The Toxicity of Secondary Lithium-Sulfur Batteries Components
Abstract
:1. Introduction
- (a)
- Batteries electric vehicles (BEV), with the devices storing the electric energy from the electric network during the charging process and feeding an electric motor of the vehicles during the discharging process;
- (b)
- Fuel cells electric vehicles (FCEV), with the polymer electrolyte fuel cells (PEFCs) producing all the electrical energy needed to the electric motor of the vehicles. The PEFCs are fed by hydrogen accumulated in a suitable system on the vehicles board or which is steadily generated from hydrocarbons or alcohols by a proper generation system;
- (c)
- Hybrid electric vehicles (HEVs), with batteries and/or PEFCs integrated into a hybrid system feeding the electric motor of the vehicles with or without ICE.
2. Results and Discussion
2.1. Materials Used to Limit the Shuttle-Effect in Li-S Cells
2.2. Cell Configuration
2.3. Materials for the Cathode
2.4. Binders
2.5. Electrolyces
2.5.1. Liquid Electrolytes
Ether-Based Electrolytes
Solvents
Lithium Salts
Electrolytes Based on Ionic Liquids
Carbonate-Based Electrolytes
2.5.2. Solid Electrolytes
Solid Polymer Electrolytes
Gel Polymer Electrolytes
Non-Polymer Electrolytes
2.5.3. Electrolyte Additives
Lithium Nitrates
Polysulphides
Phosphorus Pentasulfide
2.6. Anode Materials
2.6.1. Lithium Anode
2.6.2. Protected Anodes from Metallic Lithium
2.7. Separators
3. Materials and Methods
4. Summary
Funding
Conflicts of Interest
References
- De Lorenzo, G.; Andaloro, L.; Sergi, F.; Napoli, G.; Ferraro, M.; Antonucci, V. Numerical simulation model for the preliminary design of hybrid electric city bus power train with polymer electrolyte fuel cell. Int. J. Hydrog. Energy 2014, 39, 12934–12947. [Google Scholar] [CrossRef]
- De Luca, D.; Fragiacomo, P.; De Lorenzo, G.; Czarnetzki, W.T.; Schneider, W. Strategies for Dimensioning Two-Wheeled Fuel Cell Hybrid Electric Vehicles Using Numerical Analysis Software. Fuel Cells 2016, 16, 628–639. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Astorino, E.; Chippari, G.; De Lorenzo, G.; Czarnetzki, W.T.; Schneider, W. Dynamic modeling of a hybrid electric system based on an anion exchange membrane fuel cell. Cogent. Eng. 2017, 4, 1357891. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Zhou, T.; Yang, K.; Wei, S.; Tang, N.; Dang, N.; Li, H.; Qiu, X.; Chen, L. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery. Nano Energy 2016, 27, 313–319. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, M.K.; Kim, H.S.; Lee, B.M. Risk assessment of lithium-ion battery explosion: Chemical leakages. J. Toxicol. Environ. Health Part B 2018, 21, 370–381. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.Z.; Chung, S.H.; Zu, C.X.; Su, Y.S. Rechargeable lithium-sulfur batteries. Chem Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Son, Y.; Lee, J.S.; Son, Y.; Jang, J.H.; Cho, J. Recent advances in lithium sulfide cathode materials and their use in lithium sulfur batteries. Adv. Energy Mater. 2015, 5, 1500110. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Li, T.; Gao, X.; Yuan, C. Life cycle assessment of lithium sulfur battery for electric vehicles. J. Power Sources 2017, 343, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Mikhaylik, Y.; Kovalev, I.; Schock, R.; Kumaresan, K.; Xu, J.; Affinito, J. High energy rechargeable Li-S cells for EV application. status, remaining problems and solutions. ECS Trans. 2010, 25, 23–34. [Google Scholar] [CrossRef]
- Herbert, D.; Ulam, J. Electric dry cells and storage batteries. U.S. Patent 3,043,896, 10 July 1962. [Google Scholar]
- Rao, M.L.B. Organic electrolyte cells. U.S. Patent 3,413,154, 26 November 1968. [Google Scholar]
- Chem-Supply PTY Ltd. Sulfur. CAS No.: 7704-34-9. Safety Data Sheet. (ABN 19008264211). 2014. Available online: https://www.chemsupply.com.au/documents/ST0061CH71.pdf (accessed on 22 May 2020).
- Sigma-Aldrich. Sulfur. CAS No.: 7704-34-9. Material Safety Data Sheet, version 3.4. 2012. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- Ji, X.; Nazar, L.F. Advances in Li-S batteries. J. Mater. Chem. 2010, 20, 9821. [Google Scholar] [CrossRef]
- Barghamadi, M.; Kapoor, A.; Wen, C. A review on Li-S batteries as a high efficiency rechargeable lithium battery. J. Electrochem. Soc. 2013, 160, A1256–A1263. [Google Scholar] [CrossRef]
- Bresser, D.; Passerini, S.; Scrosati, B. Recent progress and remaining challenges in sulfur-based lithium secondary batteries—A review. Chem. Commun. 2013, 49, 10545–10562. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New approaches for high energy density lithium-sulfur battery cathodes. ACC Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.S. Challenges and prospects of lithium-sulfur batteries. ACC Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Song, M.K.; Cairns, E.J.; Zhang, Y. Lithium/sulfur batteries with high specific energy: Old challenges and new opportunities. Nanoscale 2013, 5, 2186–2204. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium-sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. Engl. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Chen, L.; Shaw, L.L. Recent advances in lithium-sulfur batteries. J. Power Sources 2014, 267, 770–783. [Google Scholar] [CrossRef]
- Xu, G.Y.; Ding, B.; Pan, J.; Nie, P.; Shen, L.F.; Zhang, X.G. High performance lithium-sulfur batteries: Advances and challenges. J. Mater. Chem. A 2014, 2, 12662–12676. [Google Scholar] [CrossRef]
- Dong, C.; Gao, W.; Jin, B.; Jiang, Q. Advances in Cathode Materials for High-Performance Lithium-Sulfur Batteries. iScience 2018, 6, 151–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul Scherrer Institut. A new generation of lithium batteries is approaching industrial implementation. Our Research/Current topics from our research/Lithium-sulfur battery, 6 March 2013. Available online: https://www.psi.ch/en/media/our-research/lithium-sulfur-battery (accessed on 20 May 2020).
- Benveniste, G.; Rallo, H.; Canals Casals, L.; Merino, A.; Amante, B. Comparison of the state of Lithium-Sulphur and lithium-ion batteries applied to electromobility. J. Environ. Manag. 2018, 226, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Fu, W.; Liang, C. Chapter 24—Lithium–Sulfur Batteries. In Handbook of Battery Materials, 2nd ed.; Daniel, C., Besenhard, J.O., Eds.; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, Germany, 2011; pp. 811–840. [Google Scholar]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic layer deposited TiO2 on a nitrogen-doped graphene/sulfur electrode for high performance lithium–sulfur batteries. Energy Env. Sci 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, X. Toxicity Mechanism of Graphene Oxide and Nitrogen-doped Graphene Quantum Dots in RBCs Revealed by Surface-Enhanced Infrared Absorption Spectroscopy. Toxicol. Res. 2015, 4, 885–894. [Google Scholar] [CrossRef]
- CamGraph Graphene Powder. Material Safety Data Sheet. Cambridge Nanosystems Ltd., 2019. Available online: https://cambridgenanosystems.com/wp-content/uploads/2015/05/CNS_CamGraph_MSDS_V1.0.pdf (accessed on 22 May 2020).
- Conductive Graphene Sheets. CAS-No.: 7782-42-5. Safety Data Sheet. Graphene Supermarket, 2016. Available online: www.graphene-supermarket.com (accessed on 20 May 2020).
- Giampiccolo, A.; Tobaldi, D.M.; Leonardi, S.G.; Murdoch, B.J.; Seabra, M.P.; Ansell, M.P.; Neri, G.; Ball, R.J. Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2. Appl. Catal. B: Environ. 2019, 243, 183–194. [Google Scholar] [CrossRef]
- Landmann, M.; Rauls, E.; Schmidt, W.G. The electronic structure and optical response of rutile, anatase and brookite TiO2. J. Phys. Condens Matter. 2012, 24, 195503. [Google Scholar] [CrossRef] [Green Version]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem 2016, 16, 762–769. [Google Scholar] [CrossRef]
- LTS Research Laboratories Inc. Titanium Oxide. Safety Data Sheet. 2017. Available online: https://www.ltschem.com/msds/TiO2.pdf (accessed on 22 May 2020).
- Zhou, G.; Zhao, Y.; Zu, C.; Manthiram, A. Free-standing TiO2 nanowire–embedded graphene hybrid membrane for advanced Li/dissolved polysulfide batteries. Nano Energy 2015, 12, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wang, G.; Wang, P.; Yang, J.; Zhang, K.; Liu, Y.; Lai, Y. High-performance lithium–sulfur batteries with a carbonized bacterial cellulose/TiO2 modified separator. J. Electroanal Chem. 2017, 788, 150. [Google Scholar] [CrossRef]
- Dourado, F.; Gama, M.; Rodrigues, A.C. A Review on the toxicology and dietetic role of bacterial cellulose. Toxicol. Rep. 2017, 4, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhao, G.; Wu, N.; Zhang, Y.; Xiang, M.; Wang, B.; Liu, H.; Wu, H. Efficient synthesis of graphene nanoscrolls for fabricating sulfur-loaded cathode and flexible hybrid interlayer toward high-performance Li–S batteries. ACS Appl. Mater. Interfaces 2016, 8, 34185. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor behavior with KCl electrolyte. J. Solid State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Lee, H.Y.; Manivannan, V.; Goodenough, J. Electrochemical capacitors with KCl electrolyte. Comptes Rendus Acad. Des. Sci. Ser. IIC Chem. 1999, 2, 565–577. [Google Scholar] [CrossRef]
- Lee, T.H.; Pham, D.T.; Sahoo, R.; Seok, J.; Luu, T.H.T.; Lee, Y.H. High energy density and enhanced stability of asymmetric supercapacitors with mesoporous MnO2@CNT and nanodot MoO3@CNT free-standing films. Energy Storage Mater. 2018, 12, 223–231. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Env. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Manganese dioxide, CID=14801. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Manganese-dioxide (accessed on 16 May 2020).
- LTS Research Laboratories, Inc. Manganese oxide CAS No.: 1317-35-7. Safety Data Sheet. Available online: https://www.ltschem.com/msds/Mn3O4.pdf (accessed on 22 May 2020).
- Liu, M.; Li, Q.; Qin, X.; Liang, G.; Han, W.; Zhou, D.; He, Y.B.; Li, B.; Kang, F. Suppressing self-discharge and shuttle effect of lithium–sulfur batteries with V2O5-decorated carbon nanofiber interlayer. Small 2017, 13, 1602539–1602545. [Google Scholar] [CrossRef]
- Monash University. Carbon fibre composites. OHS information sheet. 2009. Available online: https://www.monash.edu/ohs/info-docs/safety-topics/chemical-management/carbon-fibre-composites-ohs-information-sheet (accessed on 20 May 2020).
- Whittingham, M.S. Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 2014, 114, 11414–11440. [Google Scholar] [CrossRef]
- EVRAZ Stratcor, Inc. Vanadium Pentoxide [V2O5]. Safety Data Sheet. 2015. Available online: www.evrazstratcor.com (accessed on 20 May 2020).
- Peled, E.; Gorenshtein, A.; Segal, M.; Sternberg, Y. Rechargeable Lithium-Sulfur Battery. J. Power Sources 1989, 26, 269–271. [Google Scholar] [CrossRef]
- Liu, M.; Qin, X.; He, Y.-B.; Li, B.; Kang, F. Recent innovative configurations in high-energy lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 5222–5234. [Google Scholar] [CrossRef]
- Nole, D.A.; Moss, V. Battery employing lithium-sulphur electrodes with non-aqueous electrolyte. U.S. Patent 3,532,543, 6 October 1970. [Google Scholar]
- Eichinger, G.; Besenhard, J.O. High energy density lithium cells: Part II. Cathodes and complete cells. J. Electroanal. Chem. Interfacial Electrochem. 1976, 72, 1–31. [Google Scholar] [CrossRef]
- Rauh, R.D.; Abraham, K.M.; Pearson, G.F.; Surprenant, J.K.; Brummer, S.B. A Lithium/Dissolved Sulfur Battery with an Organic Electrolyte. J. Electrochem. Soc. 1979, 126, 523–527. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Lithium Hexafluoroarsenate, CID=9837036. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-hexafluoroarsenate (accessed on 15 May 2020).
