Electrode Kinetic Data: Geometric vs. Real Surface Area †
Abstract
1. Introduction
2. Experimental Procedure
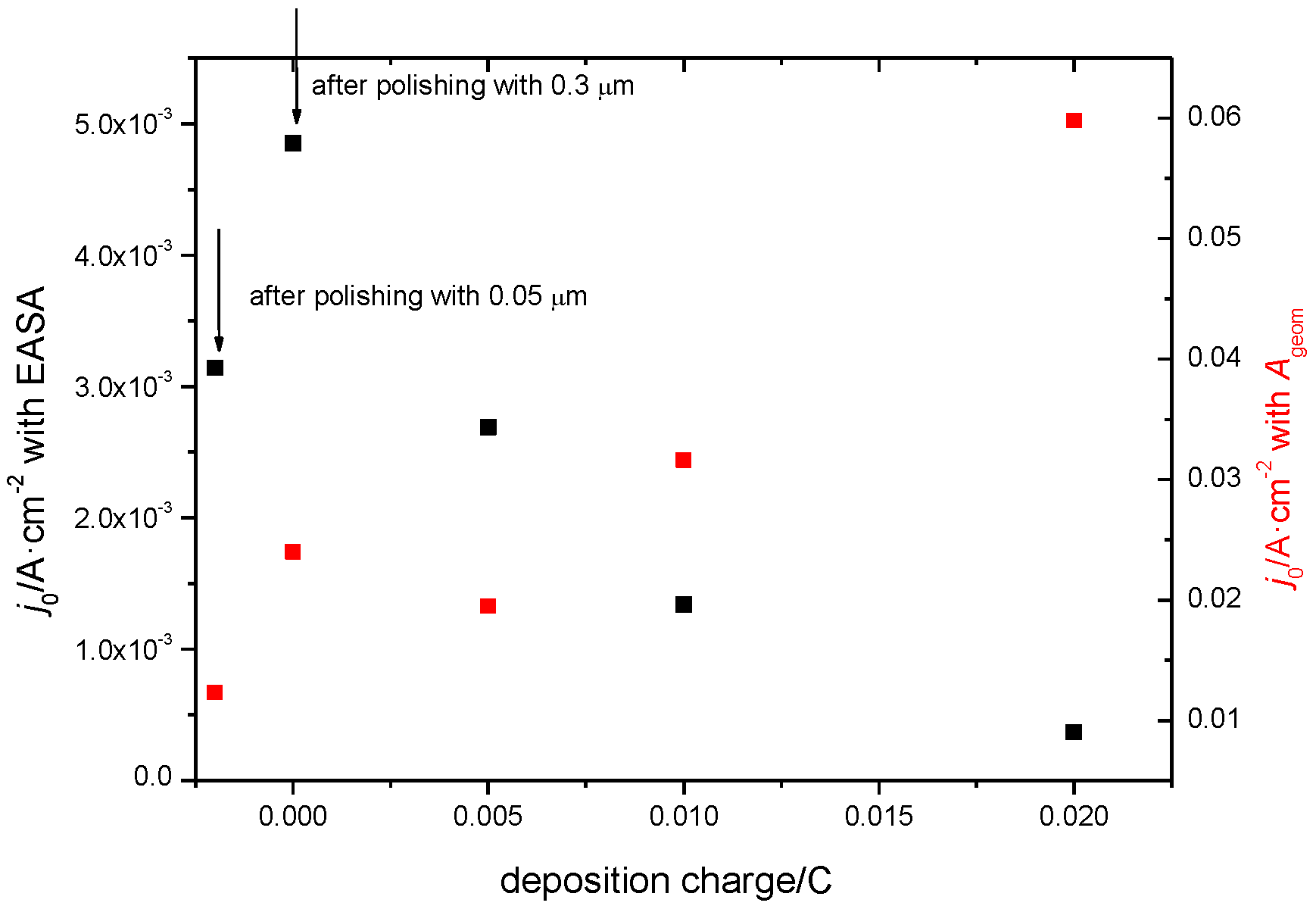
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vetter, K.J. Elektrochemische Kinetik; Springer: Berlin, Germany, 1961. [Google Scholar]
- Vetter, K.J.; Bruckenstein, S. Electrochemical Kinetics; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Zhang, L.; Han, L.; Liu, H.; Liu, X.; Luo, J. Potential-Cycling Synthesis of Single Platinum Atoms for Efficient Hydrogen Evolution in Neutral Media. Angew. Chem. Int. Ed. 2017, 56, 13694–13698. [Google Scholar] [CrossRef]
- Bard, A.J.; Inzelt, G.; Scholz, F. (Eds.) Electrochemical Dictionary; Springer: Heidelberg, Germany, 2012; p. 216. [Google Scholar]
- Schweiss, R.; Meiser, C.; Goh, F.W.T. Steady-State Measurements of Vanadium Redox-Flow Batteries to Study Particular Influences of Carbon Felt Properties. ChemElectroChem 2017, 4, 1969–1974. [Google Scholar] [CrossRef]
- Holze, R. Impedanzmessungen an porösen Elektroden. Ph.D. Thesis, University of Bonn, Bonn, Germany, 1983. [Google Scholar]
- Holze, R.; Vielstich, W. Double-layer capacity measurements as a method to characterize porous fuel cell electrodes. Electrochim. Acta 1984, 29, 607–610. [Google Scholar] [CrossRef]
- Holze, R. Kinetics of Fast Redox Systems for Energy Storage. In Springer Handbook of Electrochemical Energy; Breitkopf, C., Swider-Lyons, K., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 591–610. [Google Scholar]
- Newman, J.; Thomas-Alyea, K.E. Electrochemical Systems, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2004. [Google Scholar]
