Comparative Study of Onion-like Carbons Prepared from Different Synthesis Routes towards Li-Ion Capacitor Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MOFs
2.3. Synthesis of OLCs
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of Precursors and Produced OLCs
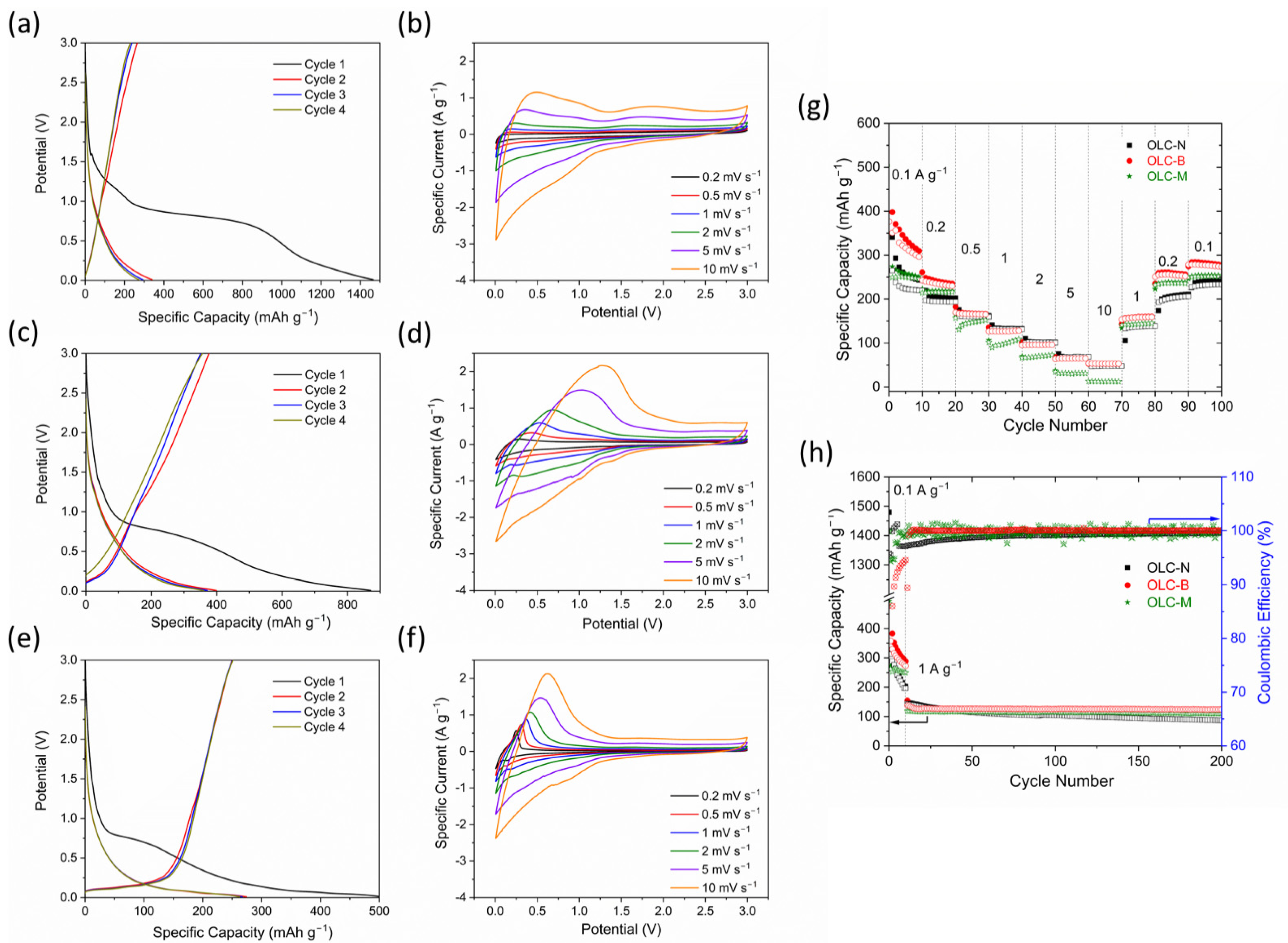
3.2. Electrochemical Performance in LIB Half Cells
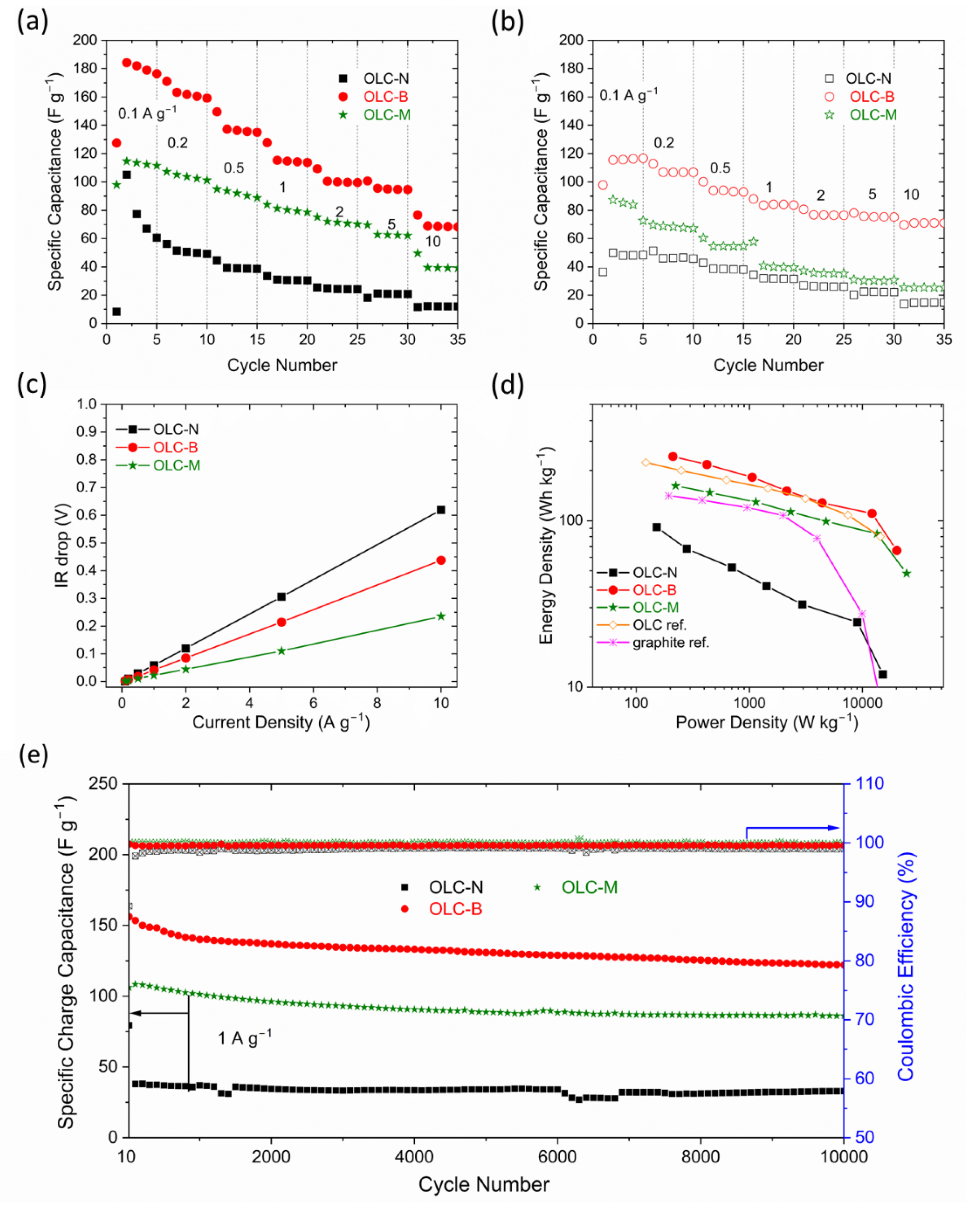
3.3. Electrochemical Performance in Full-Cell LICs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eguchi, T.; Sawada, K.; Tomioka, M.; Kumagai, S. Energy density maximization of Li-ion capacitor using highly porous activated carbon cathode and micrometer-sized Si anode. Electrochim. Acta 2021, 394, 139115. [Google Scholar] [CrossRef]
- Sehrawat, P.; Julien, C.; Islam, S.S. Carbon nanotubes in Li-ion batteries: A review. Mater. Sci. Eng. B 2016, 213, 12–40. [Google Scholar] [CrossRef]
- Kawasaki, S.; Hara, T.; Iwai, Y.; Suzuki, Y. Metallic and semiconducting single-walled carbon nanotubes as the anode material of Li ion secondary battery. Mater. Lett. 2008, 62, 2917–2920. [Google Scholar] [CrossRef]
- Di Lecce, D.; Andreotti, P.; Boni, M.; Gasparro, G.; Rizzati, G.; Hwang, J.-Y.; Sun, Y.-K.; Hassoun, J. Multiwalled carbon nanotubes anode in lithium-ion battery with LiCoO2, Li[Ni1/3Co1/3Mn1/3]O2, and LiFe1/4Mn1/2Co1/4PO4 cathodes. ACS Sustain. Chem. Eng. 2018, 6, 3225–3232. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Bai, P.; Guo, J.; Wang, M.; Kushima, A.; Su, L.; Li, J.; Brushett, F.R.; Bazant, M.Z. Interactions between lithium growths and nanoporous ceramic separators. Joule 2018, 2, 2434–2449. [Google Scholar] [CrossRef]
- Lamb, J.J.; Burheim, O.S. Lithium-ion capacitors: A review of design and active materials. Energies 2021, 14, 979. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Xu, Y.; Li, C.; Wang, K.; Sun, X.; Su, F.; Chen, C.-M.; Liu, F.; Wu, Z.-S.; et al. Recent advances on carbon-based materials for high performance lithium-ion capacitors. Batter. Supercaps 2021, 4, 407–428. [Google Scholar] [CrossRef]
- Permana, A.D.C.; Omar, A.; Guillermo Gonzalez-Martinez, I.; Oswald, S.; Giebeler, L.; Nielsch, K.; Mikhailova, D. MOF-derived onion-like carbon with superior surface area and porosity for high performance lithium-ion capacitors. Batter. Supercaps 2022, n/a, e202100353. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef]
- Bu, I.Y.Y. Synthesis of graphitic carbon nano-onions for dye sensitized solar cells. Sol. Energy 2014, 105, 236–242. [Google Scholar] [CrossRef]
