Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods
Abstract
:1. Introduction
2. Experimental
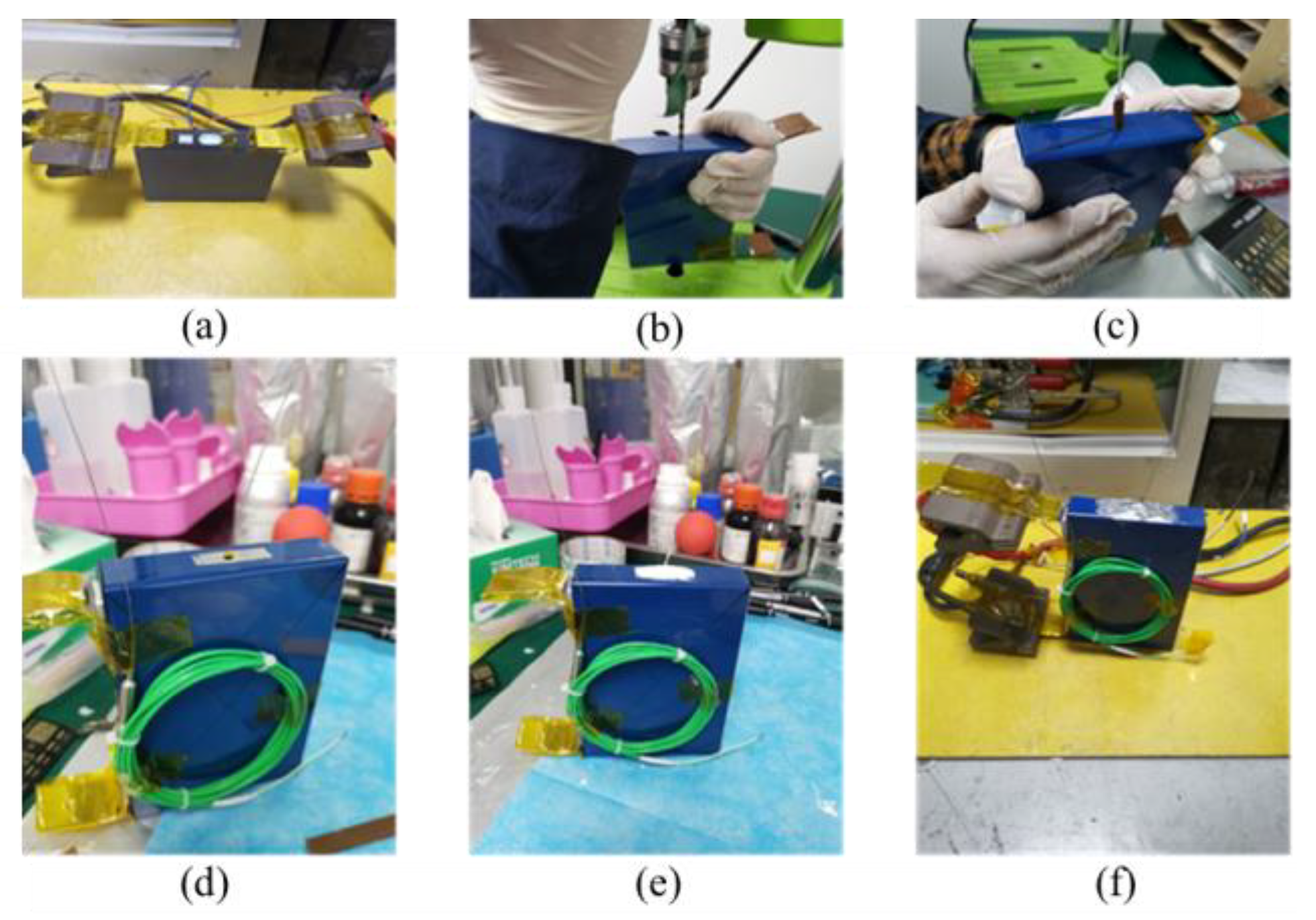