- Ganassin, A.; Maljusch, A.; Colic, V.; Spanier, L.; Brandl, K.; Schuhmann, W.; Bandarenka, A. Benchmarking the Performance of Thin-Film Oxide Electrocatalysts for Gas Evolution Reactions at High Current Densities. ACS Catal. 2016, 6, 3017–3024. [Google Scholar] [CrossRef]
- Holze, R.; Vielstich, W. The Kinetics of Oxygen Reduction at Porous Teflon-Bonded Fuel Cell Electrodes. J. Electrochem. Soc. 1984, 131, 2298–2303. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Singh, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids, 2nd ed.; Academic Press: Oxford, UK, 2014. [Google Scholar]
- Condon, J.B. Surface Area and Porosity Determinations by Physisorption, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Watzele, S.; Hauenstein, P.; Liang, Y.; Garlyyev, B.; Scieszka, D.; Bandarenka, A. Determination of real electro-active surface area of common metal oxide catalysts for the OER. Paper S21. In Proceedings of the 69th ISE-Meeting, Bologna, Italy, 2–7 September 2018. [Google Scholar]
- Trasatti, S.; Petrii, O.A. Real Surface-Area Measurements in Electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Watt-Smith, M.J.; Friedrich, J.M.; Rigby, S.P.; Ralph, T.R.; Walsh, F.C. Determination of the electrochemically active surface area of Pt/C PEM fuel cell electrodes using different adsorbates. J. Phys. D Appl. Phys. 2008, 41, 74004. [Google Scholar] [CrossRef]
- Binninger, T.; Fabbri, E.; Koetz, R.; Schmidt, T.J. Determination of the Electrochemically Active Surface Area of Metal-Oxide Supported Platinum Catalyst. J. Electrochem. Soc. 2014, 161, H121–H128. [Google Scholar] [CrossRef]