- Marmorstein, D.; Yu, T.H.; Striebel, K.A.; McLarnon, F.R.; Hou, J.; Cairns, E.J. Electrochemical performance of lithium/sulfur cells with three different polymer electrolytes. J. Power Sources 2000, 89, 219–226. [Google Scholar] [CrossRef]
- Jeon, B.H.; Yeon, J.H.; Kim, K.M.; Chung, I.J. Preparation and electrochemical properties of lithium–sulfur polymer batteries. J. Power Sources 2002, 109, 89–97. [Google Scholar] [CrossRef]
- Park, J.H.; Yeo, S.Y.; Park, J.K.; Lee, Y.M. Electrochemical Performance of Lithium Sulfur Batteries with Plasticized Polymer Electrolytes based on P(VdF-co-HFP). J. Korean Electrochem. Soc. 2010, 13, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.S.; Choi, J.W.; Ahn, J.H.; Cho, G.B.; Ahn, H.J. The Electrochemical Properties of Poly(acrylonitrile) Polymer Electrolyte for Li/S Battery. Mater. Sci. Forum 2006, 510, 50–53. [Google Scholar] [CrossRef]
- Shin, J.; Jung, B.; Jeong, S.; Kim, K.; Ahn, H.; Cho, K.; Ahn, J. Electrochemical and interfacial properties of (PEO)10LiCF3SO3−Al2O3 nanocomposite polymer electrolytes using ball milling. Met. Mater. Int. 2004, 10, 177–183. [Google Scholar] [CrossRef]
- Kolosnitsyn, V.S.; Karaseva, E.V.; Amineva, N.A.; Batyrshina, G.A. Cycling Lithium–Sulfur Batteries. Russ. J. Electrochem. 2002, 38, 329–331. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, K.W.; Ahn, H.J.; Ahn, J.H. Electrochemical properties and interfacial stability of (PEO)10LiCF3SO3–TinO2n−1 composite polymer electrolytes for lithium/sulfur battery. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2002, 95, 148–156. [Google Scholar] [CrossRef]
- Zhu, X.J.; Wen, Z.Y.; Gu, Z.H.; Lin, Z.X. Electrochemical characterization and performance improvement of lithium/sulfur polymer batteries. J. Power Sources 2005, 139, 269–273. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, J.H.; Cheruvally, G.; Ahn, J.H.; Kim, K.W.; Ahn, H.J.; Kim, J.U. Microporous Poly(vinylidene fluoride-co-hexafluoropropylene) Polymer Electrolytes for Lithium/Sulfur Cells. J. Ind. Eng. Chem. 2006, 12, 939–949. [Google Scholar]
- Jeong, S.S.; Lim, Y.T.; Choi, Y.J.; Cho, G.B.; Kim, K.W.; Ahn, H.J.; Cho, K.K. Electrochemical properties of lithium sulfur cells using PEO polymer electrolytes prepared under three different mixing conditions. J. Power Sources 2007, 174, 745–750. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.W.; Ahn, J.H.; Cheruvally, G. Secondary Batteries—Lithium rechargeable systems: Lithium-sulfur. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Hassoun, J.; Scrosati, B. Moving to a Solid-State Configuration: A Valid Approach to Making Lithium-Sulfur Batteries Viable for Practical Applications. Adv. Mater. 2010, 22, 5198–5201. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, J.; Scrosati, B. A High-Performance Polymer Tin Sulfur Lithium Ion Battery. Angew. Chem. Int. Ed. 2010, 49, 2371–2374. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Ohtomo, T.; Mizuno, F.; Tadanaga, K.; Tatsumisago, M. All-solid-state Li/S batteries with highly conductive glass-ceramic electrolytes. Electrochem. Commun. 2003, 5, 701–705. [Google Scholar] [CrossRef]
- Hayashi, A.; Ohtsubo, R.; Ohtomo, T.; Mizuno, F.; Tatsumisago, M. All-solid-state rechargeable lithium batteries with Li2S as a positive electrode material. J. Power Sources 2008, 183, 422–426. [Google Scholar] [CrossRef]
- Kobayashi, T.; Imade, Y.; Shishihara, D.; Homma, K.; Nagao, M.; Watanabe, R.; Yokoi, T.; Yamada, A.; Kanno, R.; Tatsumi, T. All solid-state battery with sulfur electrode and thio-LISICON electrolyte. J. Power Sources 2008, 182, 621–625. [Google Scholar] [CrossRef]
- Machida, N.; Kobayashi, K.; Nishikawa, Y.; Shigematsu, T. Electrochemical properties of sulfur as cathode materials in a solid-state lithium battery with inorganic solid electrolytes. Solid State Ion. 2004, 175, 247–250. [Google Scholar] [CrossRef]
- Machida, N.; Shigematsu, T. An All-solid-state Lithium Battery with Sulfur as Positive Electrode Materials. Chem. Lett. 2004, 33, 376–377. [Google Scholar] [CrossRef]
- Pradel, A.; Ribes, M. Electrical properties of lithium conductive silicon sulfide glasses prepared by twin roller quenching. Solid State Ion. 1986, 18, 351–355. [Google Scholar] [CrossRef]
- Minami, K.; Mizuno, F.; Hayashi, A.; Tatsumisago, M. Lithium ion conductivity of the Li2S–P2S5 glass-based electrolytes prepared by the melt quenching method. Solid State Ion. 2007, 178, 837–841. [Google Scholar] [CrossRef]
- Hedya, S.A.; Avula, A.; Swoboda, H.D. Lithium Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499992/ (accessed on 20 May 2020).
- Central Drug House (P) Ltd. Lithium Metal. CAS No.: 7439-93-2. Material Safety Data Sheet. 2020. Available online: www.cdhfinechemical.com (accessed on 20 May 2020).
- Ji, X.L.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.C.; Xu, Y.H.; Wang, C.S. Sulfur-impregnated disordered carbon nanotubes cathode for lithium-sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Gu, L.; Zhao, N.-H.; Yin, Y.-X.; Zhou, L.-J.; Guo, Y.-G.; Wan, L.-J. Smaller sulfur molecules promise better lithium-sulfur batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Zhang, F.X.; Jin, J. Interface chemistry guided long-cycle-life Li-S battery. Nano Lett. 2013, 13, 4206–4211. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef]
- She, Z.W.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.-C.; Cui, Y. Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium-sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar]
- Zhou, W.D.; Yu, Y.C.; Chen, H.; DiSalvo, F.J.; Abruña, H.D. Yolk-shell structure of polyaniline-coated sulfur for lithium-sulfur batteries. J. Am. Chem. Soc. 2013, 135, 16736–16743. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.; Cha, J.J.; Wu, H.; Vosgueritchian, M.; Yao, Y.; Bao, Z.; Cui, Y. Improving the performance of lithium-sulfur batteries by conductive polymer coating. ACS Nano 2011, 5, 9187–9193. [Google Scholar] [CrossRef]
- Chen, H.W.; Wang, C.; Dai, Y.; Qiu, S.; Yang, J.; Lu, W.; Chen, L. Rational design of cathode structure for high rate performance lithium-sulfur batteries. Nano Lett. 2015, 15, 5443–5448. [Google Scholar] [CrossRef]
- She, Z.W.; Yu, J.H.; Li, W.; Hsu, P.-C.; Wang, H.; Sun, Y.; Yao, H.; Zhang, Q.; Cui, Y. Two-dimensional layered transition metal disulphides for effective encapsulation of high-capacity lithium sulphide cathodes. Nat. Commun. 2014, 5, 5017. [Google Scholar]
- Su, Y.S.; Manthiram, A. Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.M.; Pei, S.; Li, L.; Wang, D.-W.; Wang, S.; Huang, K.; Yin, L.-C.; Li, F.; Cheng, H.-M. A graphene-pure-sulfur sandwich structure for ultrafast, long-life lithium-sulfur batteries. Adv. Mater. 2014, 26, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Fan, J.M.; Zheng, M.S.; Dong, Q.F. A novel synergistic composite with multi-functional effects for high-performance Li-S batteries. Energy Env. Sci. 2016, 9, 1998–2004. [Google Scholar] [CrossRef]
- Rehman, S.; Gu, X.; Khan, K.; Mahmood, N.; Yang, W.; Huang, X.; Guo, S.; Hou, Y. 3D vertically aligned and interconnected porous carbon nanosheets as sulfur immobilizers for high performance lithium-sulfur batteries. Adv. Energy Mater. 2016, 6, 1502518. [Google Scholar] [CrossRef]
- Pei, F.; An, T.; Zang, J.; Zhao, X.; Fang, X.; Zheng, M.; Dong, Q.; Zheng, N. From hollow carbon spheres to N-doped hollow porous carbon bowls: Rational design of hollow carbon host for Li-S batteries. Adv. Energy Mater. 2016, 6, 1502539. [Google Scholar] [CrossRef]
- Lenntech BV. Chemical properties of carbon—Health effects of carbon—Environmental effects of carbon. 2020. Available online: https://www.lenntech.com/periodic/elements/c.htm (accessed on 20 May 2020).
- Sun, J.; Huang, Y.Q.; Wang, W.K.; Yu, Z.B.; Wang, A.B.; Yuan, K.G. Application of gelatin as a binder for the sulfur cathode in lithium-sulfur batteries. Electrochim. Acta 2008, 53, 7084–7088. [Google Scholar] [CrossRef]
- Frischmann, P.D.; Hwa, Y.; Cairns, E.J.; Helms, B.A. Redox-Active Supramolecular Polymer Binders for Lithium−Sulfur Batteries That Adapt Their Transport Properties in Operando. Chem. Mater. 2016, 28, 7414–7421. [Google Scholar] [CrossRef] [Green Version]
- Kurt J Lesker Company. Aluminum (pieces). CAS No.: 7429-90-5. Safety Data Sheet. 2018. Available online: http://www.nano.pitt.edu/sites/default/files/MSDS/Metals/Al-Aluminium.pdf (accessed on 22 May 2020).
- Exley, C. The toxicity of aluminium in humans. Morphologie 2016, 100, 51–55. [Google Scholar] [CrossRef]
- Duan, X.; Han, Y.; Li, Y.; Chen, Y. Improved capacity retention of low cost sulfur cathodes enabled by a novel starch binder derived from food. RSC Adv. 2014, 4, 60995–61000. [Google Scholar] [CrossRef]
- Lacey, M.J.; Jeschull, F.; Edström, K.; Brandell, D. Why PEO as a binder or polymer coating increases capacity in the Li–S system. Chem. Commun. 2013, 49, 8531–8533. [Google Scholar] [CrossRef]
- Pan, J.; Xu, G.; Ding, B.; Han, J.; Dou, H.; Zhang, X. Enhanced electrochemical performance of sulfur cathodes with a water-soluble binder. RSC Adv. 2015, 5, 13709–13714. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Shao, H.; Wang, W.; Gao, M.; Li, C.; Zhang, H.; Wang, A.; Huang, Y. Chitosan as a functional additive for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 15235–15240. [Google Scholar] [CrossRef]
- Li, G.; Ling, M.; Ye, Y.; Li, Z.; Guo, J.; Yao, Y.; Zhu, J.; Lin, Z.; Zhang, S. Acacia senegal–inspired bifunctional binder for longevity of lithium-sulfur batteries. Adv. Energy Mater. 2015, 5, 1500878. [Google Scholar] [CrossRef]
- Li, G.; Cai, W.; Liu, B.; Li, Z. A multi functional binder with lithium ion conductive polymer and polysulfide absorbents to improve cycleability of lithium–sulfur batteries. J. Power Sources 2015, 294, 187–192. [Google Scholar] [CrossRef]
- Polyethylene Oxide. American Polymer Standards Corporation. 2019. Available online: http://www.ampolymer.com/SDS/PolyethyleneOxideSDS.html (accessed on 20 May 2020).
- Gelatin. Drugbank. 2020. Available online: https://www.drugbank.ca/drugs/DB11242 (accessed on 20 May 2020).
- Toxicity Profile for Polyvinylpyrrolidone. 1991. Available online: https://www.bibra-information.co.uk/downloads/toxicity-profile-for-polyvinylpyrrolidone-1991/ (accessed on 20 May 2020).
- Carl Roth GmbH + Co KG. Polyvinylpyrrolidone K 30 extra pure. CAS No.: 9003-39-8. Safety Data Sheet. 2018. Available online: www.carlroth.de (accessed on 20 May 2020).
- Anderson, D.M.; Brydon, W.G.; Eastwood, M.A.; Sedgwick, D.M. Dietary effects of sodium alginate in humans. Food Addit. Contam. 1991, 8, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Abdelgadir, A.E. Assessment of Toxicity and Influence of Three Types of Gum Arabic on Blood Glucose, Body Weight, Total Protein and Lipid Profile on Diabetic Rats. Int. J. Adv. Res. 2013, 1, 13–19. [Google Scholar]
- Thermo Fisher Scientific. Gum Arabic. CAS No.: 9000-01-5. Safety Data Sheet. 2018. Available online: https://www.fishersci.com/store/msds?partNumber=AC258850010&productDescription=GUM+ARABIC+1KG&vendorId=VN00032119&countryCode=US&language=en (accessed on 22 May 2020).
- Carl Roth GmbH + Co KG. Gum Arabic, spray dried. CAS No.: 9000-01-5. Safety Data Sheet, Version: 1.0. 2019. Available online: https://www.carlroth.com/downloads/sdb/en/4/SDB_4159_GB_EN.pdf (accessed on 22 May 2020).
- Inderherbergh, J. Polyvinylidene Fluoride (PVDF) Appearance, General Properties and Processing. Ferroelectrics 1991, 115, 295–302. [Google Scholar] [CrossRef]
- Fluorochem Ltd. Poly(vinylidene fluoride). CAS No.: 24937-79-9. Safety Data Sheet. 2011. Available online: http://www.fluorochem.co.uk/System/DownloadSDS?fileName=(EN)007211_1.00.pdf (accessed on 22 May 2020).
- Feng, M.; Qu, R.; Habteselassie, M.; Wu, J.; Yang, S.; Sun, P.; Huang, Q.; Wang, Z. Hepatic Transcriptome Responses in Mice (Mus musculus) Exposed to the Nafion Membrane and Its Combustion Products. PLoS ONE 2015, 10, e0128591. [Google Scholar] [CrossRef] [Green Version]
- Nafion Cast Membranes. Article Information Sheet, Version 4.0. Chemours, Ref. 150000003570. 2015. Available online: https://www.nafion.com/en/products/sulfonic-membranes (accessed on 22 May 2020).