- McDonough, J.K.; Frolov, A.I.; Presser, V.; Niu, J.; Miller, C.H.; Ubieto, T.; Fedorov, M.V.; Gogotsi, Y. Influence of the structure of carbon onions on their electrochemical performance in supercapacitor electrodes. Carbon 2012, 50, 3298–3309. [Google Scholar] [CrossRef]
- Wu, G.; Dai, C.; Wang, D.; Li, D.; Li, N. Nitrogen-doped magnetic onion-like carbon as support for Pt particles in a hybrid cathode catalyst for fuel cells. J. Mater. Chem. 2010, 20, 3059–3068. [Google Scholar] [CrossRef]
- Han, D.; Hu, H.; Liu, B.; Song, G.; Yan, H.; Di, J. CuCo2O4 nanoparticles encapsulated by onion-like carbon layers: A promising solution for high-performance lithium ion battery. Ceram. Int. 2016, 42, 12460–12466. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Liu, E.; He, C.; Shi, C.; Du, X.; Hauge, R.H.; Zhao, N. Synthesis of hollow carbon nano-onions and their use for electrochemical hydrogen storage. Carbon 2012, 50, 3513–3521. [Google Scholar] [CrossRef]
- Wanninayake, N.; Qianxiang, A.; Thomas, M.; Kodithuwakku, U.S.; Hoque, A.; Guzman, M.I.; Guiton, B.; Risko, C.; Kim, D.Y. Nitrogen and Sulfur Co-Doped Carbon Nano-Onions for Efficient Electrochemical Conversion of Carbon Dioxide. ECS Meet. Abstr. 2019, MA2019-01, 1591. [Google Scholar] [CrossRef]
- Han, C.; Bo, X.; Zhang, Y.; Li, M.; Guo, L. One-pot synthesis of nitrogen and sulfur co-doped onion-like mesoporous carbon vesicle as an efficient metal-free catalyst for oxygen reduction reaction in alkaline solution. J. Power Sources 2014, 272, 267–276. [Google Scholar] [CrossRef]
- Frasconi, M.; Maffeis, V.; Bartelmess, J.; Echegoyen, L.; Giordani, S. Highly surface functionalized carbon nano-onions for bright light bioimaging. Methods Appl. Fluoresc. 2015, 3, 044005. [Google Scholar] [CrossRef]
- Pérez-Ojeda, M.E.; Castro, E.; Kröckel, C.; Lucherelli, M.A.; Ludacka, U.; Kotakoski, J.; Werbach, K.; Peterlik, H.; Melle-Franco, M.; Chacón-Torres, J.C.; et al. Carbon Nano-onions: Potassium Intercalation and Reductive Covalent Functionalization. J. Am. Chem. Soc. 2021, 143, 18997–19007. [Google Scholar] [CrossRef]
- Gu, W.; Peters, N.; Yushin, G. Functionalized carbon onions, detonation nanodiamond and mesoporous carbon as cathodes in Li-ion electrochemical energy storage devices. Carbon 2013, 53, 292–301. [Google Scholar] [CrossRef]
- Fleischmann, S.; Jäckel, N.; Zeiger, M.; Krüner, B.; Grobelsek, I.; Formanek, P.; Choudhury, S.; Weingarth, D.; Presser, V. Enhanced electrochemical energy storage by nanoscopic decoration of endohedral and exohedral carbon with vanadium oxide via atomic layer deposition. Chem. Mater. 2016, 28, 2802–2813. [Google Scholar] [CrossRef]