- Maksimov, Y.M.; Podlovchenko, B.I. Use of silver adatoms for the determination of the electrochemically active surface area of polycrystalline gold. Mendeleev Commun. 2017, 27, 64–66. [Google Scholar] [CrossRef]
- Watzele, S.; Bandarenka, A.S. Quick Determination of Electroactive Surface Area of Some Oxide Electrode Materials. Electroanalysis 2016, 28, 2394–2399. [Google Scholar] [CrossRef]
- Wiberg, G.K.H.; Mayrhofer, K.J.J.; Arenz, M. Investigation of the Oxygen Reduction Activity on Silver—A Rotating Disc Electrode Study. Fuel Cells 2010, 10, 575–581. [Google Scholar] [CrossRef]
- Fang, L.L.; Tao, Q.; Li, M.F.; Liao, L.W.; Chen, D.; Chen, Y.X. Determination of the Real Surface Area of Palladium Electrode. Chin. J. Chem. Phys. 2010, 23, 543–548. [Google Scholar] [CrossRef]
- Ge, Y.; Xie, X.; Roscher, J.; Holze, R.; Qu, Q. How to measure and report the capacity of electrochemical double layers, supercapacitors, and their electrode materials. J. Solid State Electr. 2020, 24, 3215–3230. [Google Scholar] [CrossRef]
- Tilak, B.V.; Rader, C.G.; Rangarajan, S.K. Techniques for Characterizing Porous Electrodes. I. Determination of the Double Layer Capacity. J. Electrochem. Soc. 1977, 124, 1879–1886. [Google Scholar] [CrossRef]
- Gagnon, E.G. The triangular voltage sweep method for determining double-layer capacity of porous-electrodes 3. Porous nickel in potassium hydroxide. J. Electrochem. Soc. 1974, 121, 512–515. [Google Scholar] [CrossRef]
- Gagnon, E.G. Determination of double-layer capacity of porous nickel in KOH in presence of Faradaic current. J. Appl. Electrochem. 1976, 6, 95–98. [Google Scholar] [CrossRef]