- SynQuest Laboratories, Inc. Nafion NR50. Safety Data Sheet 6198308. 2019. Available online: www.synquestlabs.com (accessed on 20 May 2020).
- Sigma-Aldrich. PAMAM dendrimer. Safety Data Sheet, version 3.10. 2016. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sulis Sato, S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In Vivo Distribution and Toxicity of PAMAM Dendrimers in the Central Nervous System Depend on Their Surface Chemistry. Mol. Pharm. 2013, 10, 249–260. [Google Scholar] [CrossRef]
- Cryan, S.A.; Holohan, A.; Donohue, R.; Darcy, R.; O’Driscoll, C. Cell transfection with polycationic cyclodextrin vectors. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2004, 21, 625–633. [Google Scholar] [CrossRef]
- Varan, G.; Benito, J.M.; Mellet, C.O.; Bilensoy, E. Development of polycationic amphiphilic cyclodextrin nanoparticles for anticancer drug delivery. Beilstein J. Nanotechnol. 2017, 8, 1457–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerloczy, A.; Hoshino, T.; Pitha, J. Safety of oral cyclodextrins: Effects of hydroxypropyl cyclodextrins, cyclodextrin sulfates and cationic cyclodextrins on steroid balance in rats. J. Pharm. Sci. 1994, 83, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Terao, K. Poly(acrylic acid) (PAA). In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- World Health Organization. Acrylic Acid Environmental Health Criteria; No 191; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Spectrum Chemical Mfg. Corp. Carboxymethyl Cellulose Sodium. CAS No.: 9004-32-4. Safety Data Sheet. 2015. Available online: https://www.spectrumchemical.com (accessed on 20 May 2020).
- Mondal, M.I.; Yeasmin, M.S. Toxicity study of food-grade carboxymethyl cellulose synthesized from maize husk in Swiss albino mice. Int. J. Biol. Macromol. 2016, 92, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.L.; Yunginger, J.W.; Dunn, W.F.; Jones, R.T.; Hunt, L.W. Anaphylaxis induced by the carboxymethylcellulose component of injectable triamcinolone acetonide suspension (Kenalog). Ann. Allergy Asthma Immunol. 1995, 74, 163–166. [Google Scholar]
- Healy, M. Is common food additive to blame for rising rates of bowel disease? Los Angeles Times. 25 February 2015. Available online: https://www.latimes.com/science/sciencenow/la-sci-sn-metabolic-bowel-emulsifiers-20150225-story.html (accessed on 22 May 2020).
- Martino, J.V.; van Limbergen, J.; Cahill, L.E. The Role of Carrageenan and Carboxymethylcellulose in the Development of Intestinal Inflammation. Front. Pediatrics 2017, 5, 96. [Google Scholar] [CrossRef] [Green Version]
- Fieldturf, T. Debunking the Myth of SBR Dangers Tire Crumb Rubber Use in Artificial Turf Fields: The Latest in a Long List of Scare Tactics; ADEME/ALIAPUR/FIELDTURF TARKETT; Däckindustrin: Upplands Väsby, Sweeden, 2004. [Google Scholar]
- Kim, N.-I.; Lee, C.-B.; Seo, J.-M.; Lee, W.-J.; Roh, Y.-B. Correlation between positive-electrode morphology and sulfur utilization in lithium-sulfur battery. J. Power Sources 2004, 132, 209–212. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Ahn, H.-J.; Kim, K.-W.; Ahn, J.-H.; Cho, K.-K.; Nam, T.-H.; Kim, J.-U.; Cho, G.-B. Discharge behavior of lithium/sulfur cell with TEGDME based electrolyte at low temperature. J. Power Sources 2006, 163, 201–206. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, S. New approaches to improve cycle life characteristics of lithium-sulfur cells. Electrochem. Commun. 2007, 9, 249–254. [Google Scholar] [CrossRef]
- Wang, W.K.; Wang, Y.; Huang, Y.Q.; Huang, C.J.; Yu, Z.B.; Zhang, H.; Wang, A.B.; Yuan, K.G. The electrochemical performance of lithium–sulfur batteries with LiClO DOL/DME electrolyte. J. Appl. Electrochem. 2010, 40, 321–325. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.Q.; Wang, W.K.; Yu, Z.B.; Wang, A.B.; Yuan, K.G. Preparation and electrochemical characterization of the porous sulfur cathode using a gelatin binder. Electrochem. Commun. 2008, 10, 930–933. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Huang, Y.Q.; Wang, W.K.; Huang, C.J.; Wang, Y.; Yu, Z.B.; Zhang, H. Influence of pH of Gelatin Solution on Cycle Performance of the Sulfur Cathode. J. Electrochem. Soc. 2010, 157, A443–A446. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M. PTFE-coated non-stick cookware and toxicity concerns: A perspective. Environ. Sci. Pollut. Res. Int. 2017, 24, 23436–23440. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Hamada, O.; Sasaki, A.; Ikeda, M. Polymer fume fever. BMJ Case Reports 2012, bcr2012007790. Available online: https://pubmed.ncbi.nlm.nih.gov/23230259/ (accessed on 22 May 2020).
- HaloPolymer Kirovo-Chepetsk, LLC. Polytetrafluoroethylene. CAS No.: 9002-84-0. Safety Data Sheet. 2017. Available online: www.halopolymer.com (accessed on 20 May 2020).
- BrookeTaylor, S.; Verger, P. Polyvinyl Alcohol. WHO Food Additives Series: 52, JECFA 52. Inchem. 2004. Available online: http://www.inchem.org/documents/jecfa/jecmono/v52je09.htm (accessed on 20 May 2020).
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- American Polymer Standards Corporation. Polyvinyl Alcohol. CAS No.: 9002-89-5. 2014. Available online: http://www.ampolymer.com/SDS/PolyvinylAlcoholSDS.html (accessed on 20 May 2020).
- Peled, E.; Goor, M.; Schektman, I.; Mukra, T.; Shoval, Y.; Golodnitsky, D. The Effect of Binders on the Performance and Defradation of the Lithium/Sulfur Battery Assembled in the Discharged State. J. Electrochem. Soc. 2017, 164, A5001–A5007. [Google Scholar] [CrossRef]
- American Polymer Standards Corporation. Vinylidene Fluoride-Hexafluoropropylene PVDF-HFP2. CAS No.: 9011-17-0. Safety Data Sheet. 2014. Available online: http://www.ampolymer.com/SDS/VinylideneFluoride-HexafluoropropyleneSDS.html (accessed on 22 May 2020).
- BASF SE. Lupasol PS. Safety Data Sheet. 2016. Available online: https://www.chempoint.com/products/download?grade=4849&type=sds (accessed on 22 May 2020).
- Johnson Matthey Company Inc. Polyaniline. Material Safety Data Sheet. Alfa Aesar, Johnson Matthey Catalog Company, 2009. Available online: www.alfa.com (accessed on 20 May 2020).
- Ibarra, L.E.; Tarres, L.; Bongiovanni, S.; Barbero, C.A.; Kogan, M.J.; Rivarola, V.A.; Bertuzzi, M.L.; Yslas, E.I. Assessment of polyaniline nanoparticles toxicity and teratogenicity in aquatic environment using Rhinella arenarum model. Ecotoxicol. Environ. Saf. 2015, 114, 84–92. [Google Scholar] [CrossRef]
- Humpolíček, P.; Radaszkiewicz, K.A.; Capáková, Z.; Pacherník, J.; Bober, P.; Kašpárková, V.; Rejmontová, P.; Lehocký, M.; Ponížil, P.; Stejskal, J. Polyaniline cryogels: Biocompatibility of novel conducting macroporous material. Sci. Rep. 2018, 8, 135. [Google Scholar] [CrossRef]
- He, X.; Yan, B.; Zhang, X.; Liu, Z.; Bresser, D.; Wang, J.; Wang, R.; Cao, X.; Su, Y.; Jia, H.; et al. Fluorine-free water-in-ionomer electrolytes for sustainable lithium-ion batteries. Nat. Commun. 2018, 9, 5320. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Hu, L.; Li, H.; Zhai, T. Aqueous Binder Enhanced High-Performance GeP5 Anode for Lithium-Ion Batteries. Front. Chem. 2018, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- BK Giulini GmbH. Lithium polyacrylate. Safety Data Sheet. 2015. Available online: www.icl-pp.com (accessed on 20 May 2020).
- Zhang, S.S. Binder Based on Polyelectrolyte for High Capacity Density Lithium/Sulfur Battery. J. Electrochem. Soc. 2012, 159, A1226–A1229. [Google Scholar] [CrossRef]
- GuideChem. Poly(acrylamide-co-diallyl dimethyl ammonium chloride). CAS No.: 26590-05-6. Safety Data Sheet. 2017. Available online: www.guidechem.com (accessed on 20 May 2020).
- Exon, J.H. A review of the toxicology of acrylamide. J. Toxicol. Env. Health B Crit. Rev. 2006, 9, 397–412. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. CID=33286. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diallyldimethylammonium-chloride (accessed on 11 May 2020).
- Hwa, Y.; Frischmann, P.D.; Helms, B.A.; Cairns, E.J. Aqueous-Processable Redox-Active Supramolecular Polymer Binders for Advanced Lithium/Sulfur Cells. Chem. Mater. 2018, 30, 685–691. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, W.; Lei, T.; Dai, L.; Chen, B.; Wu, C.; Xiong, J. A Novel Polar Copolymer Design as a Multi-Functional Binder for Strong Affinity of Polysulfides in Lithium-Sulfur Batteries. Nanoscale Res. Lett. 2017, 12, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lee, S.-Y.; Wu, S.-C.; Chen, H.; Tsai, L.-L.; Tzeng, J.-J.; Lin, C.-H.; Lin, Y.-M. Synthesis and Characterization of Polycaprolactone-Based Polyurethanes for the Fabrication of Elastic Guided Bone Regeneration Membrane. Biomed. Res. Int. 2018, 3240571. [Google Scholar] [CrossRef] [Green Version]
- Cary, R.; Dobson, S.; Delic, J. 1,2-Diaminoethane (Ethylenediamine). Concise International Chemical Assessment Document 15; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Cheng, M.; Liu, Y.; Guo, X.; Wu, Z.; Chen, Y.; Li, J.; Li, L.; Zhong, B. A novel binder-sulfonated polystyrene for the sulfur cathode of Li-S batteries. Ionics 2017, 23, 2251–2258. [Google Scholar] [CrossRef]
- Rogers, F.B.; Li, S.C. Acute colonic necrosis associated with sodium polystyrene sulfonate (Kayexalate) enemas in a critically ill patient: Case report and review of the literature. J. Trauma 2001, 51, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. Improved cyclability of liquid electrolyte lithium/sulfur batteries by optimizing electrolyte/sulfur ratio. Energies 2012, 5, 5190–5197. [Google Scholar] [CrossRef]
- Carbone, L.; Gobet, M.; Peng, J.; Devany, M.; Scrosati, B.; Greenbaum, S.; Hassoun, J. Comparative study of ether-based electrolytes for application in lithium–sulfur battery. ACS Appl. Mater. Interfaces 2015, 7, 13859–13865. [Google Scholar] [CrossRef]
- Gao, J.; Lowe, M.A.; Kiya, Y.; Abruña, H.D. Effects of liquid electrolytes on the charge-discharge performance of rechargeable lithium/sulfur batteries: Electrochemical and in-situ X-ray absorption spectroscopic studies. J. Phys. Chem. C 2011, 115, 25132–25137. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Kim, H.-S.; Jeong, C.-S. Electrochemical properties of binary electrolytes for lithium-sulfur batteries. Bull. Korean Chem. Soc. 2011, 32, 3682–3686. [Google Scholar] [CrossRef] [Green Version]
- Aurbach, D.; Youngman, O.; Dan, P. The electrochemical-behavior of 1,3-dioxolane-LiClO4 solutions. 2. Contaminated solutions. Electrochim. Acta 1990, 35, 639–655. [Google Scholar] [CrossRef]
- Aurbach, D.; Youngman, O.; Gofer, Y.; Meitav, A. The electrochemical-behavior of 1,3-dioxolane-LiCLO4 solutions. 1. Uncontaminated solutions. Electrochim. Acta 1990, 35, 625–638. [Google Scholar] [CrossRef]
- Gofer, Y.; Ely, Y.E.; Aurbach, D. Surface-chemistry of lithium in 1,3-dioxolane. Electrochim. Acta 1992, 37, 1897–1899. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. CID=3816071. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-bis_trifluoromethyl_sulfonyl_azanide#datasheet=LCSS (accessed on 8 May 2020).
- New Jersey Department of Health and Senior Services. Dimethyl Ether. CAS No.: 115-10-6. Hazardous Substance Fact Sheet; 1996. Available online: http://www.nj.gov/health/eoh/rtkweb/documents/fs/0758.pdf (accessed on 22 May 2020).
- Praxair, Inc. Dimethyl Ether. CAS No.: 115-10-6. Safety Data Sheet P-4589. 2016. Available online: www.praxair.com (accessed on 20 May 2020).
- ECHA. 1,3-dioxolane. CAS No.: 646-06-0. 2013. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/15807/7/13 (accessed on 20 May 2020).
- Central Drug House (P) Ltd. 1,3-dioxolane. CAS No.: 646-06-0. Safety Data SheetCAS No.: 646-06-0. Safety Data Sheet. 2019. Available online: www.cdhfinechemical.com (accessed on 20 May 2020).