- Fleischmann, S.; Tolosa, A.; Zeiger, M.; Krüner, B.; Peter, N.J.; Grobelsek, I.; Quade, A.; Kruth, A.; Presser, V. Vanadia–titania multilayer nanodecoration of carbon onions via atomic layer deposition for high performance electrochemical energy storage. J. Mater. Chem. A 2017, 5, 2792–2801. [Google Scholar] [CrossRef]
- Fleischmann, S.; Zeiger, M.; Jäckel, N.; Krüner, B.; Lemkova, V.; Widmaier, M.; Presser, V. Tuning pseudocapacitive and battery-like lithium intercalation in vanadium dioxide/carbon onion hybrids for asymmetric supercapacitor anodes. J. Mater. Chem. A 2017, 5, 13039–13051. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Li, X.; Wang, Z.; Wang, J.; Wang, Y.; Yan, Z.; Zhang, D. Graphitic carbon balanced between high plateau capacity and high rate capability for lithium ion capacitors. J. Mater. Chem. A 2017, 5, 15302–15309. [Google Scholar] [CrossRef]
- Chen, J.; Yang, B.; Li, H.; Ma, P.; Lang, J.; Yan, X. Candle soot: Onion-like carbon, an advanced anode material for a potassium-ion hybrid capacitor. J. Mater. Chem. A 2019, 7, 9247–9252. [Google Scholar] [CrossRef]
- Klose, M.; Pinkert, K.; Zier, M.; Uhlemann, M.; Wolke, F.; Jaumann, T.; Jehnichen, P.; Wadewitz, D.; Oswald, S.; Eckert, J.; et al. Hollow carbon nano-onions with hierarchical porosity derived from commercial metal organic framework. Carbon 2014, 79, 302–309. [Google Scholar] [CrossRef]
- Moussa, G.; Matei Ghimbeu, C.; Taberna, P.-L.; Simon, P.; Vix-Guterl, C. Relationship between the carbon nano-onions (CNOs) surface chemistry/defects and their capacitance in aqueous and organic electrolytes. Carbon 2016, 105, 628–637. [Google Scholar] [CrossRef]
- Hu, X.; Lou, X.; Li, C.; Ning, Y.; Liao, Y.; Chen, Q.; Mananga, E.S.; Shen, M.; Hu, B. Facile synthesis of the Basolite F300-like nanoscale Fe-BTC framework and its lithium storage properties. RSC Adv. 2016, 6, 114483–114490. [Google Scholar] [CrossRef]
- Han, L.; Qi, H.; Zhang, D.; Ye, G.; Zhou, W.; Hou, C.; Xu, W.; Sun, Y. A facile and green synthesis of MIL-100(Fe) with high-yield and its catalytic performance. New J. Chem. 2017, 41, 13504–13509. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Aslan, M.; Weingarth, D.; Presser, V. Understanding structure and porosity of nanodiamond-derived carbon onions. Carbon 2015, 84, 584–598. [Google Scholar] [CrossRef]