- Gagnon, E.G. The Triangular Voltage Sweep Methode for Determining Doubly-Layer Capacity of Porous Electrodes. J. Electrochem. Soc. 1973, 120, 1052–1056. [Google Scholar] [CrossRef]
- Wu, Y.; Holze, R. Electrocatalysis at Electrodes for Vanadium Redox Flow Batteries. Batteries 2018, 4, 47. [Google Scholar] [CrossRef]
- Ahn, J.; Holze, R. Bifunctional electrodes for an integrated water-electrolysis and hydrogen-oxygen fuel cell with a solid polymer electrolyte. J. Appl. Electrochem. 1992, 22, 1167–1174. [Google Scholar] [CrossRef]
- Friedl, J.; Stimming, U. Determining Electron Transfer Kinetics at Porous Electrodes. Electrochim. Acta 2017, 227, 235–245. [Google Scholar] [CrossRef]
- Friedl, J.; Bauer, C.M.; Rinaldi, A.; Stimming, U. Electron transfer kinetics of the VO2+/VO2+-Reaction on multi-walled carbon nanotubes. Carbon 2013, 63, 228–239. [Google Scholar] [CrossRef]
- Sun, S.; Li, H.; Xu, Z.J. Impact of Surface Area in Evaluation of Catalyst Activity. Joule 2018, 2, 1024–1027. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, H.X.; Schwingenschlögl, U.; Alshareef, H.N. Plasma-Assisted Synthesis of NiCoP for Efficient Overall Water Splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Li, S.; Du, Y.; Han, X.; Xu, P. How to Reliably Report the Overpotential of an Electrocatalyst. ACS Energy Lett. 2020, 5, 1083–1087. [Google Scholar] [CrossRef]
- Holze, R. Underpotential deposit electrocatalysis of fast redox reactions for electrochemical energy storage systems. J. Solid State Electr. 1998, 2, 73–77. [Google Scholar] [CrossRef]