- Ryu, H.S.; Ahn, H.J.; Kim, K.W.; Ahn, J.H.; Lee, J.Y.; Cairns, E.J. Self-discharge of lithium–sulfur cells using stainless-steel current-collectors. J. Power Sources 2005, 140, 365–369. [Google Scholar] [CrossRef]
- Tobishima, S.-I.; Yamamoto, H.; Matsuda, M. Study on the reduction species of sulfur by alkali metals in nonaqueous solvents. Electrochim. Acta 1997, 42, 1019–1029. [Google Scholar] [CrossRef]
- Yamin, H.; Gorenshtein, A.; Penciner, J.; Sternberg, Y.; Peled, E. Lithium Sulfur Battery Oxidation/Reduction Mechanisms of Polysulfides in THF Solutions. J. Electrochem. Soc. 1988, 135, 1045–1048. [Google Scholar] [CrossRef]
- Kim, S.; Jung, Y.J.; Park, S.J. Effects of imidazolium salts on discharge performance of rechargeable lithium–sulfur cells containing organic solvent electrolytes. J. Power Sources 2005, 152, 272–277. [Google Scholar] [CrossRef]
- Ryu, H.S.; Ahn, H.J.; Kim, K.W.; Ahn, J.H.; Cho, K.K.; Nam, T.H. Self-discharge characteristics of lithium/sulfur batteries using TEGDME liquid electrolyte. Electrochim. Acta 2006, 52, 1563–1566. [Google Scholar] [CrossRef]
- Choi, J.W.; Cheruvally, G.; Kim, D.S.; Ahn, J.H.; Kim, K.W.; Ahn, H.J. Rechargeable lithium/sulfur battery with liquid electrolytes containing toluene as additive. J. Power Sources 2008, 183, 441–445. [Google Scholar] [CrossRef]
- Shim, J.; Striebel, K.A.; Cairns, E.J. The lithium/sulfur rechargeable cell. J. Electrochem. Soc. 2002, 149, A1321. [Google Scholar] [CrossRef]
- Barchasz, C.; Lepretre, J.C.; Patoux, S.; Alloin, F. Electrochemical properties of ether-based electrolytes for lithium/sulfur rechargeable batteries. Electrochim. Acta 2013, 89, 737–743. [Google Scholar] [CrossRef]
- Nimon, Y.; Visco, S.J.; Chu, M.-Y. Dioxolane as a proctector for lithium electrodes. U.S. Patent 6,225,002, 1 May 2001. [Google Scholar]
- Zhao, X.; Cheruvally, G.; Kim, C.; Cho, K.-K.; Ahn, H.-J.; Kim, K.-W.; Ahn, J.-H. Lithium/Sulfur Secondary Batteries: A Review. J. Electrochem. Sci. Technol. 2016, 7, 97–114. [Google Scholar] [CrossRef]
- Barghamadi, M. Electrochemistry and speciation of sulfur/polysulfides in ionic liquid-based lithium sulfur batteries. Ph.D. Thesis, Swinburne University of Technology, Melbourne, Australia, 2016. [Google Scholar]
- Barchasz, C.; Lepretre, J.C.; Patoux, S.; Alloin, F. Revisiting TEGDME/DIOX binary electrolytes for lithium/sulfur batteries: Importance of solvation ability and additives. J. Electrochem. Soc. 2013, 160, A430–CA436. [Google Scholar] [CrossRef]
- Kim, S.; Jung, Y.; Lim, H.S. The effect of solvent component on the discharge performance of lithium–sulfur cell containing various organic electrolytes. Electrochim. Acta 2004, 50, 889–892. [Google Scholar] [CrossRef]
- Song, M.S.; Han, S.C.; Kim, H.S.; Kim, J.H.; Kim, K.T.; Kang, Y.M.; Ahn, H.J.; Dou, S.X.; Lee, J.Y. Effects of Nanosized Adsorbing Material on Electrochemical Properties of Sulfur Cathodes for Li/S Secondary Batteries. J. Electrochem. Soc. 2004, 151, A791–A795. [Google Scholar] [CrossRef]
- Fowles, J.; Boatman, R.; Bootman, J.; Lewis, C.; Morgott, D.; Rushton, E.; van Rooij, J.; Banton, M. A review of the toxicological and environmental hazards and risks of tetrahydrofuran. Crit. Rev. Toxicol. 2013, 43, 811–828. [Google Scholar] [CrossRef]
- Carl Roth GmbH + Co KG. Tetrahydrofuran. CAS No.: 109-99-9. Safety Data Sheet. 2016. Available online: www.carlroth.de (accessed on 20 May 2020).
- Kruse, J.A. Methanol and Ethylene Glycol Intoxication. Crit. Care Clin. 2012, 28, 661–711. [Google Scholar] [CrossRef]
- Carl Roth GmbH + Co KG. Ethylene glycol. CAS No.: 107-21-1. Safety Data Sheet, Version 4.0. 2015. Available online: www.carlroth.de (accessed on 20 May 2020).
- Triethylene glycol. The MAK-Collection Part I. MAK Value Documentations. 2014. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/3527600418.mb11227e4214 (accessed on 20 May 2020).
- National Center for Biotechnology Information. PubChem Database. Triglyme, CID=8189. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Triglyme (accessed on 12 May 2020).
- Glycol Ethers Charter. Glycol Ethers Online. 2020. Available online: https://www.glycol-ethers.eu/glycol-ethers-charter/ (accessed on 20 May 2020).
- Kupczewska-Dobecka, M. Bis(2-methoxyethyl) ether. Podstawy I Metod. Oceny Środowiska Pr. 2011, 4, 43–69. [Google Scholar]
- 1,2-bis(2-methoxyethoxy)ethane (Triglyme). Proposal for Identification of a Substance as a Category 1A or 1B CMR, PBT, vPvB or a Substance of an Equivalent Level of Concern, Annex XV—Identification of Triglyme (TEGDME) as SVHC, Belgian Federal Public Service (FPS) Health, Food Chain Safety and Environment, Risk Management Service. 2020. Available online: https://echa.europa.eu/documents/10162/7c7d5fc7-53a1-e4d8-55fb-9229d5248c57 (accessed on 22 May 2020).
- Melody, B.J.; Kinard, J.T.; Wheeler, D.A. Anodizing electrolyte and its use. U.S. Patent 5716511A, 10 February 1998. [Google Scholar]
- Sigma-Aldrich. Polyethylene Glycol 2000 Dimethyl Ether. CAS No.: 24991-55-7. Material Safety Data Sheet, Version 1.5. 2006. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- 2-Ethoxyethyl ether. Chemical Book. 2017. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_cb4669548.htm (accessed on 20 May 2020).
- Diethylene Glycol Dimethyl Ether. Concise International Chemical Assessment Document 41; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- National Center for Biotechnology Information. PubChem Database. Diglyme, CID=8150. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diglyme (accessed on 19 May 2020).
- Gillett, A.; Waugh, D.G.; Lawrence, J. 7—Laser surface modification of polymeric surfaces for microbiological applications. In Laser Surface Modification of Biomaterials; Vilar, R., Ed.; Woodhead Publishing: Sawston/Cambridge, UK, 2016; pp. 197–220. [Google Scholar]
- Schier, J.; Barr, D.; Li, Z.; Wolkin, A.; Baker, S.; Lewis, L.; McGeehin, M. Diethylene Glycol in Health Products Sold Over-the-Counter and Imported from Asian Countries. J. Med. Toxicol. J. Am. Coll. Med. Toxicol. 2010, 7, 33–38. [Google Scholar]
- Choi, J.W.; Kim, J.K.; Cheruvally, G.; Ahn, J.H.; Ahn, H.J.; Kim, K.W. Rechargeable lithium/sulfur battery with suitable mixed liquid electrolytes. Electrochim. Acta 2007, 52, 2075–2082. [Google Scholar] [CrossRef]
- Methyl acetate. Wiley Online Library. 2002. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/3527600418.mb7920e0018 (accessed on 20 May 2020).
- Eastman Chemical Company. Eastman Methyl Acetate. Safety Data Sheet, Version 2.2. 2016. Available online: http://ws.eastman.com/ProductCatalogApps/PageControllers/MSDS_PC.aspx?Product=71001121 (accessed on 22 May 2020).
- Medical Management Guidelines for Toluene [C6H5CH3]. CAS 108-88-3. Toxic Substances Portal—Toluene. Agency for Toxic Substances and Disease Registry; 2019. Available online: https://www.atsdr.cdc.gov/MMG/MMG.asp?id=157&tid=29 (accessed on 20 May 2020).
- LabChem Inc. Toluene. CAS-No.: 108-88-3. Safety Data Sheet, Version 1.1. 2014. Available online: www.labchem.com (accessed on 20 May 2020).
- Gamma-Butyrolactone (GBL). Critical Review Report Agenda item 4.3. Expert Committee on Drug Dependence. In Proceedings of the Thirty-sixth Meeting of the World Health Organization, Geneva, Switzerland, 16–20 June 2014. [Google Scholar]
- Gamma-Butyrolactone (GBL). Material Safety Data Sheet. Fred Holmberg & Co AB, 2011. Available online: http://www.holmberg.se/upload/product/files/msds-gamma-butyrolactone-gbl-eng-2011-10-07---641.pdf (accessed on 22 May 2020).
- Cho, C.W.; Pham, T.P.; Jeon, Y.C.; Vijayaraghavan, K.; Choe, W.S.; Yun, Y.S. Toxicity of imidazolium salt with anion bromide to a phytoplankton Selenastrum capricornutum: Effect of alkyl-chain length. Chemosphere 2007, 69, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Biczak, R.; Pawlowska, B.; Balczewski, P.; Rychter, P. The role of the anion in the toxicity of imidazolium ionic liquids. J. Hazard. Mater. 2014, 274C, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich. Tetrabutylammonium hexafluorophosphate. Safety Data Sheet, Version 5.0. 2012. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- Chapter 5.3.1.—Lithium Salts. Future Lithium-ion Batteries. In Energy and Environment Series; Eftekhari, A. (Ed.) Royal Society of Chemistry: Cambridge, UK, 2019; p. 361. [Google Scholar]
- Sigma-Aldrich. Lithium trifluoromethanesulfonate. Safety Data Sheet, Version 6.0. 2019. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- Zhu, J. Advanced Separator Selection and Design for High-Performance Lithium-Sulfur Batteries; North Carolina State University: Raleigh, NC, USA, 2016. [Google Scholar]
- Foropoulos, M.; Lee, H.S.; Sun, X.; Yang, X.Q.; Moodenbaugh, A.R.; McBreen, J.; Fischer, D.A.; Fu, Z. Formation of SEI on cycled lithium-ion battery cathodes: Soft X-ray absorption study. Electrochem. SolidState Lett. 2002, 5, A22–A25. [Google Scholar]
- Foropoulos, J.; Desmarteau, D.D. Synthesis, properties, and reactions of bis((trifluoromethyl)sulfonyl) imide, (Cf3so2)2nh. Inorg. Chem 1984, 23, 3720–3723. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Scheers, J.; Fantini, S.; Johansson, P. A review of electrolytes for lithium-sulphur batteries. J. Power Sources 2014, 255, 204–218. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Bis (Trifluoromethane) Sulfonimide Lithium Salt. Safety Data Sheet, Version 6.0. 2019. Available online: www.sigmaaldrich.com (accessed on 20 May 2020).
- Guerfi, A.; Duchesne, S.; Kobayashi, Y.; Vijh, A.; Zaghib, K. LiFePO4 and graphite electrodes with ionic liquids based on bis(fluorosulfonyl)imide (FSI) (-) for Li-ion batteries. J. Power Sources 2008, 175, 866–873. [Google Scholar] [CrossRef]
- Dudley, J.T.; Wilkinson, D.P.; Thomas, G.; LeVae, R.; Woo, S.; Blom, H.; Horvath, C.; Juzkow, M.W.; Denis, B.; Juric, P.; et al. Conductivity of electrolytes for rechargeable lithium batteries. J. Power Sources 1991, 35, 59–82. [Google Scholar] [CrossRef]
- Xu, K.; Angell, C.A. Sulfone-based electrolytes for lithium-ion batteries. J. Electrochem. Soc. 2002, 149, A920–A926. [Google Scholar] [CrossRef]
- KISHIDA Chemical CO Ltd. 1mol/L LiFSI EMI-FSI. Safety Data Sheet T794E-2,09/05/2017. 2017. Available online: http://www.kishida.co.jp/product/catalog/msds/id/20641/code/LBG-02315e.pdf (accessed on 22 May 2020).
- Watson International Ltd. Lithium bis(fluorosulfonyl)imide, ultra dry. CAS No.: 171611-11-3. Safety Data Sheet. 2017. Available online: https://www.watson-int.com/wp-content/uploads/2017/10/MSDS-of-LiFSI-171611-11-3.pdf (accessed on 22 May 2020).
- Fisher Scientific. Alfa Aesar Lithium 4,5-dicyano-2-(trifluoromethyl)imidazole, 95%. CAS 761441-54-7. 2020. Available online: https://www.fishersci.com/shop/products/lithium-4-5-dicyano-2-trifluoromethyl-imidazole-95/AAH3408103 (accessed on 20 May 2020).
- National Center for Biotechnology Information. PubChem Database. Lithium perchlorate, CID=23665649. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-perchlorate (accessed on 20 May 2020).
- Lithium perchlorate sc-215260. Material Safety Data Sheet. Santa Cruz Biotechnology Inc., 2009. Available online: http://datasheets.scbt.com/sc-215260.pdf (accessed on 22 May 2020).