- Chen, J.; Deng, S.Z.; Chen, J.; Yu, Z.X.; Xu, N.S. Graphitization of nanodiamond powder annealed in argon ambient. Appl. Phys. Lett. 1999, 74, 3651–3653. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, M.; de Asua, I.; Ruano, D.; Diaz, K. Direct synthesis, structural features, and enhanced catalytic activity of the Basolite F300-like semiamorphous Fe-BTC framework. Cryst. Growth Des. 2015, 15, 4498–4506. [Google Scholar] [CrossRef]
- Rivera-Torrente, M.; Filez, M.; Hardian, R.; Reynolds, E.; Seoane, B.; Coulet, M.-V.; Oropeza Palacio, F.E.; Hofmann, J.P.; Fischer, R.A.; Goodwin, A.L.; et al. Metal-organic frameworks as catalyst supports: Influence of Lattice disorder on metal nanoparticle formation. Chem. Eur. J. 2018, 24, 7498–7506. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.-Y.; Seo, Y.-K.; Chang, J.-S.; Grenèche, J.-M.; Margiolaki, I.; Férey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(iii) carboxylate with large pores. Chem. Commun. 2007, 27, 2820–2822. [Google Scholar] [CrossRef]
- Tan, F.; Liu, M.; Li, K.; Wang, Y.; Wang, J.; Guo, X.; Zhang, G.; Song, C. Facile synthesis of size-controlled MIL-100(Fe) with excellent adsorption capacity for methylene blue. Chem. Eng. J. 2015, 281, 360–367. [Google Scholar] [CrossRef]
- Msahel, A.; Galiano, F.; Pilloni, M.; Russo, F.; Hafiane, A.; Castro-Muñoz, R.; Kumar, V.B.; Gedanken, A.; Ennas, G.; Porat, Z.e.; et al. Exploring the effect of iron metal-organic framework particles in polylactic acid membranes for the azeotropic separation of organic/organic mixtures by pervaporation. Membranes 2021, 11, 65. [Google Scholar] [CrossRef]
- Delhaes, P. Graphite and Precursors; CRC Press: Boca Raton, FL, USA, 2000; Volume 1. [Google Scholar]
- Mao, C.; Kong, A.; Wang, Y.; Bu, X.; Feng, P. MIL-100 derived nitrogen-embodied carbon shells embedded with iron nanoparticles. Nanoscale 2015, 7, 10817–10822. [Google Scholar] [CrossRef]
- Hassel, O.; Mark, H. Über die Kristallstruktur des Graphits. Z. Phys. 1924, 25, 317–337. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Rummeli, M.H.; Gemming, T.; Lange, H.; Huczko, A. Catalyst-free synthesis of onion-like carbon nanoparticles. New Carbon Mater. 2010, 25, 1–8. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Presser, V. Carbon Nanomaterials; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Cebik, J.; McDonough, J.K.; Peerally, F.; Medrano, R.; Neitzel, I.; Gogotsi, Y.; Osswald, S. Raman spectroscopy study of the nanodiamond-to-carbon onion transformation. Nanotechnology 2013, 24, 205703. [Google Scholar] [CrossRef]