- Herrmann, J. Entwicklung und Anwendung einer elektrochemischen Methode zur Untersuchung schneller zwischengelagerter Reaktionen in turbulenter Rohrströmung. Ph.D. Thesis, University of Bonn, Bonn, Germany, 1983. [Google Scholar]
- Herrmann, J.; Schmidt, H.; Vielstich, W. Electrochemical investigations of a fast chemical step between two charge transfer reactions. Z. Phys. Chem. NF 1984, 139, 83–96. [Google Scholar] [CrossRef]
- Iwasita, T.; Schmickler, W.; Herrmann, J.; Vogel, U. The Kinetic Parameters of the Fe(CN)63-/4- Redox System: New Results with the Ring Electrode in Turbulent Pipe Flow. J. Electrochem. Soc. 1983, 130, 2026–2032. [Google Scholar] [CrossRef]
- Bohnenkamp, K.; Engell, H.J. Messungen der Impedanz der Phasengrenze Germanium-Elektrolyt. Z. Elektrochem. 1957, 61, 1184–1196. [Google Scholar]
- Bauer, H.H.; Spritzer, M.S.; Elving, P.J. Double-Layer capacity at a pyrolytic graphite disk electrode. J. Electroanal. Chem. 1968, 17, 299–306. [Google Scholar] [CrossRef]
- Randin, J.P.; Yeager, E. Differential Capacitance Study of Stress-Annealed Pyrolytic Graphite Electrodes. J. Electrochem. Soc. 1971, 118, 711–714. [Google Scholar] [CrossRef]
- Wu, Y.; Holze, R. Electrochemical Energy Conversion and Storage; WILEY-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Rabbow, T.J.; Whitehead, A.H. Deconvolution of electrochemical double layer capacitance between fractions of active and total surface area of graphite felts. Carbon 2017, 111, 782–788. [Google Scholar] [CrossRef]
- Kania, S.; Holze, R. Beschleunigung schneller Redoxreaktionen durch upd-modifizierte Elektroden? In GDCh-Monograhie; Stimming, U., Lehnert, W., Eds.; GDCh: Frankfurt, Germany, 1996; Volume 9, pp. 439–445. [Google Scholar]
