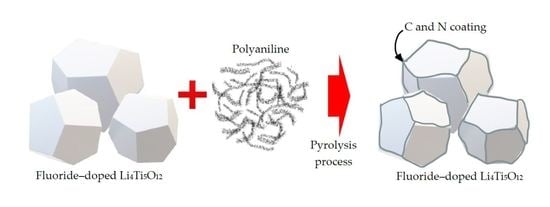
Direct Double Coating of Carbon and Nitrogen on Fluoride-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries
Abstract
:1. Introduction
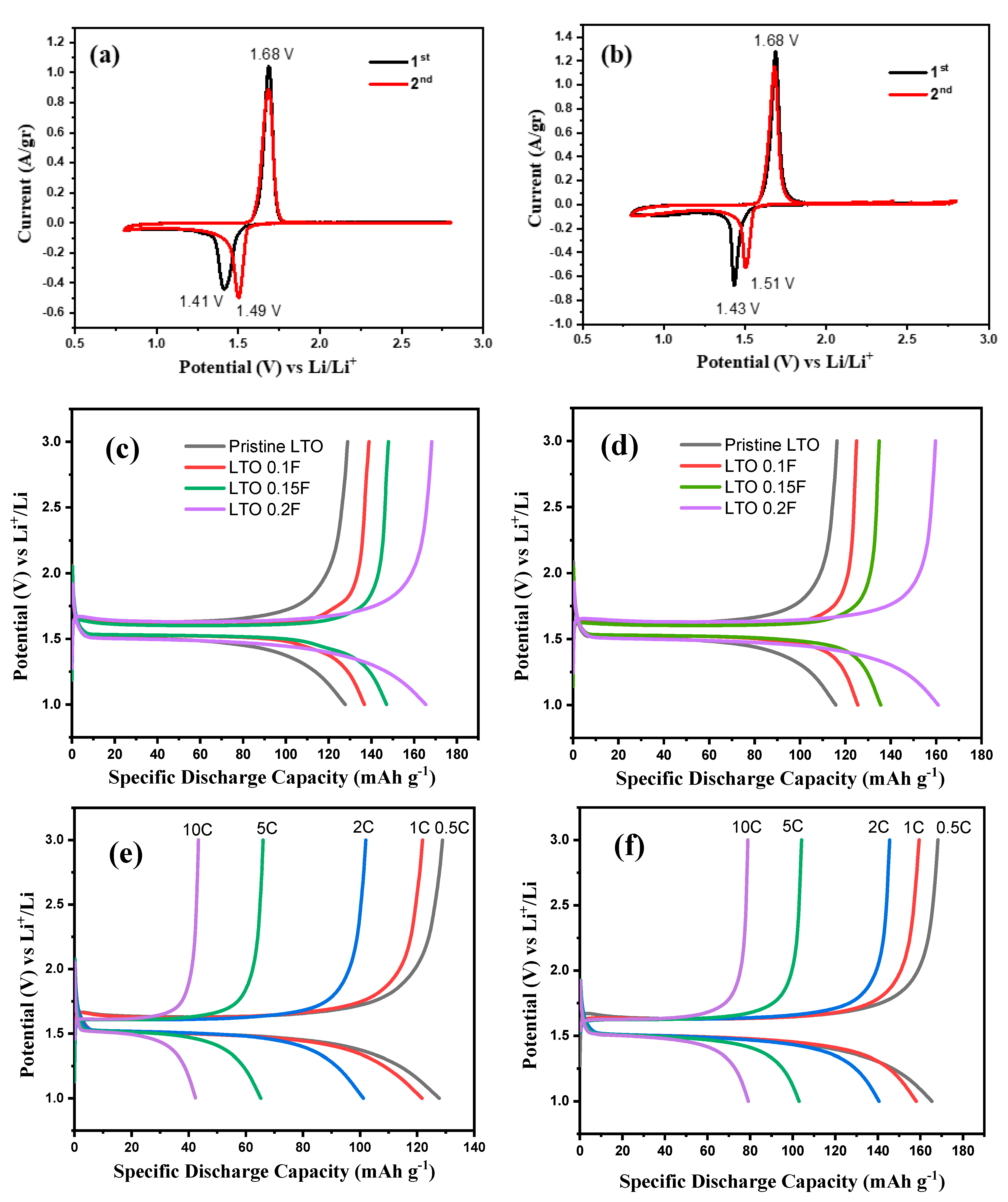
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Double-Coated F-Doped LTO
4.2. Material Characterization
4.3. Electrochemical Measurement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Radovanovic, P.V.; Cui, B. Advances in spinel Li4Ti5O12 anode materials for lithium-ion batteries. New J. Chem. 2015, 39, 38–63. [Google Scholar] [CrossRef]
- Fong, R.; von Sacken, U.; Dahn, J.R. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 1990, 137, 2009–2013. [Google Scholar] [CrossRef]
- Bai, P.; Li, J.; Brushett, F.R.; Bazant, M.Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229. [Google Scholar] [CrossRef] [Green Version]
- Kasnatscheew, J.; Placke, T.; Streipert, B.; Rothermel, S.; Wagner, R.; Meister, P.; Laskovic, I.C.; Winter, M. A Tutorial into Practical Capacity and Mass Balancing of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A2479–A2486. [Google Scholar] [CrossRef]
- Vikram Babu, B.; Vijaya Babu, K.; Tewodros Aregai, G.; Seeta Devi, L.; Madhavi Latha, B.; Sushma Reddi, M.; Samatha, K.; Veeraiah, V. Structural and electrical properties of Li4Ti5O12 anode material for lithium-ion batteries. Results Phys. 2018, 9, 284–289. [Google Scholar] [CrossRef]
- Chen, M.; Li, W.; Shen, X.; Diao, G. Fabrication of Core–Shell α-Fe2O3@ Li4Ti5O12 Composite and Its Application in the Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 4514–4523. [Google Scholar] [CrossRef]
- Shen, L.; Uchaker, E.; Zhang, X.; Cao, G. Hydrogenated Li4Ti5O12 Nanowire Arrays for High Rate Lithium Ion Batteries. Adv. Mater. 2012, 24, 6502–6506. [Google Scholar] [CrossRef]
- Kang, E.; Jung, Y.S.; Kim, G.-H.; Chun, J.; Wiesner, U.; Dillon, A.C.; Kim, J.K.; Lee, J. Highly Improved Rate Capability for a Lithium-Ion Battery Nano-Li4Ti5O12 Negative Electrode via Carbon-Coated Mesoporous Uniform Pores with a Simple Self-Assembly Method. Adv. Funct. Mater. 2011, 21, 4349–4357. [Google Scholar] [CrossRef]