- Yuan, L.X.; Feng, J.K.; Ai, X.P.; Cao, Y.L.; Chen, S.L.; Yang, H.X. Improved dischargeability and reversibility of sulfur cathode in a novel ionic liquid electrolyte. Electrochem. Commun. 2006, 8, 610–614. [Google Scholar] [CrossRef]
- Wang, J.; Chew, S.Y.; Zhao, Z.W.; Ashraf, S.; Wexler, D.; Chen, J.; Ng, S.H.; Chou, S.L.; Liu, H.K. Sulfur–mesoporous carbon composites in conjunction with a novel ionic liquid electrolyte for lithium rechargeable batteries. Carbon 2008, 46, 229–235. [Google Scholar] [CrossRef]
- Shin, J.H.; Cairns, E.J. N-Methyl-(n-butyl)pyrrolidinium bis(trifluoromethanesulfonyl)imide-LiTFSI–poly(ethylene glycol) dimethyl ether mixture as a Li/S cell electrolyte. J. Power Sources 2008, 177, 537–545. [Google Scholar] [CrossRef]
- Central Drug House (P) Ltd. Dimethyl Carbonate. CAS No.: 616-38-6. Material Safety Data Sheet. 2019. Available online: www.cdhfinechemical.com (accessed on 20 May 2020).
- Kuenen, H.J.; Mengers, H.J.; van der Ham, A.G.J.; Kiss, A.A. Novel Process for Conversion of CO2 to Dimethyl Carbonate using Catalytic Membrane Reactors. In Computer Aided Chemical Engineering; Kravanja, Z., Bogataj, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 991–996. [Google Scholar]
- Shahzad, S.; Shah, A.; Kowsari, E.; Iftikhar, F.J.; Nawab, A.; Piro, B.; Akhter, M.S.; Rana, U.A.; Zou, Y. Ionic Liquids as Environmentally Benign Electrolytes for High-Performance Supercapacitors. Glob. Chall. 2019, 3, 1800023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigma-Aldrich. 1-Butyl-1-methylpiperidinium bis(trifluoromethylsulfonyl)imide. Material Safety Data Sheet, Version 8.1. 2020. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- Park, J.-W.; Yamauchi, K.; Takashima, E.; Tachikawa, N.; Ueno, K.; Dokko, K.; Watanabe, M. Solvent effect of room temperature ionic liquids on electro- chemical reactions in lithium-sulfur batteries. J. Phys. Chem C 2013, 117, 4431–4440. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Diethylmethyl(2-methoxyethyl)ammonium bis(trifluoromethylsulfonyl)imide. Material Safety Data Sheet, Version 6.0. 2019. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- Sigma-Aldrich. 1-ethyl-3-methylimidazoliumbis (trifluoromethanesulfonyl) imide. CAS No: 174899-82-2. Safety Data Sheet. 2019. Available online: www.sigmaaldrich.com (accessed on 20 May 2020).
- Santa Cruz Biotechnology Inc. 1-ethyl-3-methylimidazoliumbis (trifluoromethanesulfonyl) imide. Material Safety Data Sheet sc-251507. 2009. Available online: http://datasheets.scbt.com/sc-251507.pdf (accessed on 22 May 2020).
- Tachikawa, N.; Yamauchi, K.; Takashima, E.; Park, J.W.; Dokko, K.; Watanabe, M. Reversibility of electrochemical reactions of sulfur supported on inverse opal carbon in glyme-Li salt molten complex electrolytes. Chem. Commun. 2011, 47, 8157–8159. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yin, Y.X.; Xin, S.; Su, J.; Guo, Y.G.; Wan, L.J. High-safety lithium-sulfur battery with prelithiated Si/C anode and ionic liquid electrolyte. Electrochim. Acta 2013, 91, 58–61. [Google Scholar] [CrossRef]
- TCI Chemicals. 1-Allyl-1-methylpyrrolidinium Bis(trifluoromethanesulfonyl)imide. CAS No.: 1059624-23-5. 2020. Available online: https://www.tcichemicals.com/SG/en/p/A3089 (accessed on 20 May 2020).
- Sigma-Aldrich. 1-Ethyl-3-methylimidazolium bis(pentafluoroethylsulfonyl)imide. Material Safety Data Sheet, Version 6.1. 2019. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- Sigma-Aldrich. 1-Butyl-3-methylimidazolium hexafluorophosphate. Material Safety Data Sheet, Version 6.0. 2019. Available online: www.sigma-aldrich.com (accessed on 20 May 2020).
- Wang, J.; Wang, Y.W.; He, X.M.; Ren, J.G.; Jiang, C.Y.; Wan, C.R. Electrochemical characteristics of sulfur composite cathode materials in rechargeable lithium batteries. J. Power Sources 2004, 138, 271–273. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, J.; Xie, J.Y.; Xu, N.X. A novel conductive polymer-sulfur composite cathode material of rechargeable lithium batteries. Adv. Mater. 2002, 14, 963–996. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, J.; Wan, C.R.; Du, K.; Xie, J.Y.; Xu, N.X. Sulfur composite cathode materials for rechargeable lithium batteries. Adv. Funct. Mater. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, L.; Ling, Z.J.; Yang, J.; Wan, C.R.; Jiang, C.Y. Polymer lithium cells with sulfur composites as cathode materials. Electrochim. Acta 2003, 48, 1861–1867. [Google Scholar] [CrossRef]
- Hassoun, J.; Sun, Y.-K.; Scrosati, B.J. Rechargeable lithium sulfide electrode for a polymer tin/sulfur lithium ion battery. Power Sources 2011, 196, 343–348. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Env. Sci. 2010, 3, 1531. [Google Scholar] [CrossRef]
- Polyacrylonitrile Encyclopædia. In Encyclopædia Britannica; Britannica Inc: London, UK, 2014.
- National Center for Biotechnology Information. PubChem Database. Acrylonitrile, CID=7855. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acrylonitrile (accessed on 20 May 2020).
- National Center for Biotechnology Information. PubChem Database. Ethylene carbonate, CID=7303. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ethylene-carbonate (accessed on 12 May 2020).
- National Center for Biotechnology Information. PubChem Database. Diethyl carbonate, CID=7766. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diethyl-carbonate (accessed on 12 May 2020).
- National Center for Biotechnology Information. PubChem Database. Propylene carbonate, CID=7924. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Propylene-carbonate (accessed on 12 May 2020).
- LTS Research Laboratories Inc. Lithium Hexafluorophosphate. Safety Data Sheet. 2015. Available online: https://www.ltschem.com/msds/LiPF6.pdf (accessed on 22 May 2020).
- Lee, Y.M.; Choi, N.; Park, J.H.; Park, J. Electrochemical performance of lithium/sulfur batteries with protected Li anodes. J. Power Sources 2003, 119, 964–972. [Google Scholar] [CrossRef]
- Rao, M.; Geng, X.; Li, X.; Hu, S.; Li, W. Lithium-sulfur cell with combining carbon nanofibers–sulfur cathode and gel polymer electrolyte. J. Power Sources 2012, 212, 179–185. [Google Scholar] [CrossRef]
- Nagao, M.; Hayashi, A.; Tatsumisago, M. Sulfur–carbon composite electrode for all-solid-state Li/S battery with Li2S-P2S5 solid electrolyte. Electrochim. Acta 2011, 56, 6055–6059. [Google Scholar] [CrossRef]
- Unemoto, A.; Yasaku, S.; Nogami, G.; Tazawa, M.; Taniguchi, M.; Matsuo, M.; Ikeshoji, T.; Orimo, S. Development of bulk-type all-solid-state lithium-sulfur battery using LiBH4 electrolyte. Appl. Phys. Lett. 2014, 105, 083901. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Y.; Zhou, G.; Yu, G.; Manthiram, A. Durability of the Li1+xTi2–xAlx(PO4)3 Solid Electrolyte in Lithium–Sulfur Batteries. ACS Energy Lett. 2016, 1, 1080–1085. [Google Scholar] [CrossRef]
- Han, F.; Yue, J.; Fan, X.; Gao, T.; Luo, C.; Ma, Z.; Suo, L.; Wang, C. High-Performance All-Solid-State Lithium–Sulfur Battery Enabled by a Mixed-Conductive Li2S Nanocomposite. Nano Lett. 2016, 16, 4521–4527. [Google Scholar] [CrossRef]
- Nagao, M.; Imade, Y.; Narisawa, H.; Kobayashi, T.; Watanabe, R.; Yokoi, T.; Tatsumi, T.; Kanno, R. All-solid-state Li-sulfur batteries with mesoporous electrode and thio-LISICON solid electrolyte. J. Power Sources 2013, 222, 237–242. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polimer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Kim, K.M.; Park, N.G.; Ryu, K.S.; Chang, S.H. Characteristics of PVdF-HFP/TiO2 composite membrane electrolytes prepared by phase inversion and conventional casting methods. Electrochim. Acta 2006, 51, 5636–5644. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Gosselink, D.; Doan, T.N.; Sadhu, M.; Cheang, H.J.; Chen, P. Polymer electrolytes for lithium/sulfur batteries. Membranes 2012, 2, 553–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SABIC Innovative Plastics. Polyphenylene ether. Material Safety Data Sheet. 2008. Available online: www.sabic-ip.com (accessed on 20 May 2020).
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for Tissue Engineering and Regenerative Medicine. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 462–482. [Google Scholar]
- ToxTown. Polyvinyl Chloride [PVC]. 2017. Available online: https://toxtown.nlm.nih.gov/chemicals-and-contaminants/polyvinyl-chloride-pvc (accessed on 20 May 2020).
- Ensinger Inc. Tecaflon PVDF. Safety Data Sheet. 2015. Available online: http://www.ensinger-online.com/modules/nonpublic/customersheet/openpdf.php?pdf=TECAFLON%20PVDF%20SDS%20-%202015-min.pdf (accessed on 22 May 2020).
- Carbosynth Ltd. Poly(vinylidene fluoride-co-hexafluoropropylene). CAS No.: 9011-17-0. Safety Data Sheet. 2018. Available online: www.carbosynth.com (accessed on 20 May 2020).
- Weston, J.E.; Steele, B.C.H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt poly(ethylene-oxide) polymer electrolytes. Solid State Ion. 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Jeon, B.H.; Yeon, J.H.; Chung, I.J. Preparation and electrical properties of lithium-sulfur-composite polymer batteries. J. Mater. Process. Technol. 2003, 143, 93–97. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Persi, L.; Ronci, F.; Scrosati, B. Transport and interfacial properties of composite polymer electrolytes. Electrochim. Acta 2000, 45, 1481–1490. [Google Scholar] [CrossRef]
- Chung, S.H.; Wang, Y.; Persi, L.; Croce, F.; Greenbaum, S.G.; Scrosati, B.; Plichta, E. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J. Power Sources 2001, 9, 644–648. [Google Scholar] [CrossRef]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M.A. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2001, 46, 2457–2461. [Google Scholar] [CrossRef]
- Ahn, J.H.; Wang, G.X.; Liu, H.K.; Dou, S.X. Nanoparticle-dispersed PEO polimer electrolytes for Li batteries. J. Power Sources 2003, 119, 422–426. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Jayathilaka, P.A.R.D.; Bokalawala, R.S.P.; Albinsson, I.; Mellander, B.-E. Effect of concentration and grain size of alumina filler on the ionic conductivity enhancement of the (PEO) 9LiCF3SO3:Al2O3 composite polymer electrolyte. J. Power Sources 2003, 119, 409–414. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, C.L.; Venkateswarlu, M.; Hwang, B.J. Influence of TiO2 nano-particles on the transport properties of composite polymerel ectrolyte for lithium-ion batteries. J. Power Sources 2005, 146, 397–401. [Google Scholar] [CrossRef]
- Sergent, J.A.; Paget, V.; Chevillard, S. Toxicity and genotoxicity of nano-SiO2 on human epithelial intestinal HT-29 cell line. Ann. Occup. Hyg. 2012, 56, 622–630. [Google Scholar] [PubMed] [Green Version]
- Carl Roth GmbH + Co KG. Silicon dioxide. Safety Data Sheet, Version 1.0. 2016. Available online: www.carlroth.de (accessed on 20 May 2020).
- Arefian, Z.; Pishbin, F.; Negahdary, M.; Ajdary, M. Potential toxic effects of Zirconia Oxide nanoparticles on liver and kidney factors. Biomed. Res. 2015, 26, 89–97. [Google Scholar]
- LTS Research Laboratories Inc. Zirconium oxide. Safety Data Sheet. 2015. Available online: https://www.ltschem.com/msds/ZrO2.pdf (accessed on 22 May 2020).
- National Center for Biotechnology Information. PubChem Database. Alumina, CID=14769. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alumina (accessed on 21 May 2020).
- LTS Research Laboratories Inc. Lithium Aluminate. Safety Data Sheet. 2016. Available online: https://www.ltschem.com/msds/LiAlO2.pdf (accessed on 22 May 2020).