- Kania, S.; Holze, R. On the adsorption and redox catalysis of the oxalate anion and oxalato complexes on gold and metal-modified gold electrodes. Electrochim. Acta 2003, 48, 945–950. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and its Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
- Yuan, X.Z.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer: London, UK, 2010. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy, 2nd ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Holze, R. Electrode impedance measurements: A versatile tool for electrochemists. Bull. Electrochem. 1994, 10, 56–67. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy; WILEY-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Will, F.G.; Hess, H.J. Morphology and Capacity of a Cadmium Electrode. J. Electrochem. Soc. 1973, 120, 1. [Google Scholar] [CrossRef]
- Will, F.G. A self-contained miniature hydrogen reference electrode. J. Electrochem. Soc. 1986, 133, 454–455. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Holze, R.; Shukla, A.K. The Cadmium Electrode Revisited: Kinetic Data. J. Electrochem. Soc. 2017, 164, A3858–A3861. [Google Scholar] [CrossRef]
- Liu, Y.; Wiek, A.; Dzhagan, V.; Holze, R. Improved Electrochemical Behavior of Amorphous Carbon-Coated Copper/CNT Composites as Negative Electrode Material and Their Energy Storage Mechanism. J. Electrochem. Soc. 2016, 163, A1247–A1253. [Google Scholar] [CrossRef]
- Jovic, V.D. Available online: https://www.gamry.com/application-notes/EIS/correct-value-of-cdl/ (accessed on 27 July 2022).