2.1. Preparation for Internal Temperature Measurement
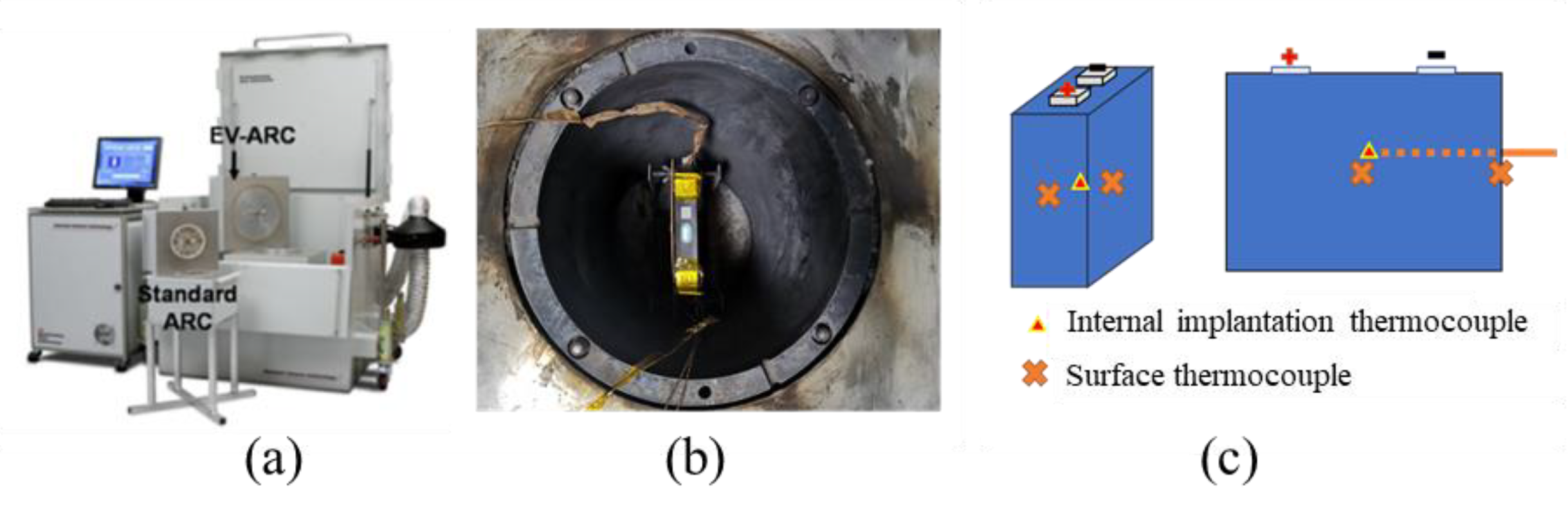
2.2. EV-ARC Test
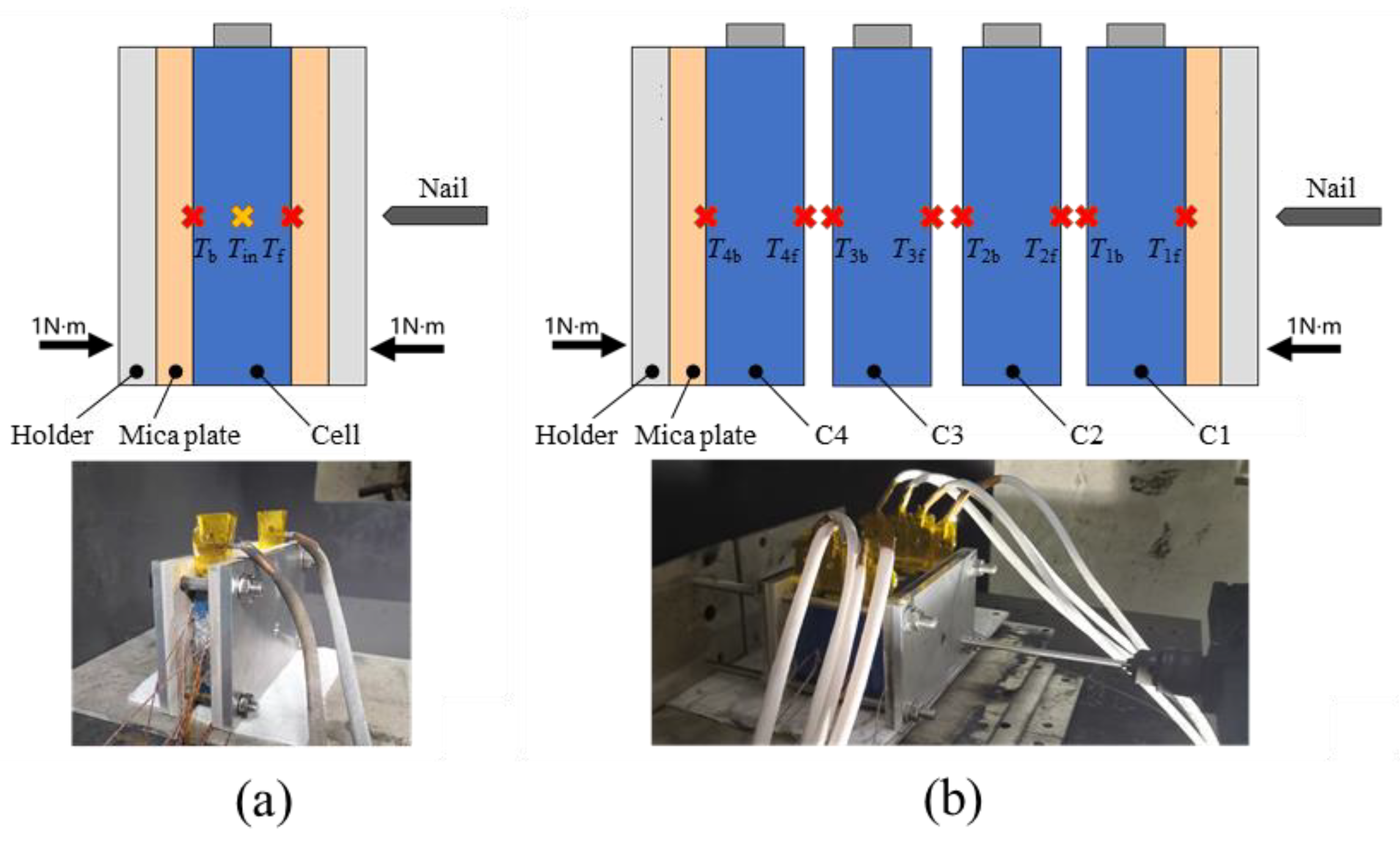