- Croce, F.; Sacchetti, S.; Scrosati, B. Advanced, high-performance composite polymer electrolytes for lithium batteries. J. Power Sources 2006, 161, 560–564. [Google Scholar] [CrossRef]
- Croce, F.; Settimi, L.; Scrosati, B. Super acid ZrO2-added, composite polymer electrolytes with improved transport properties. Electrochem. Commun. 2006, 8, 364–368. [Google Scholar] [CrossRef]
- Liang, X.; Wen, Z.; Liu, Y.; Zhang, H.; Huang, L.; Jin, J. Highly dispersed sulphur in ordered mesoporous carbon sphere as a composite cathode for rechargeable polymer Li/S battery. J. Power Sources 2011, 196, 3655–3658. [Google Scholar] [CrossRef]
- Xu, J.J.; Ye, H. Polymer gel electrolytes based on oligomeric polyether/cross-linked PMMA blends prepared via in situ polymerization. Electrochem. Commun. 2005, 7, 829–835. [Google Scholar] [CrossRef]
- Shin, J.H.; Jung, S.S.; Kim, K.W.; Ahn, H.J.; Ahn, J.H. Preparation and characterization of plasticized polimer electrolytes based on the PVdF-HFP copolymer for lithium/sulphur battery. J. Mater. Sci. Mater. Electron. 2002, 13, 727–733. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, J.; Xie, J.Y.; Xu, N.X.; Li, Y. Sulfur-carbon nano-composite as cathode for rechargeable lithium battery based on gel electrolyte. Electrochem. Commun. 2002, 4, 499–502. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Ahn, H.-J.; Kim, K.-W.; Ahn, J.-H.; Lee, J.-Y. Discharge process of Li/PVdF/S cells at room temperature. J. Power Sources 2006, 153, 360–364. [Google Scholar] [CrossRef]
- Zhang, S.S.; Tran, D.T. How a gel polymer electrolyte affects performance of lithium/sulphur batteries. Electrochim. Acta 2013, 114, 296–302. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Tetraglyme, CID=8925. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetraglyme (accessed on 13 May 2020).
- Lenntech. Chemical properties of tin—Health effects of tin—Environmental effects of tin. Available online: https://www.lenntech.com/periodic/elements/sn.htm (accessed on 13 May 2020).
- Digital Fire Reference Library. Zirconium Compounds Toxicity. Available online: https://digitalfire.com/4sight/hazards/ceramic_hazard_zirconium_compounds_toxicity_373.html (accessed on 13 May 2020).
- Liu, M.; Zhou, D.; He, Y.; Fu, Y.; Qin, X.; Miao, C.; Du, H.; Li, B.; Yang, Q.; Lin, Z. Novel gel polymer electrolyte for high-performance lithium–sulfur batteries. Nano Energy 2016, 22, 278–289. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, H.; Ren, Y.; Zhou, D.; Kang, F.; Zhao, T. In-situ Fabrication of a Freestanding Acrylate-based Hierarchical Electrolyte for Lithium-sulfur Batteries. Electrochim. Acta 2016, 213, 871–878. [Google Scholar] [CrossRef]
- NTP Report on the Toxicology Studies of Pentaerythritol Triacrylate (Technical Grade) (CAS NO. 3524-68-3). In F344/N Rats, B6C3F1 Mice, and Genetically Modified (FVB Tg.AC HEMIZYGOUS) Mice (Dermal Studies); NTP GMM 4. NIH Publication No. 06-4451; National Toxicology Program: Durham, NC, USA, 2005.
- National Center for Biotechnology Information. PubChem Database. Pentaerythritol tetraacrylate, CID=62556. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pentaerythritol-tetraacrylate (accessed on 21 May 2020).
- Adachi, G.Y.; Imanaka, N.; Aono, H. Fast Li-circle plus conducting ceramic electrolytes. Adv. Mater. 1996, 8, 127–135. [Google Scholar] [CrossRef]
- Robertson, A.D.; West, A.R.; Ritchie, A.G. Review of crystalline lithium-ion conductors suitable for high temperature battery applications. Solid State Ion. 1997, 104, 1–11. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 2006, 12, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Stramare, S.; Thangadurai, V.; Weppner, W. Lithium lanthanum titanates: A review. Chem. Mater. 2003, 15, 3974–3990. [Google Scholar] [CrossRef]
- Le, D.T. Influence of Lithium Content on the Structure and Ionic Conductivity of Perovskite La(2-3)-xLi3xTiO3 made by Double Mechanical Alloying Method. Commun. Phys. 2014, 24, 33–39. [Google Scholar]
- Noah Technologies Corporation SDS. Lithium Orthosilicate. Safety Data Sheet No. 272. 2019. Available online: https://noahtech.com/data/safety.13373.pdf (accessed on 22 May 2020).
- Materion Advanced Chemicals Inc. Lithium orthosilicate [Li4SiO4]. Safety Data Sheet. 2020. Available online: https://www.materion.com/api/materion/Msds/Download?fileName=2MJ_LITHIUM%20ORTHOSILICATE%20(LI4SIO4)_SDS-EU_EU%20English.pdf (accessed on 22 May 2020).
- Hayashi, A.; Hama, S.; Morimoto, H.; Tatsumisago, M.; Minami, T. Preparation of Li2S-P2S5 amorphous solid electrolytes by mechanical milling. J. Am. Ceram. Soc. 2001, 84, 477–479. [Google Scholar] [CrossRef]
- Hayashi, A.; Ohtomo, T.; Mizuno, F.; Tadanaga, K.; Tatsumisago, M. Rechargeable lithium batteries, using sulfur-based cathode materials and Li2S-P2S5 glass-ceramic electrolytes. Electrochim. Acta 2004, 50, 893–897. [Google Scholar] [CrossRef]
- Nagao, M.; Hayashi, A.; Tatsumisago, M. Fabrication of favorable interface between sulfide solid electrolyte and Li metal electrode for bulk-type solid-state Li/S battery. Electrochem. Commun. 2012, 22, 177–180. [Google Scholar] [CrossRef]
- Teragawa, S.; Aso, K.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Preparation of Li2S-P2S5 solid electrolyte from N-methylformamide solution and application for all-solid-state lithium battery. J. Power Sources 2014, 248, 939–942. [Google Scholar] [CrossRef] [Green Version]
- Agostini, M.; Aihara, Y.; Yamada, T.; Scrosati, B.; Hassoun, J. A lithium–sulfur battery using a solid, glass-type P2S5–Li2S electrolyte. Solid State Ion. 2013, 244, 48–51. [Google Scholar] [CrossRef]
- Yamada, T.; Ito, S.; Omoda, R.; Watanabe, T.; Aihara, Y.; Agostini, M.; Ulissi, U.; Hassoun, J.; Scrosati, B. All Solid-State Lithium–Sulfur Battery Using a Glass-Type P2S5–Li2S Electrolyte: Benefits on Anode Kinetics. J. Electrochem. Soc. 2015, 162, A646–A651. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Morimoto, H.; Tatsumisago, M.; Minami, T. High lithium ion conductivity of glass-ceramics derived from mechanically milled glassy powders. Chem. Lett. 2001, 84, 872–873. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Minami, T.; Tatsumisago, M. Formation of superionic crystals from mechanically milled Li2S-P2S5 glasses. Electrochem. Commun. 2003, 5, 111–114. [Google Scholar] [CrossRef]
- MTI Corporation. Li7P3S11 LPS. Safety Data Sheet. 2018. Available online: http://www.mtixtl.com/sds/LPS_SDS.pdf (accessed on 22 May 2020).
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Nagata, H.; Chikusa, Y. A lithium sulfur battery with high power density. J. Power Sources 2014, 264, 206–210. [Google Scholar] [CrossRef]
- MTI Corporation EQ-Lib-LGPS LGPS (Li10GeP2S12) Powder with High Ion-Conductivity. Specification and Safety Data Sheet. 2018. Available online: http://www.mtixtl.com/sds/LGPS_SDS.pdf (accessed on 22 May 2020).
- Liu, Z.C.; Fu, W.J.; Payzant, E.A.; Yu, X.; Wu, Z.L.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous high ionic conductivity of nanoporous beta-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.C.; Dudney, N.J.; Liang, C.D. Lithium superionic sulfide cathode for all-solid lithium-sulfur batteries. ACS Nano 2013, 7, 2829–2833. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.C.; Fu, W.J.; Dudney, N.J.; Liang, C.D. Lithium polysulfidophosphates: A family of lithium-conducting sulfur-rich compounds for lithium-sulfur batteries. Angew. Chem. Int Ed. 2013, 52, 7460–7463. [Google Scholar] [CrossRef] [PubMed]
- NEI Corporation. LPS-Lithium Phosphorus Sulfide (β-Li3PS4) powder. Safety Data Sheet. 2020. Available online: https://neicorporation.com/msds/Li3PS4_Lithium_Phosphorus_Sulfide_SDS.pdf (accessed on 22 May 2020).
- LTS Research Laboratories Inc. Lithium Phosphorus Sulfide. Safety Data Sheet. 2017. Available online: https://www.ltschem.com/msds/Li3PS4.pdf (accessed on 22 May 2020).
- Liu, Z.; Huang, F.; Yang, J.; Wang, B.; Sun, J. New lithium ion conductor, thio-LISICON lithium zirconium sulfide system. Solid State Ion. 2008, 179, 1714–1716. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, F.; Yang, J.; Wang, Y.; Sun, J. Preparation of new lithium ion composite electrolyte 3Li4SiS4–0.5La2S3 by mechanical milling. Solid State Sci. 2008, 10, 1429–1433. [Google Scholar] [CrossRef]
- LTS Research Laboratories Inc. Silicon Sulfide. Safety Data Sheet. 2015. Available online: https://www.ltschem.com/msds/SiS2.pdf (accessed on 22 May 2020).
- Indium, RSC. Available online: https://www.rsc.org/periodic-table/element/49/indium (accessed on 20 May 2020).
- NEI Corporation. LPSCl—Lithium Phosphorus Sulfur Chloride (Li6PS5Cl) powder. Safety Data Sheet. 2020. Available online: https://neicorporation.com/msds/Li6PS5Cl_Lithium_Phosphorus_Sulfur_Chloride_SDS.pdf (accessed on 22 May 2020).
- Aurbach, D.; Pollak, E.; Elazari, R.; Salitra, G.; Kelley, C.S.; Affinito, J. On the surface chemical aspects of very high energy density, rechargeable Li-sulfur batteries. J. Electrochem. Soc. 2009, 156, A694. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Lithium nitrate, CID=10129889. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-nitrate (accessed on 8 May 2020).
- Demir-Cakan, R.; Morcrette, M.; Gangulibabu, B.; Guéguen, A.; Dedryvère, R.; Tarascon, J.-M. Li-S batteries: Simple approaches for superior performance. Energy Env. Sci. 2013, 6, 176–182. [Google Scholar] [CrossRef]
- Xiong, S.Z.; Xie, K.; Diao, Y.; Hong, X.B. On the role of polysulfides for a stable solid electrolyte interphase on the lithium anode cycled in lithium-sulfur batteries. J. Power Sources 2013, 236, 181–187. [Google Scholar] [CrossRef]
- Xiong, S.; Xie, K.; Diao, Y.; Hong, X. Characterization of the solid electrolyte interphase on lithium anode for preventing the shuttle mechanism in lithium–sulfur batteries. J. Power Sources 2014, 246, 840–845. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Lithium sulfide (Li2S), CID=64734. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-sulfide-_Li2S (accessed on 8 May 2020).
- Lin, Z.; Liu, Z.; Fu, W.; Dudney, N.J.; Liang, C. Phosphorous pentasulfide as a novel additive for high-performance lithium-sulfur batteries. Adv. Funct. Mater. 2013, 23, 1064–1069. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Phosphorus pentasulfide, CID=14817. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phosphorus-pentasulfide (accessed on 8 May 2020).
- Morris, C. MIT researchers discover two forms of lithium dendrite formation. Charged Electric Vehicles Magazine. 21 September 2016. Newswire The Tech. Available online: https://chargedevs.com/newswire/mit-researchers-discover-two-forms-of-dendrite-formation/ (accessed on 8 May 2020).
- Liu, M.; Zhou, D.; Jiang, H.; Ren, Y.; Kang, F.; Zhao, T. A highly-safe lithium-ion sulfur polymer battery with SnO2 anode and acrylate-based gel polymer electrolyte. Nano Energy 2016, 28, 97–105. [Google Scholar] [CrossRef]
- Pu, X.; Yang, G.; Yu, C. Safe and reliable operation of sulfur batteries with lithiated silicon. Nano Energy 2014, 9, 318–324. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, A.; Huang, Y.; Yuan, K.; Yu, Z.; Qiu, J.; Yang, Y. Improved cycle stability and high security of Li-B alloy anode for lithium–sulfur battery. J. Mater. Chem. A 2014, 2, 11660–11665. [Google Scholar] [CrossRef]
- Agostini, M.; Hassoun, J.; Liu, J.; Jeong, M.; Nara, H.; Momma, T.; Osaka, T.; Sun, Y.; Scrosati, B. A Lithium-Ion Sulfur Battery Based on a Carbon-Coated Lithium-Sulfide Cathode and an Electrodeposited Silicon-Based Anode. ACS Appl. Mater. Interfaces 2014, 6, 10924–10928. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Agostini, M.; Caballero, A.; Morales, J.; Hassoun, J. A long-life lithium ion sulfur battery exploiting high performance electrodes. Chem. Commun. 2015, 51, 14540–14542. [Google Scholar] [CrossRef] [PubMed]
- Alfa Aesar Thermo Fisher Scientific Chemicals Inc. Indium(III) iodide. CAS 13510-35-5. Safety Data Sheet. 2018. Available online: www.alfa.com (accessed on 20 May 2020).
- Sigma-Aldrich. Poly(3,4-ethylenedioxythiophene)-blockpoly(ethylene glycol) solution. Material Safety Data Sheet, Version 6.0. 2020. Available online: www.sigma-aldrich.com (accessed on 22 May 2020).