- Fu, L.; Qu, Q.; Holze, R.; Wu, Y. A comment on the need to distinguish between cell and electrode impedances. J. Solid State Electr. 2019, 23, 717–724. [Google Scholar] [CrossRef]
- Cooper, I.L.; Harrison, J.A.; Sandbach, D.R. Measurement and interpretation of double-layer capacity at solid metal-electrolyte interface. Electrochim. Acta 1978, 23, 527–532. [Google Scholar] [CrossRef]
- Cooper, I.L.; Harrison, J.A. A note on the caclculation and experimental-measurement of the double-layer capacity. Electrochim. Acta 1984, 29, 1165–1168. [Google Scholar] [CrossRef]
- Leikis, D.; Panin, V.; Rybalka, K. On the measurement of the electric double layer capacity at a polycrystalline cadmium electrode. J. Electroanal. Chem. 1972, 40, 9–12. [Google Scholar] [CrossRef]
- Kerner, Z.; Pajkossy, T.; Kibler, L.A.; Kolb, D.M. The double layer capacity of Pt(100) in aqueous perchlorate solutions. Electrochem. Commun. 2002, 4, 787–789. [Google Scholar] [CrossRef]
- Leiva, E.; Schmickler, W. New theories for the electric double layer at a metal/electrolyte solution interface. Proc. Indian Acad. Sci. Chem. Sci. 1986, 97, 267–296. [Google Scholar] [CrossRef]
- Parsons, R. Electrical Double Layer: Recent Experimental and Theoretical Developments. Chem. Rev. 1990, 90, 813–826. [Google Scholar] [CrossRef]
- Conway, B.E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Bai, L.; Gao, L.; Conway, B.E. Problem of Insitu Real-Area Determination in Evaluation of Performance of Rough or Porous, Gas-Evolving Electrocatalysts. 2. Unfolding of the Electrochemically Accessible Surface of Rough or Porous-Electrodes—A Case-Study with an Electrodeposited Porous Pt Electrode. J. Chem. Soc. Faraday Trans. 1993, 89, 243–249. [Google Scholar]