- Rouzaud, J.N.; Oberlin, A.; Beny-Bassez, C. Carbon films: Structure and microtexture (optical and electron microscopy, Raman spectroscopy). Thin Solid Films 1983, 105, 75–96. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Bogdanov, K.; Fedorov, A.; Osipov, V.; Enoki, T.; Takai, K.; Hayashi, T.; Ermakov, V.; Moshkalev, S.; Baranov, A. Annealing-induced structural changes of carbon onions: High-resolution transmission electron microscopy and Raman studies. Carbon 2014, 73, 78–86. [Google Scholar] [CrossRef]
- Gruen, D.M.; Shenderova, O.A.; Vul’, A.Y. Synthesis, Properties and Applications of Ultrananocrystalline Diamond: Proceedings of the NATO ARW on Synthesis, Properties and Applications of Ultrananocrystalline Diamond, St. Petersburg, Russia, from 7 to 10 June 2004; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Klose, M.; Reinhold, R.; Pinkert, K.; Uhlemann, M.; Wolke, F.; Balach, J.; Jaumann, T.; Stoeck, U.; Eckert, J.; Giebeler, L. Hierarchically nanostructured hollow carbon nanospheres for ultra-fast and long-life energy storage. Carbon 2016, 106, 306–313. [Google Scholar] [CrossRef]
- Codorniu Pujals, D.; Arias de Fuentes, O.; Desdín García, L.F.; Cazzanelli, E.; Caputi, L.S. Raman spectroscopy of polyhedral carbon nano-onions. Appl. Phys. A 2015, 120, 1339–1345. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Jian, Z.; Jiang, H.; Razink, J.J.; Stickle, W.F.; Neuefeind, J.C.; Ji, X. Defective hard carbon anode for Na-ion batteries. Chem. Mater. 2018, 30, 4536–4542. [Google Scholar] [CrossRef]
- Zólyomi, V.; Koltai, J.; Kürti, J. Resonance Raman spectroscopy of graphite and graphene. Phys. Status Solidi (B) 2011, 248, 2435–2444. [Google Scholar] [CrossRef]
- Ma, Z.; Zhuang, Y.; Deng, Y.; Song, X.; Zuo, X.; Xiao, X.; Nan, J. From spent graphite to amorphous sp2+sp3 carbon-coated sp2 graphite for high-performance lithium ion batteries. J. Power Sources 2018, 376, 91–99. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Zeng, Y.; Fan, C.; Do, D.D.; Nicholson, D. Evaporation from an Ink-bottle pore: Mechanisms of adsorption and desorption. Ind. Eng. Chem. Res. 2014, 53, 15467–15474. [Google Scholar] [CrossRef]
- Petit, T.; Arnault, J.-C.; Girard, H.A.; Sennour, M.; Bergonzo, P. Early stages of surface graphitization on nanodiamond probed by x-ray photoelectron spectroscopy. Phys. Rev. B 2011, 84, 233407. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, X.; He, D.; Zhang, J. Preparation and study of carbon nano-onion for lithium storage. Mater. Chem. Phys. 2013, 139, 333–337. [Google Scholar] [CrossRef]