- Jung, H.-G.; Myung, S.-T.; Yoon, C.S.; Son, S.-B.; Oh, K.H.; Amine, K.; Scrosati, B.; Sun, Y.-K. Microscale spherical carbon-coated Li4Ti5O12 as ultra high power anode material for lithium batteries. Energy Environ. Sci. 2011, 4, 1345–1351. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Streipert, B.; Röser, S.; Wagner, R.; Cekic Laskovic, I.; Winter, M. Determining oxidative stability of battery electrolytes: Validity of common electrochemical stability window (ESW) data and alternative strategies. Phys. Chem. Chem. Phys. 2017, 19, 16078–16086. [Google Scholar] [CrossRef]
- Li, B.; Han, C.; He, Y.-B.; Yang, C.; Du, H.; Yang, Q.-H.; Kang, F. Facile synthesis of Li4Ti5O12/C composite with super rate performance. Energy Environ. Sci. 2012, 5, 9595–9602. [Google Scholar] [CrossRef]
- Zhu, G.-N.; Liu, H.-J.; Zhuang, J.-H.; Wang, C.-X.; Wang, Y.-G.; Xia, Y.-Y. Carbon-coated nano-sized Li4Ti5O12 nanoporous micro-sphere as anode material for high-rate lithium-ion batteries. Energy Environ. Sci. 2011, 4, 4016–4022. [Google Scholar] [CrossRef]
- Zhu, G.-N.; Wang, Y.-G.; Xia, Y.-Y. Ti-based compounds as anode materials for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6652–6667. [Google Scholar] [CrossRef]
- Yi, T.-F.; Liu, H.; Zhu, Y.-R.; Jiang, L.-J.; Xie, Y.; Zhu, R.-S. Improving the high rate performance of Li4Ti5O12 through divalent zinc substitution. J. Power Sources 2012, 215, 258–265. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, J.; Wang, W.; Huang, Y.; Feng, Z.; Zhang, Z.; Tang, Z. Preparation and effects of Mg-doping on the electrochemical properties of spinel Li4Ti5O12 as anode material for lithium ion battery. Mater. Chem. Phys. 2010, 123, 510–515. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Zhu, Z.; Lin, J.; Tian, Z.; Wang, R. Structural and electrochemical characteristics of Li4−xAlxTi5O12 as anode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 7079–7083. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, Y.; Jia, D.; Bao, S.-J.; Guo, Z.P. Preparation and characterization of novel spinel Li4Ti5O12−xBrx anode materials. Electrochim. Acta 2009, 54, 4772–4776. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, B.; Ji, G.; Lee, J.Y. Carbon-Encapsulated F-Doped Li4Ti5O12 as a High Rate Anode Material for Li+ Batteries. ACS Nano 2013, 7, 10870–10878. [Google Scholar] [CrossRef]
- Huang, Y.; Qi, Y.; Jia, D.; Wang, X.; Guo, Z.; Cho, W. Il Synthesis and electrochemical properties of spinel Li4Ti5O12−xClxanode materials for lithium-ion batteries. J. Solid State Electrochem. 2012, 16, 2011–2016. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, C.; Zhang, P.; Zhao, R.; Lu, J.; Chen, M. Fluoride doping Li4Ti5O12 nanosheets as anode materials for enhanced rate performance of lithium-ion batteries. J. Electroanal. Chem. 2018, 815, 123–129. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Li, Y.; Chen, Y.; Luo, T. Electrochemical performance of Li4Ti5O12/carbon nanotubes/graphene composite as an anode material in lithium-ion batteries. Int. J. Hydrogen Energy 2017, 42, 7195–7201. [Google Scholar] [CrossRef]
- Wei, A.; Li, W.; Zhang, L.; Ren, B.; Bai, X.; Liu, Z. Enhanced electrochemical performance of a LTO/N-doped graphene composite as an anode material for Li-ion batteries. Solid State Ion. 2017, 311, 98–104. [Google Scholar] [CrossRef]
- Mo, L.; Zheng, H. Solid coated Li4Ti5O12 (LTO) using polyaniline (PANI) as anode materials for improving thermal safety for lithium ion battery. Energy Rep. 2020, 6, 2913–2918. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.-S.; Li, H.; Wang, Z.; Chen, L. Porous Li4Ti5O12 Coated with N-Doped Carbon from Ionic Liquids for Li-Ion Batteries. Adv. Mater. 2011, 23, 1385–1388. [Google Scholar] [CrossRef]