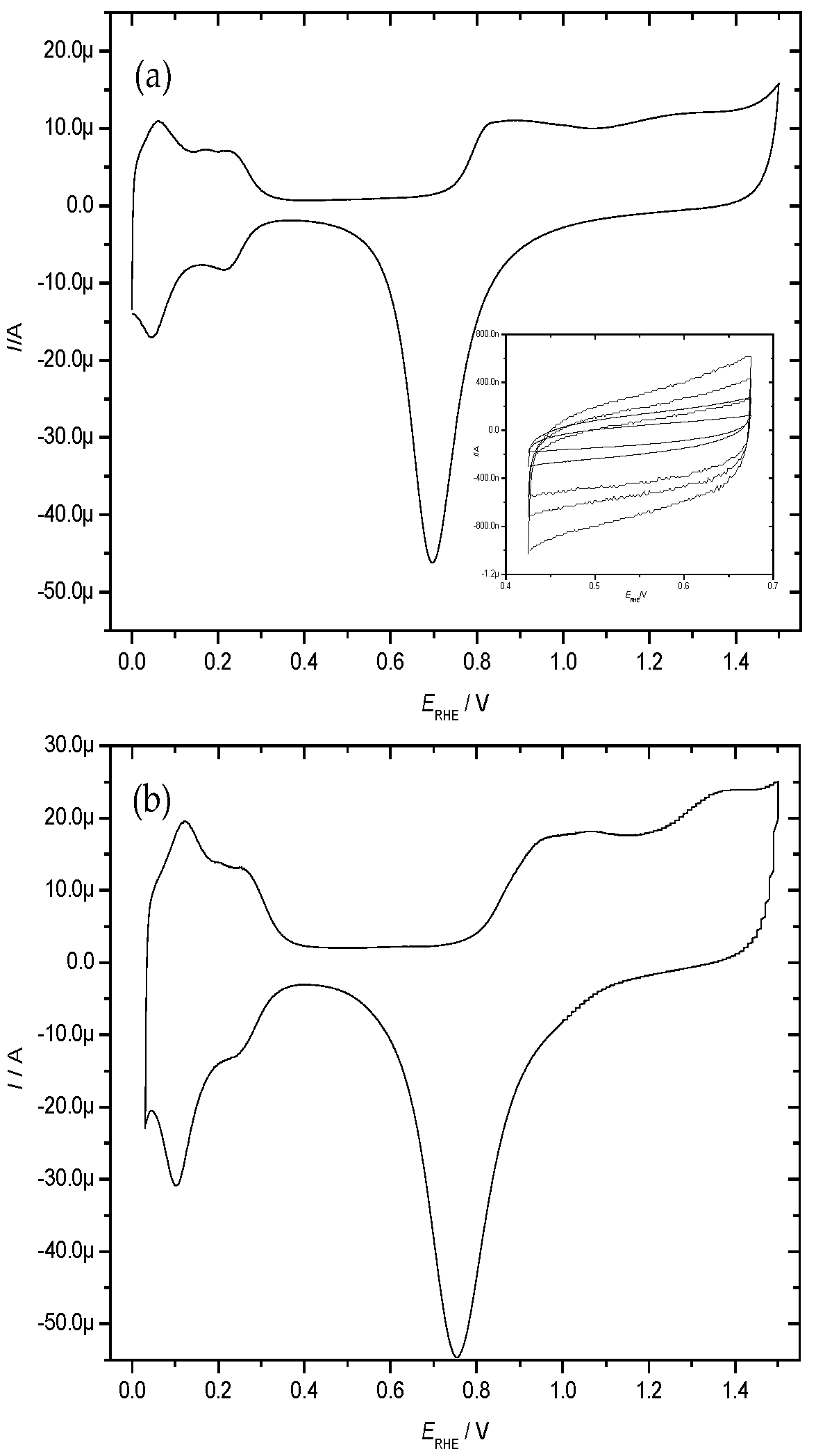
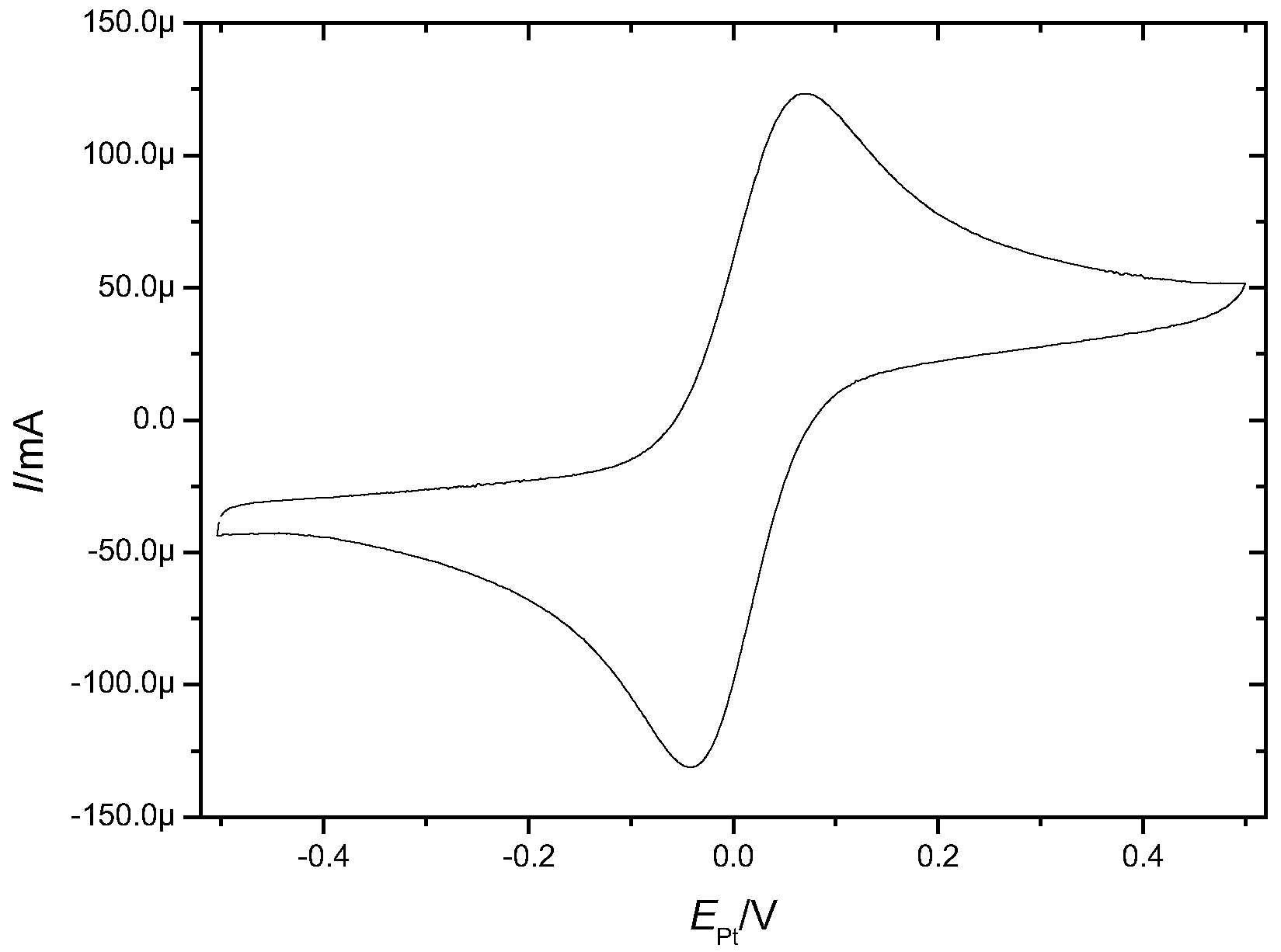
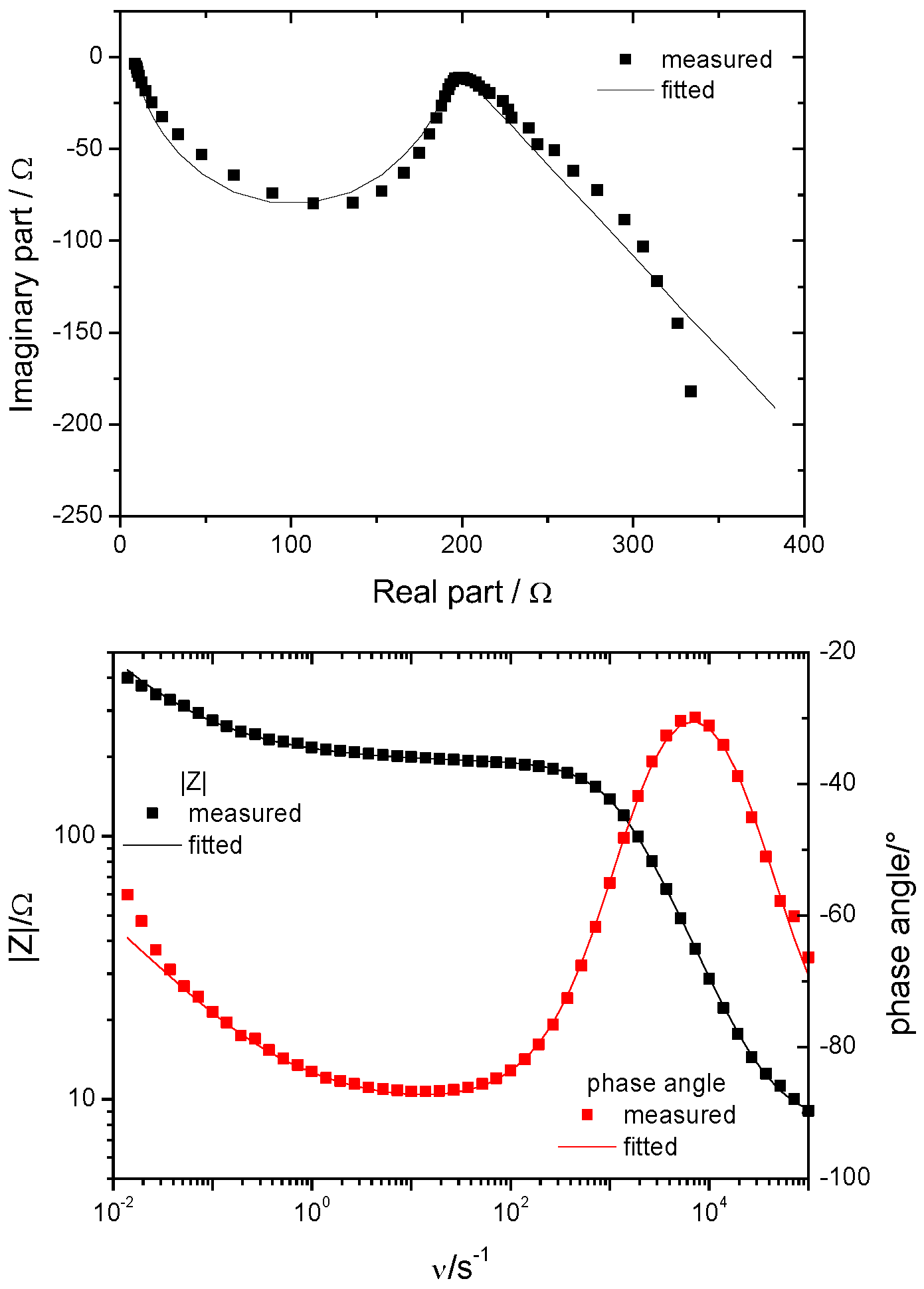
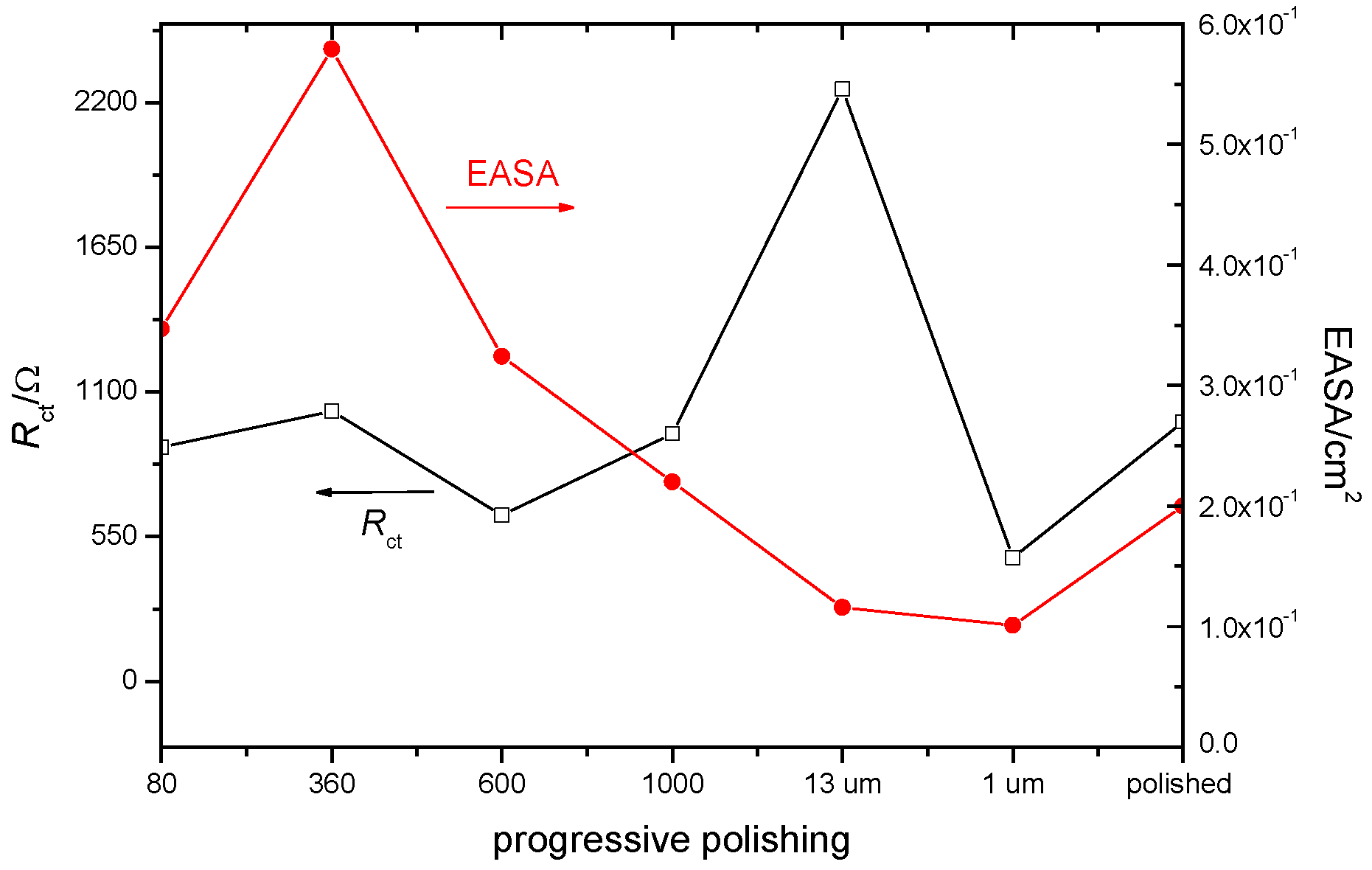

| Treatment | EASACV/cm2 | EASAimp/cm2 | RfCV |
|---|---|---|---|
| Polishing 1 μm | 1.75·10−1 | 3.2·10−1 | 5.57 |
| 80 grit | 3.47·10−1 | 6.34·10−1 | 11.06 |
| 360 grit | 5.79·10−1 | 9.79·10−1 | 18.44 |
| 600 grit | 3.24·10−1 | 1.2 | 10.33 |
| 1000 grit | 2.20·10−1 | 4.12·10−1 | 7.00 |
| Polishing 13 μm | 1.16·10−1 | 0.98·10−1 | 3.69 |
| Polishing 1 μm | 1.01·10−1 | 1.12·10−1 | 3.21 |
| Treatment | EASA/cm2 | Rf |
|---|---|---|
| Polishing 0.3 μm | 1.56·10−1 | 4.96 |
| Deposition 5 mC | 2.28·10−1 | 7.26 |
| + Deposition 5 mC | 7.41·10−1 | 23.58 |
| + Deposition 10 mC | 5.12 | 162.88 |
| Separate experiment | ||
| Polishing 0.05 μm | 1.24·10−1 | 3.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Holze, R. Electrode Kinetic Data: Geometric vs. Real Surface Area. Batteries 2022, 8, 146. https://doi.org/10.3390/batteries8100146
Xie X, Holze R. Electrode Kinetic Data: Geometric vs. Real Surface Area. Batteries. 2022; 8(10):146. https://doi.org/10.3390/batteries8100146
Chicago/Turabian StyleXie, Xuan, and Rudolf Holze. 2022. "Electrode Kinetic Data: Geometric vs. Real Surface Area" Batteries 8, no. 10: 146. https://doi.org/10.3390/batteries8100146
APA StyleXie, X., & Holze, R. (2022). Electrode Kinetic Data: Geometric vs. Real Surface Area. Batteries, 8(10), 146. https://doi.org/10.3390/batteries8100146