- Omichi, K.; Ramos-Sanchez, G.; Rao, R.; Pierce, N.; Chen, G.; Balbuena, P.B.; Harutyunyan, A.R. Origin of excess irreversible capacity in lithium-ion batteries based on carbon nanostructures. J. Electrochem. Soc. 2015, 162, A2106–A2115. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, T.; Zhang, D.; Zhang, W.; Zhang, H.; Liu, R.; Yao, M.; Liu, B. Remarkable cycle-activated capacity increasing in onion-like carbon nanospheres as lithium battery anode material. Nanotechnology 2016, 28, 035704. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Dong, J.; Ye, T.; Hao, J.; Yang, Y.; Jiang, X.; Kang, X.; Bando, Y.; Wang, X. Curving effects of concave dodecahedral nanocarbons enable enhanced Li-ion storage. J. Mater. Chem. A 2018, 6, 14894–14902. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Xiong, J.; Ou, X.; Su, W.; Zhong, G.; Yang, C. Activated amorphous carbon with high-porosity derived from Camellia pollen grains as anode materials for lithium/sodium ion batteries. Front. Chem. 2018, 6, 366. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhao, M.-Q.; Chen, C.; Pentecost, A.; Maleski, K.; Mathis, T.; Zhang, X.-Q.; Zhang, Q.; Jiang, J.; Gogotsi, Y. Nanodiamonds suppress the growth of lithium dendrites. Nat. Commun. 2017, 8, 336. [Google Scholar] [CrossRef]
- Portet, C.; Yushin, G.; Gogotsi, Y. Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotubes in electrical double layer capacitors. Carbon 2007, 45, 2511–2518. [Google Scholar] [CrossRef]
- Pereira, L.F.C.; Savić, I.; Donadio, D. Thermal conductivity of one-, two- and three-dimensional sp2 carbon. New J. Phys. 2013, 15, 105019. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, G.; Xu, L.; Lian, J.; Bao, J.; Zhao, Y.; Qiu, J.; Li, H. Defect-rich N-doped porous carbon derived from soybean for high rate lithium-ion batteries. Appl. Surf. Sci. 2018, 451, 298–305. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, L.; Abbas, M.A.; Bang, J.H. The influence of surface area, porous structure, and surface state on the supercapacitor performance of titanium oxynitride: Implications for a nanostructuring strategy. Phys. Chem. Chem. Phys. 2017, 19, 21140–21151. [Google Scholar] [CrossRef]
- Sun, F.; Gao, J.; Zhu, Y.; Pi, X.; Wang, L.; Liu, X.; Qin, Y. A high performance lithium ion capacitor achieved by the integration of a Sn-C anode and a biomass-derived microporous activated carbon cathode. Sci. Rep. 2017, 7, 40990. [Google Scholar] [CrossRef]
