||||||||
| Cu-alloy | 29Si: 54.0 | 61Ni: 4.05 1 | 59Co:0.07 | 55Mn:0.35 | 7Li:0.81 | 139La 0.01 | ||||||||||
| Slag | 29Si: 5.54 2 | 61Ni: 0.57 | 59Co:0.01 | 55Mn:0.03 | 7Li:0.12 | 139La:0.001 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dańczak, A.; Klemettinen, L.; Kurhila, M.; Taskinen, P.; Lindberg, D.; Jokilaakso, A. Behavior of Battery Metals Lithium, Cobalt, Manganese and Lanthanum in Black Copper Smelting. Batteries 2020, 6, 16. https://doi.org/10.3390/batteries6010016
Dańczak A, Klemettinen L, Kurhila M, Taskinen P, Lindberg D, Jokilaakso A. Behavior of Battery Metals Lithium, Cobalt, Manganese and Lanthanum in Black Copper Smelting. Batteries. 2020; 6(1):16. https://doi.org/10.3390/batteries6010016
Chicago/Turabian StyleDańczak, Anna, Lassi Klemettinen, Matti Kurhila, Pekka Taskinen, Daniel Lindberg, and Ari Jokilaakso. 2020. "Behavior of Battery Metals Lithium, Cobalt, Manganese and Lanthanum in Black Copper Smelting" Batteries 6, no. 1: 16. https://doi.org/10.3390/batteries6010016
APA StyleDańczak, A., Klemettinen, L., Kurhila, M., Taskinen, P., Lindberg, D., & Jokilaakso, A. (2020). Behavior of Battery Metals Lithium, Cobalt, Manganese and Lanthanum in Black Copper Smelting. Batteries, 6(1), 16. https://doi.org/10.3390/batteries6010016