- National Center for Biotechnology Information. PubChem Database. Trilithium nitride, CID=520242. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Trilithium-nitride (accessed on 22 May 2020).
- Zhang, X.-W.; Li, Y.; Khan, S.A.; Fedkiw, P.S. Inhibition of lithium dendrites by fumed silica-based composite electrolytes. J. Electrochem. Soc. 2004, 151, A1257–A1263. [Google Scholar] [CrossRef]
- Tatsuma, T.; Taguchi, M.; Oyama, N. Inhibition effect of covalently cross-linked gel electrolytes on lithium dendrite formation. Electrochim. Acta 2001, 46, 1201–1205. [Google Scholar] [CrossRef]
- Steiger, J.; Richter, G.; Wenk, M.; Kramer, D.; Mönig, R. Comparison of the growth of lithium filaments and dendrites under different conditions. Electrochem. Commun. 2015, 50, 11–14. [Google Scholar] [CrossRef]
- Bouchet, R. Batteries: A stable lithium metal interface. Nat. Nanotechnol. 2014, 9, 572–573. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y.; Cui, Y. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.; Zhao, C.; Huang, J.; Yang, S.; Zhang, Q. Lithium metal protection through in-situ formed solid electrolyte interphase in lithium-sulfur batteries: The role of polysulfides on lithium anode. J. Power Sources 2016, 327, 212–220. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Huang, J.-Q.; Zhang, Q. Review—Li Metal Anode in Working Lithium-Sulfur Batteries. J. Electrochem. Soc. 2018, 165, A6058–A6072. [Google Scholar] [CrossRef]
- Raymor Nanotech, division of Raymor Industries Inc. Carbon nanotube, single-walled. Safety Data Sheet. 2014. Available online: http://raymor.com/wp-content/uploads/2014/10/MSDS-SWNT-RN-020.pdf (accessed on 22 May 2020).
- Acros Organics BVBA. Graphite, Powder. CAS 7782-42-5. Material Safety Data Sheet. 2009. Available online: https://www.nwmissouri.edu/naturalsciences/sds/g/Graphite.pdf (accessed on 22 May 2020).
- LTS Research Laboratories Inc. Titanium carbide. Safety Data Sheet. 2015. Available online: https://www.ltschem.com/msds/TiC.pdf (accessed on 22 May 2020).
- AquaPhoenix Scientific. Copper, Granular, 50 Mesh. Safety Data Sheet. 2015. Available online: https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-c/S25268.pdf (accessed on 22 May 2020).
- LTS Research Laboratories Inc. Copper nickel. Safety Data Sheet. 2015. Available online: https://www.ltschem.com/msds/CuNi.pdf (accessed on 22 May 2020).
- Ladd Research. Nickel. Safety Data Sheet. 2020. Available online: https://www.laddresearch.com/lanotattachments/download/file/id/542/store/1/60810sds_1.pdf (accessed on 22 May 2020).
- LTS Research Laboratories Inc. Aluminum fluoride Safety Data Sheet. 2015. Available online: https://www.ltschem.com/msds/AlF3.pdf (accessed on 22 May 2020).
- Cheng, X.-B.; Peng, H.-J.; Huang, J.-Q.; Wei, F.; Zhang, Q. Dendrite-free Nanostructured Anode: Entrapment of Lithium in a 3D Fibrous Matrix for Ultra-Stable Lithium-Sulfur Batteries. Small 2014, 10, 4257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 10992. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Lin, D.; Zhao, J.; Lu, Z.; Liu, Y.; Liu, C.; Lu, Y.; Wang, H.; Yan, K.; Tao, X.; et al. Composite Lithium Metal Anode by Melt Infusion of Lithium Into a 3D Conducting Scaffold With Lithiophilic Coating. Proc. Natl. Acad. Sci. USA 2016, 113, 2862–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered Reduced Graphene Oxide With Nanoscale Interlayer Gaps as a Stable Host for Lithium Metal Anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Sheng, O.; Luo, J.; Yuan, H.; Fang, C.; Zhang, W.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C.; et al. 3D lithium metal embedded within lithiophilic porous matrix for stable lithium metal batteries. Nano Energy 2017, 37, 177–186. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Chen, W.; Zhou, G.; Liu, K.; Dunn, B.; Cui, Y. Conformal Lithium Fluoride Protection Layer on Three-Dimensional Lithium by Nonhazardous Gaseous Reagent Freon. Nano Lett. 2017, 17, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Takehara, Z.; Ogumi, Z.; Uchimoto, Y.; Yasuda, K.; Yoshida, H. Modification of lithium/electrolyte interface by plasma polymerization of 1,1-difluoroethene. J. Power Sources 1993, 44, 377–383. [Google Scholar] [CrossRef]
- Osaka, T.; Momma, T.; Matsumoto, Y.; Uchida, Y. Effect of carbon dioxide on lithium anode cycleability with various substrates. J. Power Sources 1997, 68, 497–500. [Google Scholar] [CrossRef]
- Osaka, T.; Momma, T.; Matsumoto, Y.; Uchida, Y. Surface Characterization of Electrodeposited Lithium Anode with Enhanced Cycleability Obtained by CO2 Addition. J. Electrochem. Soc. 1997, 144, 1709–1713. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Lithium carbonate, CID=11125. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-carbonate (accessed on 14 May 2020).
- Nimon, Y.; Chu, M.-Y.; Visco, S.J. Coated lithium electrodes. U.S. Patent 6,537,701, 25 March 2003. [Google Scholar]
- Mikhaylik, Y.V. Electrolytes for lithium–sulfur cells. U.S. Patent 7,354,680, 8 April 2008. [Google Scholar]
- Sigma-Aldrich. Aluminum oxide. Safety Data Sheet. Version 5.5. 2015. Available online: www.sigma-aldrich.com (accessed on 22 May 2020).
- Alumina Toxicology. Available online: https://digitalfire.com/4sight/hazards/ceramic_hazard_alumina_toxicology_341.html (accessed on 22 May 2020).
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanoparticle Res. 2011, 13, 3287–3299. [Google Scholar] [CrossRef]
- Cha, E.; Patel, M.D.; Park, J.; Hwang, J.; Prasad, V.; Cho, K.; Choi, W. 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li–S batteries. Nat. Nanotechnol. 2018, 13, 337–344. [Google Scholar]
- Chen, W.; Qi, W.; Lu, W.; Chaudhury, N.R.; Yuan, J.; Qin, L.; Lou, J. Direct Assessment of the Toxicity of Molybdenum Disulfide Atomically Thin Film and Microparticles via Cytotoxicity and Patch Testing. Small 2018, 14, 1702600. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Yan, K.; Li, W.; Zheng, G.; Kong, D.; She, Z.W.; Narasimhan, V.K.; Liang, Z.; Cui, Y. Improved lithium–sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode–separator interface. Energy Environ. Sci. 2014, 7, 3381–3390. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. High-performance Li–S batteries with an ultra-lightweight MWCNT-coated separator. J. Phys. Chem. Lett. 2014, 5, 1978–1983. [Google Scholar] [CrossRef]
- Fang, J.; Qin, F.; Li, J.; Zhang, K.; Liu, W.; Wang, M.; Yu, F.; Zhang, L. Improved performance of sulfur cathode by an easy and scale-up coating strategy. J. Power Sources 2015, 297, 265–270. [Google Scholar] [CrossRef]
- Chung, S.; Manthiram, A. Bifunctional Separator with a Light-Weight Carbon-Coating for Dynamically and Statically Stable Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 5299–5306. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhang, Q.; Peng, H.-J.; Liu, X.-Y.; Qian, W.-Z.; Wei, F. Ionic shield for polysulfides towards highly-stable lithium–sulfur batteries. Energy Environ. Sci. 2014, 7, 347–353. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Allegri, M.; Perivoliotis, D.K.; Bianchi, M.G.; Chiu, M.; Pagliaro, A.; Koklioti, M.A.; Trompeta, A.-F.A.; Bergamaschi, E.; Bussolati, O.; Charitidis, C.A. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicol. Rep. 2016, 3, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, I.; Fruijtier-Pölloth, C.; Ngiewih, Y.; Levy, L. Evaluating the evidence on genotoxicity and reproductive toxicity of carbon black: A critical review. Crit. Rev. Toxicol. 2018, 48, 143–169. [Google Scholar] [CrossRef] [Green Version]
- TIMCAL Belgium N.V. Ensaco 150/210/250/260/350 granular, Ensaco 150/250P, Super P, Super P-Li, C-NERGY Super C 45/65. Material Safety Data Sheet. 2011. Available online: https://ehslegacy.unr.edu/msdsfiles/24831.pdf (accessed on 22 May 2020).
- LUCENE LC180 Polyolefin Elastomer. Safety Data Sheet. LG Chem Ltd Deasan Plant, 2012. Available online: http://www.matweb.com/search/datasheettext.aspx?matguid=5cfea5ad7b944588a3818fc90bbdc12a (accessed on 22 May 2020).
- U.S. Department of Health and Human Services; Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological Profile for Aluminium. September 2008. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=191&tid=34 (accessed on 22 May 2020).
- Wang, Y.; Kaur, G.; Zysk, A.; Liapis, V.; Hay, S.; Santos, A.; Losic, D.; Evdokiou, A. Systematic In Vitro Nanotoxicity Study on Anodic Alumina Nanotubes with Engineered Aspect Ratio: Understanding Nanotoxicity by A Nanomaterial Model. Biomaterials 2015, 46, 117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Xu, B.; Wang, S.; Li, B.; Ma, J.; Gao, J.; Zuo, Y.Y.; Liu, S. Mesoporous carbon nanomaterials induced pulmonary surfactant inhibition, cytotoxicity, inflammation and lung fibrosis. J. Env. Sci. 2017, 62, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zeng, Z.; Liu, L.; Chen, J.; Zhou, H.; Huang, L.; Huang, J.; Xu, H.; Xu, Y.; Chen, Z.; et al. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale 2018, 10, 6981. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, T.W.; Miller, W.C.; McConnell, E.E.; Chevalier, J.; Hadley, J.G.; Bernstein, D.M.; Thevenaz, P.; Anderson, R. Chronic inhalation toxicity of size-separated glass fibers in Fischer 344 rats. Fundam. Appl. Toxicol. 1993, 20, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fan, S.; Fan, S.; Li, C.; Shang, Z.; Gu, M.; Liang, S.; Tian, X. In Vitro and In Vivo Toxicity of Black Phosphorus Nanosheets. J. Nanosci. Nanotechnol. 2020, 20, 659–667. [Google Scholar] [CrossRef]
- Kalgutkar Amit, S.; Scott Daniels, J. Chapter 3: Carboxylic Acids and their Bioisosteres. In Metabolism, Pharmacokinetics and Toxicity of Functional Groups: Impact of Chemical Building Blocks on ADMET; Smith, D.A., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2010; pp. 99–167. [Google Scholar]
- Yu, X.; Joseph, J.; Manthiram, A. Suppression of the polysulfide-shuttle behavior in Li–S batteries through the development of a facile functional group on the polypropylene separator. Mater. Horiz 2016, 3, 314–319. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, M.; Lv, W.; He, Y.; Wang, C.; Yun, Q.; Li, B.; Kang, F.; Yang, Q. Dense coating of Li4Ti5O12 and graphene mixture on the separator to produce long cycle life of lithium-sulfur battery. Nano Energy 2016, 30, 1–8. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J. Synthesis of Ti-based Oxide Nanoparticles and Their Electrochemical Properties. ECS Meet. Abstr. 2006, MA2006-01, 51. [Google Scholar]
- Jo, M.R.; Nam, K.M.; Lee, Y.; Song, K.; Park, J.T.; Kang, Y.M. Phosphidation of Li4Ti5O12 nanoparticles and their electrochemical and biocompatible superiority for lithium rechargeable batteries. Chem. Commun. 2011, 47, 11474–11476. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [Green Version]
- Sironval, V.; Reylandt, L.; Chaurand, P.; Ibouraadaten, S.; Palmai-Pallag, M.; Yakoub, Y.; Ucakar, B.; Rose, J.; Poleunis, C.; Vanbever, R.; et al. Respiratory hazard of Li-ion battery components: Elective toxicity of lithium cobalt oxide (LiCoO2) particles in a mouse bioassay. Arch. Toxicol. 2018, 92, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Huang, J.; Peng, H.; He, L.; Cheng, X.; Che, C.; Zhang, Q. Rational Integration of Polypropylene/Graphene Oxide/Nafion as Ternary-Layered Separator to Retard the Shuttle of Polysulfides for Lithium–Sulfur Batteries. Small 2016, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, C.; Lu, Y.; Zang, J.; Jiang, M.; Kim, D.; Zhang, X. Highly porous polyacrylonitrile/graphene oxide membrane separator exhibiting excellent anti-self-discharge feature for high-performance lithium–sulfur batteries. Carbon 2016, 101, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Sokhi, S.; Balomajumder, C.; Satapathi, S. Reusable graphene oxide nanofibers for enhanced photocatalytic activity: A detailed mechanistic study. J. Mater. Sci. 2017, 52, 5390–5403. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, J.; Kim, J.-H.; Lee, T.; Byun, H.-S. Electrospun PAN-GO composite nanofibers as water purification membranes: Research Article. J. Appl. Polym. Sci. 2017, 135, 45858. [Google Scholar] [CrossRef]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Filippousi, M.; Turner, S.; Leus, K.; Siafaka, P.I.; Tseligka, E.D.; Vandichel, M.; Nanaki, S.G.; Vizirianakis, I.S.; Bikiaris, D.N.; Van Der Voort, P.; et al. Biocompatible Zr-based nanoscale MOFs coated with modified poly (epsilon-caprolactone) as anticancer drug carriers. Int. J. Pharm. 2016, 509, 208–218. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Cunha, D.; Imbuluzqueta, E.; Ragon, F.; Serre, C.; Blanco-Prieto, M.; Horcajada, P. Cytotoxicity of nanoscaled metal-organic frameworks. J. Mater. Chem. 2014, 2, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Ruyra, A.; Yazdi, A.; Espin, J.; Carné-Sánchez, A.; Roher, N.; Lorenzo, J.; Imaz, I.; Maspoch, D. Synthesis, culture medium stability, and in vitro and in vivo zebrafish embryo toxicity of metal-organic framework nanoparticles. Chem. Eur. J. 2015, 21, 2508–2518. [Google Scholar] [CrossRef]
- Ren, F.; Yang, B.C.; Cai, J.; Jiang, Y.D.; Xu, J.; Wang, S. Toxic effect of zinc nanoscale metal-organic frameworks on rat pheochromocytoma (PC12) cells in vitro. J. Hazard. Mater. 2014, 271, 283–291. [Google Scholar] [CrossRef]
- Grall, R.; Hidalgo, T.; Delic, J.; Garcia-Marquez, A.; Chevillard, S.; Horcajada, P. In vitro biocompatibility of mesoporous metal (III.; Fe, Al, Cr) trimesate MOF nanocarriers. J. Mater. Chem. B 2015, 3, 8279–8292. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Liu, Q.; Rose, O.L.; Eden, A.; Vijay, A.; Rojanasakul, Y.; Dinu, C.Z. Toxicity screening of two prevalent metal organic frameworks for therapeutic use in human lung epithelial cells. Int. J. Nanomed. 2019, 14, 7583–7591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-H.; Chung, S.-H.; Manthiram, A. Effective Stabilization of a High-Loading Sulfur Cathode and a Lithium-Metal Anode in Li-S Batteries Utilizing SWCNT-Modulated Separators. Small 2016, 12, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Dwivedi, P.K.; Yadav, P.; Shelke, M.V. Chapter 3.8.2.—Separators for Li-ion Batteries. In Emerging Nanostructured Materials for Energy and Environmental Science, Environmental Chemistry for a Sustainable World; Saravanan, R., Mu, N., Kumar, R., Rabah, B., Eds.; Springer: New York, NY, USA, 2019; Volume 23, p. 151. [Google Scholar]
- Weng, G.-M.; Yang, B.; Liu, C.-Y.; Du, G.-Y.; Li, E.Y.; Lu, Y.-C. Asymmetric allyl-activation of organosulfides for high-energy reversible redox flow batteries. Energy Environ. Sci. 2019, 12, 2244–2252. [Google Scholar] [CrossRef]
- Mathew, D.E.; Sivalingam, G.; Murugavel, K.; Rani, G.J.; Sabu, T.; Stephan, A.M. A porous organic polymer-coated permselective separator mitigating self-discharge of lithium–sulfur batteries. Mater. Adv. 2020, 1, 648–657. [Google Scholar] [CrossRef]
- Celgard, MTI-Korea. Li-ion Battery Separator Film, Material Safety Data Sheet. 2010. Available online: http://mtikorea.co.kr/web/smh/pdf/MSDS_separator_film.pdf (accessed on 22 May 2020).