- Yang, C.; Lan, J.-L.; Liu, W.-X.; Liu, Y.; Yu, Y.-H.; Yang, X.-P. High-performance Li-ion capacitor based on an activated carbon cathode and well-dispersed ultrafine TiO2 nanoparticles embedded in mesoporous carbon nanofibers anode. ACS Appl. Mater. Interfaces 2017, 9, 18710–18719. [Google Scholar] [CrossRef]
- Zheng, J.P. Energy density theory of lithium-ion capacitors. J. Electrochem. Soc. 2021, 168, 080503. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gomez-Romero, P. All nanocarbon Li-Ion capacitor with high energy and high power density. Mater. Today Energy 2018, 8, 109–117. [Google Scholar] [CrossRef]
- Ahn, W.; Lee, D.U.; Li, G.; Feng, K.; Wang, X.; Yu, A.; Lui, G.; Chen, Z. Highly oriented graphene sponge electrode for ultra high energy density lithium ion hybrid capacitors. ACS Appl. Mater. Interfaces 2016, 8, 25297–25305. [Google Scholar] [CrossRef]
- Shan, X.-Y.; Wang, Y.; Wang, D.-W.; Li, F.; Cheng, H.-M. Armoring graphene cathodes for high-rate and long-life lithium ion supercapacitors. Adv. Energy Mater. 2016, 6, 1502064. [Google Scholar] [CrossRef]
- Du, H.; Yang, H.; Huang, C.; He, J.; Liu, H.; Li, Y. Graphdiyne applied for lithium-ion capacitors displaying high power and energy densities. Nano Energy 2016, 22, 615–622. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Huang, X.; Tan, T.L.; Xue, J.M. Low Li+ insertion barrier carbon for high energy efficient lithium-ion capacitor. ACS Appl. Mater. Interfaces 2018, 10, 1690–1700. [Google Scholar] [CrossRef]
- Moreno-Fernández, G.; Granados-Moreno, M.; Gómez-Urbano, J.L.; Carriazo, D. Phosphorus-functionalized graphene for lithium-ion capacitors with improved power and cyclability. Batter. Supercaps 2021, 4, 469–478. [Google Scholar] [CrossRef]
- Chen, M.; Le, T.; Zhou, Y.; Kang, F.; Yang, Y. Enhanced electrode matching assisted by in situ etching and co-doping toward high-rate dual-carbon lithium-ion capacitors. ACS Sustain. Chem. Eng. 2021, 9, 10054–10061. [Google Scholar] [CrossRef]
- An, Y.; Li, C.; Sun, X.; Wang, K.; Su, F.; Liu, F.; Zhang, X.; Ma, Y. Deoxygenated porous carbon with highly stable electrochemical reaction interface for practical high-performance lithium-ion capacitors. J. Phys. D Appl. Phys. 2021, 55, 045501. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, X.; Mu, J.; Liu, P.; Yuan, W.; Li, S.; Wang, X.; Fang, H.; Liu, H.; Xing, T.; et al. Robust and fast lithium storage enabled by polypyrrole-coated nitrogen and phosphorus co-doped hollow carbon nanospheres for lithium-ion capacitors. Front. Chem. 2021, 9, 760473. [Google Scholar] [CrossRef]