- Yanhua, L.; Tan, N.; Huo, D.; Ding, M.; Zhang, Y.; Liu, T.; Yu, R.; Cheng, S.; Fan, R. Fabrication of Porous N-rich Carbon Electrocatalysts from Pyrolysis of PANI-Encapsulated CeO2 for Enhanced Oxygen Reduction Reaction. J. Electrochem. Soc. 2021, 168, 44516. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, J.; Huang, T.; Sun, M. Recent Advance on Polyaniline or Polypyrrole-Derived Electrocatalysts for Oxygen Reduction Reaction. Polymers 2018, 10, 1397. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Liu, S.; Lian, Q.; Zhao, J.; Ding, W.; Yu, Z.; Huang, R.; Zou, Z. Nitrogen-doped carbon-coated hierarchical Li4Ti5O12-TiO2 hybrid microspheres as excellent high rate anode of Li-ion battery. Ceram. Int. 2017, 43, 11354–11360. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Liu, Z.; Wang, Z.; Hu, R.; Liu, J.; Ouyang, L.; Zhu, M. FeP@C Nanotube Arrays Grown on Carbon Fabric as a Low Potential and Freestanding Anode for High-Performance Li-Ion Batteries. Small 2018, 14, 1800793. [Google Scholar] [CrossRef]
- Qian, D.; Gu, Y.; Chen, Y.; Liu, H.; Wang, J.; Zhou, H. Ultra-high specific capacity of Cr3+-doped Li4Ti5O12 at 1.55 V as anode material for lithium-ion batteries. Mater. Lett. 2019, 238, 102–106. [Google Scholar] [CrossRef]
- Shu, H.; Wang, X.; Wu, Q.; Ju, B.; Liu, L.; Yang, X.; Wang, Y.; Bai, Y.; Yang, S. Ammonia Assisted Hydrothermal Synthesis of Monodisperse LiFePO4/C Microspheres as Cathode Material for Lithium Ion Batteries. J. Electrochem. Soc. 2011, 158, A1448–A1454. [Google Scholar] [CrossRef]
- Noerochim, L.; Caesarendra, W.; Habib, A.; Widyastuti; Suwarno; Ni’mah, Y.L.; Subhan, A.; Prihandoko, B.; Kosasih, B. Role of TiO2 Phase Composition Tuned by LiOH on The Electrochemical Performance of Dual-Phase Li4Ti5O12-TiO2 Microrod as an Anode for Lithium-Ion Battery. Energies 2020, 13, 5251. [Google Scholar] [CrossRef]
- Kahrizi, M.; Ghaffarinejad, A.; Daneshtalab, R. Preparation and effects of F-doping on electrochemical properties of Li4Ti5O12 as anode material for Li-ion battery. Ionics 2021, 27, 1929–1937. [Google Scholar] [CrossRef]