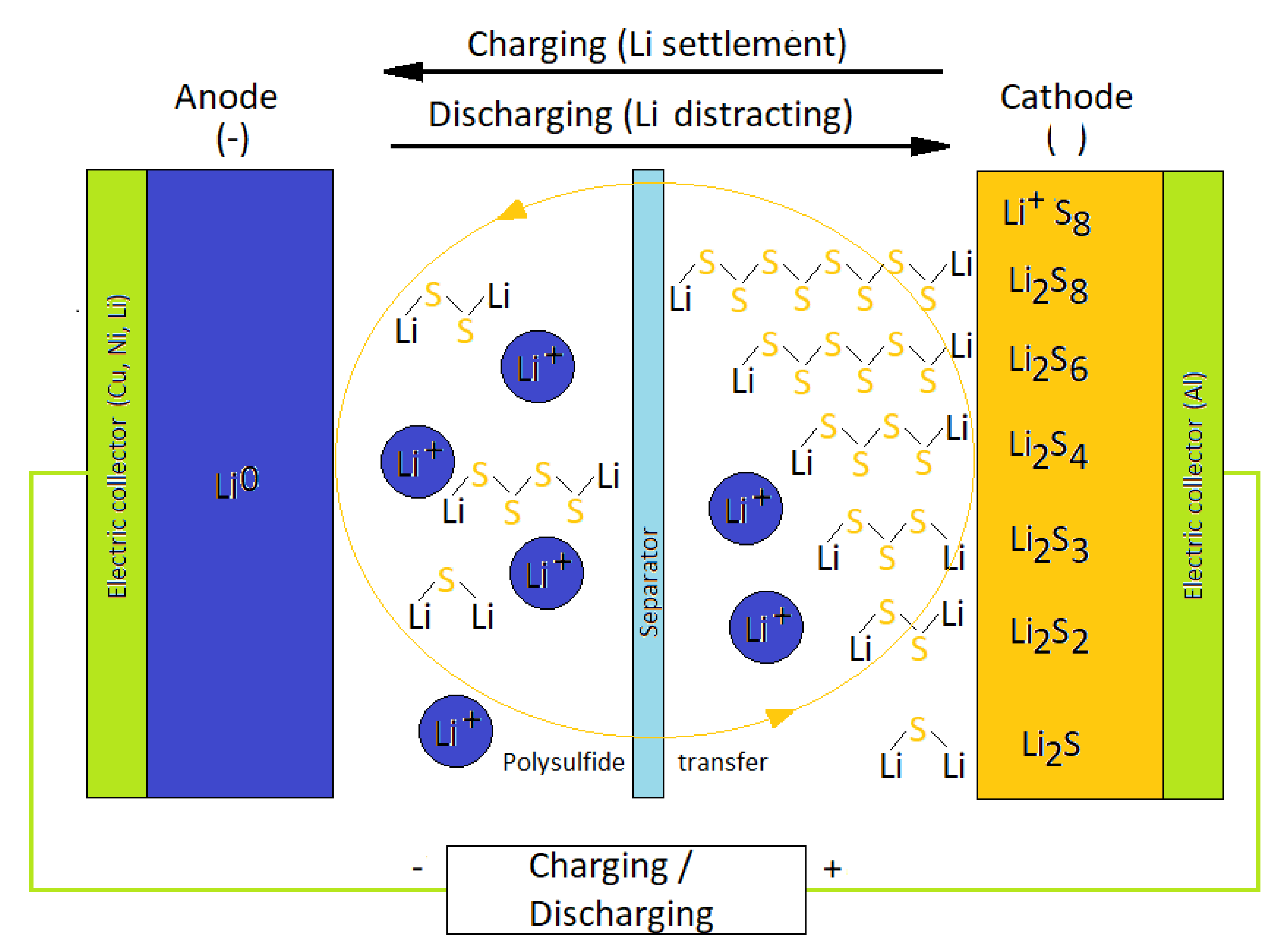

| Material | Class of EC 1272/2008 | References |
|---|---|---|
| Sulfur | H315, H228 | [14] |
| H303, H313, H315, H333 | [15] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| TiO2 | H351 | [36] |
| MnO2 | H302, H332 | [45] |
| V2O5 | H302, H332, H335, H341, H361d, H372, H411 | [50] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| LiAsF6 | H301, H319, H320, H331, H350, H360, H361, H370, H372, H400, H410 | [47] |
| Lithium Metal | H260, H314 | [78] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| polyvinyl pyrrolidone | H303 | [108] |
| gum Arabic | H319 | [112] |
| polyvinylidene fluoride | H315, H319, H335 | [114] |
| Nafion NR50 | H315, H319, H335 | [117] |
| PAMAM dendrimers | H225, H301, H311, H331, H370 | [118] |
| polyethyleneimine | H319, H302, H317, H411 | [145] |
| lithium polyacrylate | H302 | [151] |
| AMAC | H412 | [153] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| LiTFSI | H301, H311, H314, H373, H412 | [170] |
| dimethyl ether | H220, H280, H336 | [172] |
| 1,3-dioxolane | H225, H319 | [174] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| Tetrahydrofuran | H225, H302, H319, H335, H351 | [190] |
| ethylene glycol | H302, H373 | [192] |
| Triglyme | H360Df | [194] |
| DEGDME | H226, H360Df | [196] |
| TEGDME | H360Df | [197] |
| 2-ethoxyethyl ether | H227, H319 | [200] |
| Diglyme | H226, H360Df | [202] |
| methyl acetate | H319, H335, H336, H370 | [206] |
| Toluene | H225, H315, H361, H336, H373, H304, H402 | [209] |
| gamma-butyrolactone | H302, H318, H336 | [211] |
| TBAPF6 | H315, H319, H335 | [214] |
| LiCF3SO3 | H315, H319, H335 | [216] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| LiSO3CF3 | H315, H319, H335 | [216] |
| LiN(SO2CF3)2 | H301, H311, H314, H373, H412 | [222] |
| bis (fluorosulfonyl) imide | H314, H318 | [227] |
| LiTDI | H301, H315, H319, H335 | [228] |
| LiClO4 | H271, H272, H315, H319, H335 | [229] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| dimethyl carbonate | H225 | [234] |
| 1-butyl-1-methylpiperidinium bis (trifluoromethylsulfonyl) imide | H301, H311, H314 | [237] |
| 1-ethyl-3-methylimidazoliumbis (trifluoromethanesulfonyl) imide | H301 | [240] |
| 1-allyl-1-methylpyrrolidinium bis (trifluoromethanesulfonyl) imide | H315, H319 | [244] |
| 1-ethyl-3-methylimidazolium bis (pentafluoroethylsulfonyl) imide | H301 | [245] |
| 1-butyl-3-methylimidazolium hexafluorophosphate | H315, H319 | [246] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| acrylonitrile (CH2 = CHCN) | H225, H301, H311, H315, H317, H318, H331, H335, H350, H411 | [254] |
| ethylene carbonate | H302, H315, H318, H319, H335, H373 | [255] |
| diethyl carbonate | H226, H315, H319, H335 | [256] |
| propylene carbonate | H319 | [257] |
| LiPF6 | H302, H311, H314 | [258] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| SiO2 | H319, H335, H373 | [283] |
| TiO2 | H351 | [36] |
| Al2O3 | H319, H335, H372 | [286] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| Li4SiO4 | H315, H320 | [310] |
| Li7P3S11 | H228, H260, H301, H302, H332, H314, H318, H400 | [319] |
| Li10GeP2S12 | H302, H315, H319, H335 | [322] |
| β-Li3PS4 | H228, H260, H301, H314, H318, H332, H400 | [326] |
| Li3PS4 | H228, H261, H301, H302, H332, H315, H318, H335, H410 | [327] |
| SiS2 | H261, H300, H315, H319, H335 | [330] |
| Li6PS5Cl | H228, H260, H301, H314, H318, H332, H400 | [332] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| LiNO3 | H272, H302, H319 | [334] |
| Li2S | H261, H301, H314 | [338] |
| P2S5 | H228, H260, H302, H332, H400 | [340] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| Indium (III) iodide | H314, H317, H334, H361 | [347] |
| poly(3,4-ethylenedioxythiophene)-blockpoly (ethylene glycol) | H302, H226, H351 | [348] |
| Li3N | H314, H318, H260 | [349] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| carbon nanotube | H319, H335 | [357] |
| TiC | H228 | [359] |
| copper granular | H300, H330, H319, H317, H340, H373, H400 | [360] |
| CuNi | H228, H317, H319, H335, H351, H372 | [361] |
| Ni | H228, H317, H351, H372, H410 | [359] |
| AlF3 | H300, H314 | [360] |
| Material | Class of EC 1272/2008 | References |
|---|---|---|
| Li2CO3 | H302, H315, H318, H319, H335, H360, H372 | [373] |
| Al2O3 | H332 | [376] |
| Material | Li-S Cell Component | Comments |
|---|---|---|
| Gelatin | Binder | Practically non-toxic |
| Celgrad | Separator | Practically non-toxic |
| perovskite type La(2/3)xLi3xTiO3 (LLT) | Electrolyte | Ionic conducting solid materials are the least electrolytes |
| Lithium protected by DOL layer | Anode | Protecting layer limits the toxicity of pure lithium |
| Carbon + Sulfur | Cathode | C is much lower toxic than S. The content of S should be limited to the necessary minimum. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siczek, K. The Toxicity of Secondary Lithium-Sulfur Batteries Components. Batteries 2020, 6, 45. https://doi.org/10.3390/batteries6030045
Siczek K. The Toxicity of Secondary Lithium-Sulfur Batteries Components. Batteries. 2020; 6(3):45. https://doi.org/10.3390/batteries6030045
Chicago/Turabian StyleSiczek, Krzysztof. 2020. "The Toxicity of Secondary Lithium-Sulfur Batteries Components" Batteries 6, no. 3: 45. https://doi.org/10.3390/batteries6030045
APA StyleSiczek, K. (2020). The Toxicity of Secondary Lithium-Sulfur Batteries Components. Batteries, 6(3), 45. https://doi.org/10.3390/batteries6030045