- Han, L.; Kang, S.; Zhu, X.; Li, J.; Wang, Q.; Jia, X. High-Performance Lithium-Ion Capacitors Produced by Atom-Thick Carbon Cathode and Nitrogen-Doped Porous Carbon Anode. Energy Fuels 2021, 35, 16894–16902. [Google Scholar] [CrossRef]
- Li, S.; Liu, P.; Zheng, X.; Wu, M. High-performance dual carbon lithium-ion capacitors based on nitrogen-doped 2D carbon nanosheets as both anode and cathode. Electrochim. Acta 2022, 428, 140921. [Google Scholar] [CrossRef]
- Xiao, Y.; He, D.; Peng, W.; Chen, S.; Liu, J.; Chen, H.; Xin, S.; Bai, Y. Oxidized-Polydopamine-Coated Graphene Anodes and N,P Codoped Porous Foam Structure Activated Carbon Cathodes for High-Energy-Density Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2021, 13, 10336–10348. [Google Scholar] [CrossRef] [PubMed]


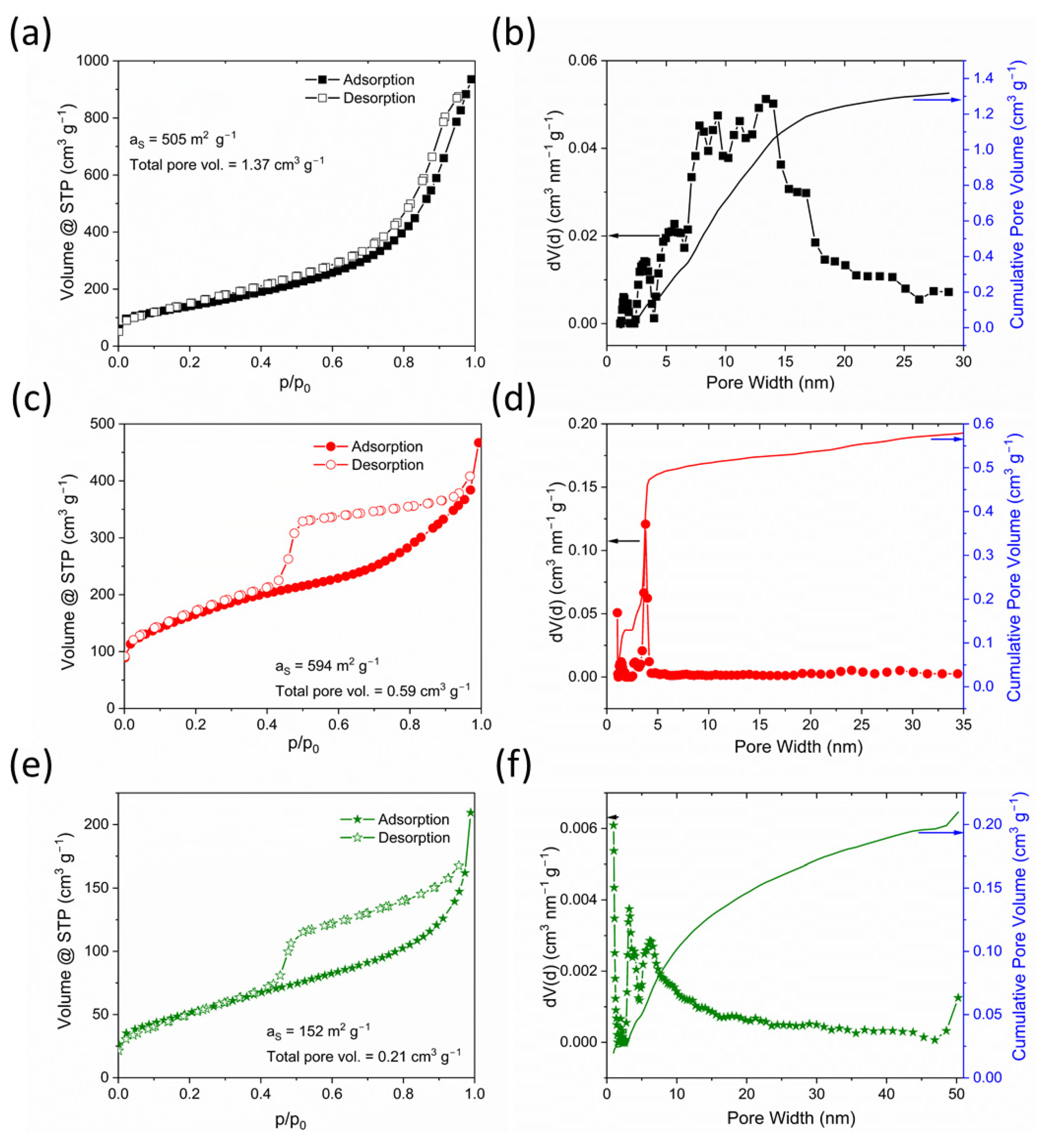

| Sample | Raman ID/IG | Specific Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | Pore VolumeQSDFT (cm3 g−1) | ||
|---|---|---|---|---|---|---|
| Micro-pores | Meso-pores | Macro-pores | ||||
| OLC-N | 0.88 | 505 | 1.37 | 0.04 | 1.33 | - |
| OLC-B | 1.23 | 594 | 0.59 | 0.13 | 0.46 | - |
| OLC-M | 0.78 | 180 | 0.23 | 0.02 | 0.19 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permana, A.D.C.; Ding, L.; Gonzalez-Martinez, I.G.; Hantusch, M.; Nielsch, K.; Mikhailova, D.; Omar, A. Comparative Study of Onion-like Carbons Prepared from Different Synthesis Routes towards Li-Ion Capacitor Application. Batteries 2022, 8, 160. https://doi.org/10.3390/batteries8100160
Permana ADC, Ding L, Gonzalez-Martinez IG, Hantusch M, Nielsch K, Mikhailova D, Omar A. Comparative Study of Onion-like Carbons Prepared from Different Synthesis Routes towards Li-Ion Capacitor Application. Batteries. 2022; 8(10):160. https://doi.org/10.3390/batteries8100160
Chicago/Turabian StylePermana, Antonius Dimas Chandra, Ling Ding, Ignacio Guillermo Gonzalez-Martinez, Martin Hantusch, Kornelius Nielsch, Daria Mikhailova, and Ahmad Omar. 2022. "Comparative Study of Onion-like Carbons Prepared from Different Synthesis Routes towards Li-Ion Capacitor Application" Batteries 8, no. 10: 160. https://doi.org/10.3390/batteries8100160
APA StylePermana, A. D. C., Ding, L., Gonzalez-Martinez, I. G., Hantusch, M., Nielsch, K., Mikhailova, D., & Omar, A. (2022). Comparative Study of Onion-like Carbons Prepared from Different Synthesis Routes towards Li-Ion Capacitor Application. Batteries, 8(10), 160. https://doi.org/10.3390/batteries8100160