- Scharner, S.; Weppner, W.; Schmid-Beurmann, P. Evidence of Two-Phase Formation upon Lithium Insertion into the Li1.33Ti1.67O4 Spinel. J. Electrochem. Soc. 1999, 146, 857–861. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, C.; Zhang, C.; Xie, Q.; Qiao, Z.; Zeng, X.; Xu, W.; Zheng, H.; Li, S.; Lin, J.; et al. CNTs-intertwined and N-doped porous carbon wrapped silicon anode for high performance lithium-ion batteries. J. Alloys Compd. 2021, 877, 160240. [Google Scholar] [CrossRef]
- Yuan, T.; Yu, X.; Cai, R.; Zhou, Y.; Shao, Z. Synthesis of pristine and carbon-coated Li4Ti5O12 and their low-temperature electrochemical performance. J. Power Sources 2010, 195, 4997–5004. [Google Scholar] [CrossRef]
- Yuan, T.; Cai, R.; Wang, K.; Ran, R.; Liu, S.; Shao, Z. Combustion synthesis of high-performance Li4Ti5O12 for secondary Li-ion battery. Ceram. Int. 2009, 35, 1757–1768. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Y.; Ji, M.; Zhang, H. Synthesis and electrochemical performance of F-doped Li4Ti5O12 for lithium-ion batteries. Electrochim. Acta 2013, 109, 645–650. [Google Scholar] [CrossRef]
- Tsai, P.; Nasara, R.N.; Shen, Y.; Liang, C.; Chang, Y.; Hsu, W.-D.; Thuy Tran, N.T.; Lin, S. Ab initio phase stability and electronic conductivity of the doped-Li4Ti5O12 anode for Li-ion batteries. Acta Mater. 2019, 175, 196–205. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, X.; Uchaker, E.; Yuan, C.; Cao, G. Li4Ti5O12 Nanoparticles Embedded in a Mesoporous Carbon Matrix as a Superior Anode Material for High Rate Lithium Ion Batteries. Adv. Energy Mater. 2012, 2, 691–698. [Google Scholar] [CrossRef]
- Huang, S.; Wen, Z.; Gu, Z.; Zhu, X. Preparation and cycling performance of Al3+ and F− co-substituted compounds Li4AlxTi5−xFyO12−y. Electrochim. Acta 2005, 50, 4057–4062. [Google Scholar] [CrossRef]
- Bai, X.; Li, W.; Wei, A.; Chang, Q.; Zhang, L.; Liu, Z. Preparation and electrochemical performance of F-doped Li4Ti5O12 for use in the lithium-ion batteries. Solid State Ion. 2018, 324, 13–19. [Google Scholar] [CrossRef]


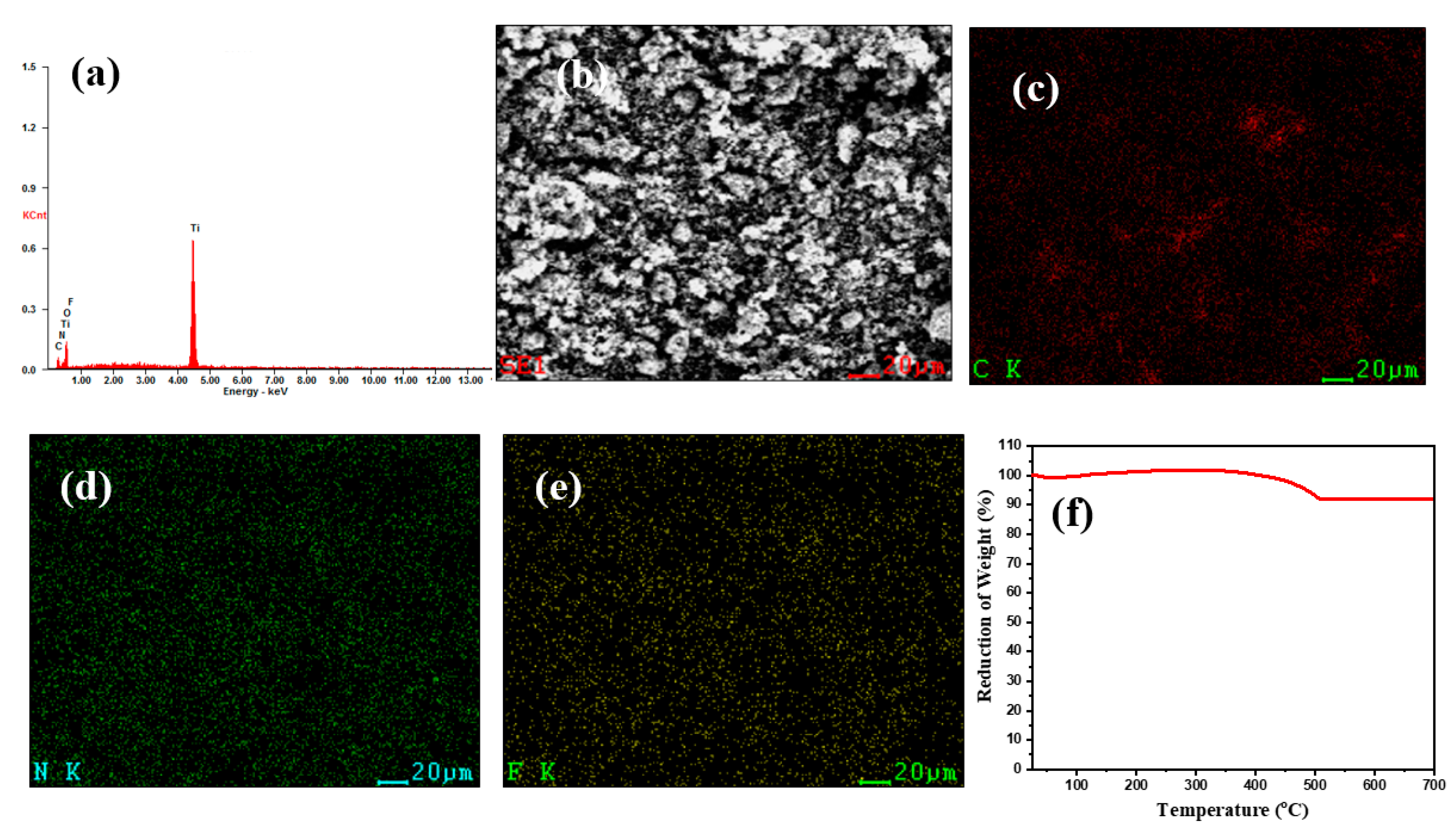

| Sample | Lattice Parameter a (Å) | Cell Volume (106 pm3) |
|---|---|---|
| Pristine LTO | 8.351 | 582.392 |
| LTO 0.1F | 8.353 | 582.810 |
| LTO 0.15F | 8.354 | 583.019 |
| LTO 0.2F | 8.355 | 583.229 |
| Materials System | Modification/Treatment | Voltage (V) | Initial Discharge Capacity (mAh g−1) | Current Density | Ref. |
|---|---|---|---|---|---|
| Li4Ti5O12−xFx (x = 0.3) | Solid-state reaction | 0.01–2.5 | 168.0 | 1.0 C for 100 cycles | [37] |
| Carbon-encapsulated F-doped Li4Ti5O12 | Hydrothermal process and solid state lithiation | 1.0–3.0 | 158 | 1.0 C for 200 cycles | [18] |
| Fluoride doping Li4Ti5O12 nanosheets | Hydrothermal process and calcination | 0.5–2.5 | 172 | 1.0 C for 20 cycles | [20] |
| Li4Ti5O11.9F0.1 | Solid-state reaction | 1.0–3.0 | 160 | 1.0 C for 5 cycles | [41] |
| Li4Ti5O11.7F0.3 | Solid-state reaction | 1.0–3.0 | 139.7 | 1.0 C for 100 cycles | [32] |
| Li4Ti5O12 0.2F | Solid-state reaction | 1.0–3.0 | 157.95 | 1.0 C for 150 cycles | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noerochim, L.; Wibowo, A.T.; Widyastuti; Subhan, A.; Prihandoko, B.; Caesarendra, W. Direct Double Coating of Carbon and Nitrogen on Fluoride-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries. Batteries 2022, 8, 5. https://doi.org/10.3390/batteries8010005
Noerochim L, Wibowo AT, Widyastuti, Subhan A, Prihandoko B, Caesarendra W. Direct Double Coating of Carbon and Nitrogen on Fluoride-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries. Batteries. 2022; 8(1):5. https://doi.org/10.3390/batteries8010005
Chicago/Turabian StyleNoerochim, Lukman, Alvalo Toto Wibowo, Widyastuti, Achmad Subhan, Bambang Prihandoko, and Wahyu Caesarendra. 2022. "Direct Double Coating of Carbon and Nitrogen on Fluoride-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries" Batteries 8, no. 1: 5. https://doi.org/10.3390/batteries8010005
APA StyleNoerochim, L., Wibowo, A. T., Widyastuti, Subhan, A., Prihandoko, B., & Caesarendra, W. (2022). Direct Double Coating of Carbon and Nitrogen on Fluoride-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries. Batteries, 8(1), 5. https://doi.org/10.3390/batteries8010005