2.3. Nail Penetration Abuse Test
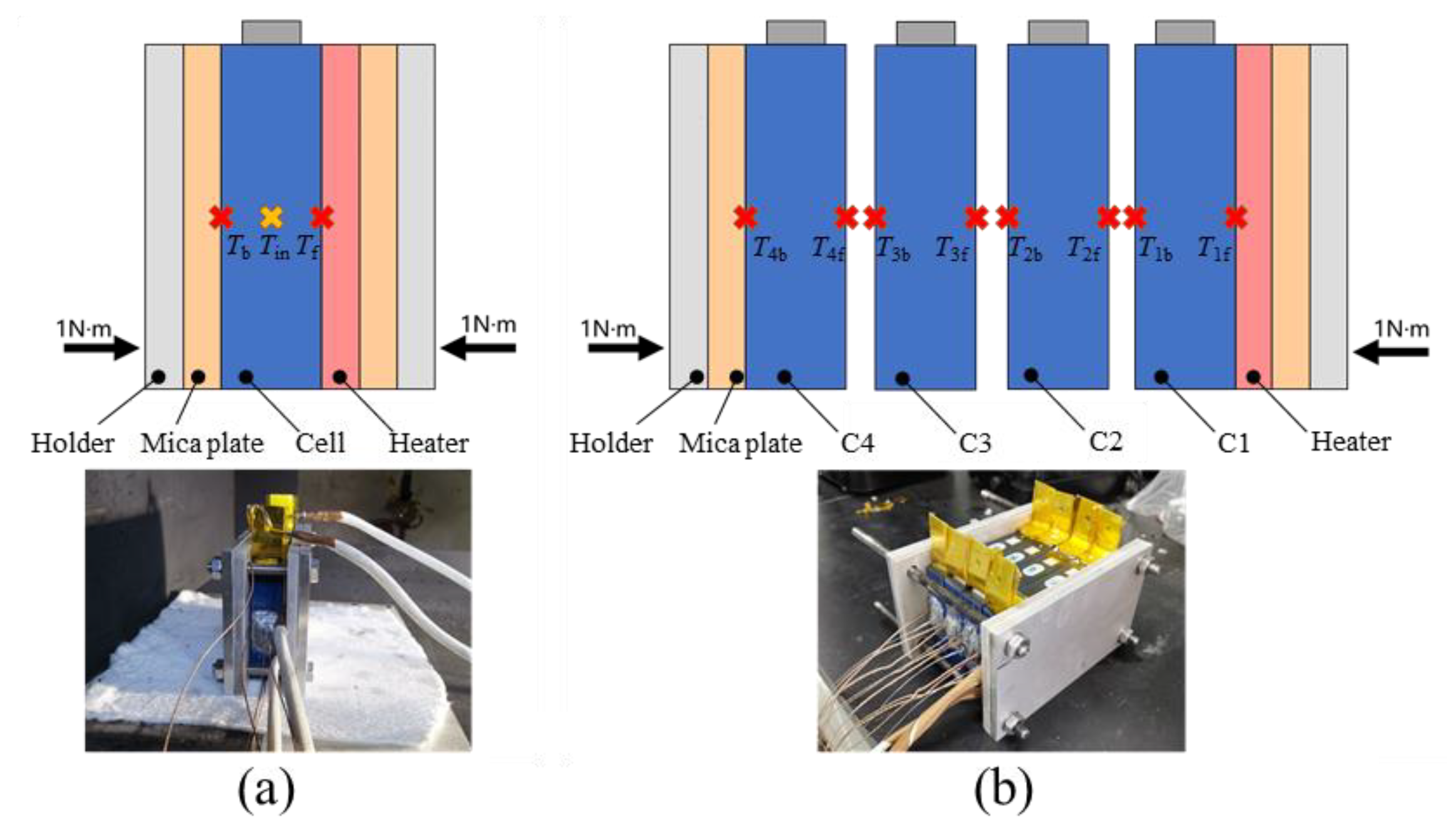
2.4. Side Heating Abuse Test
2.5. Overcharge Abuse Test
3. Results and Discussion
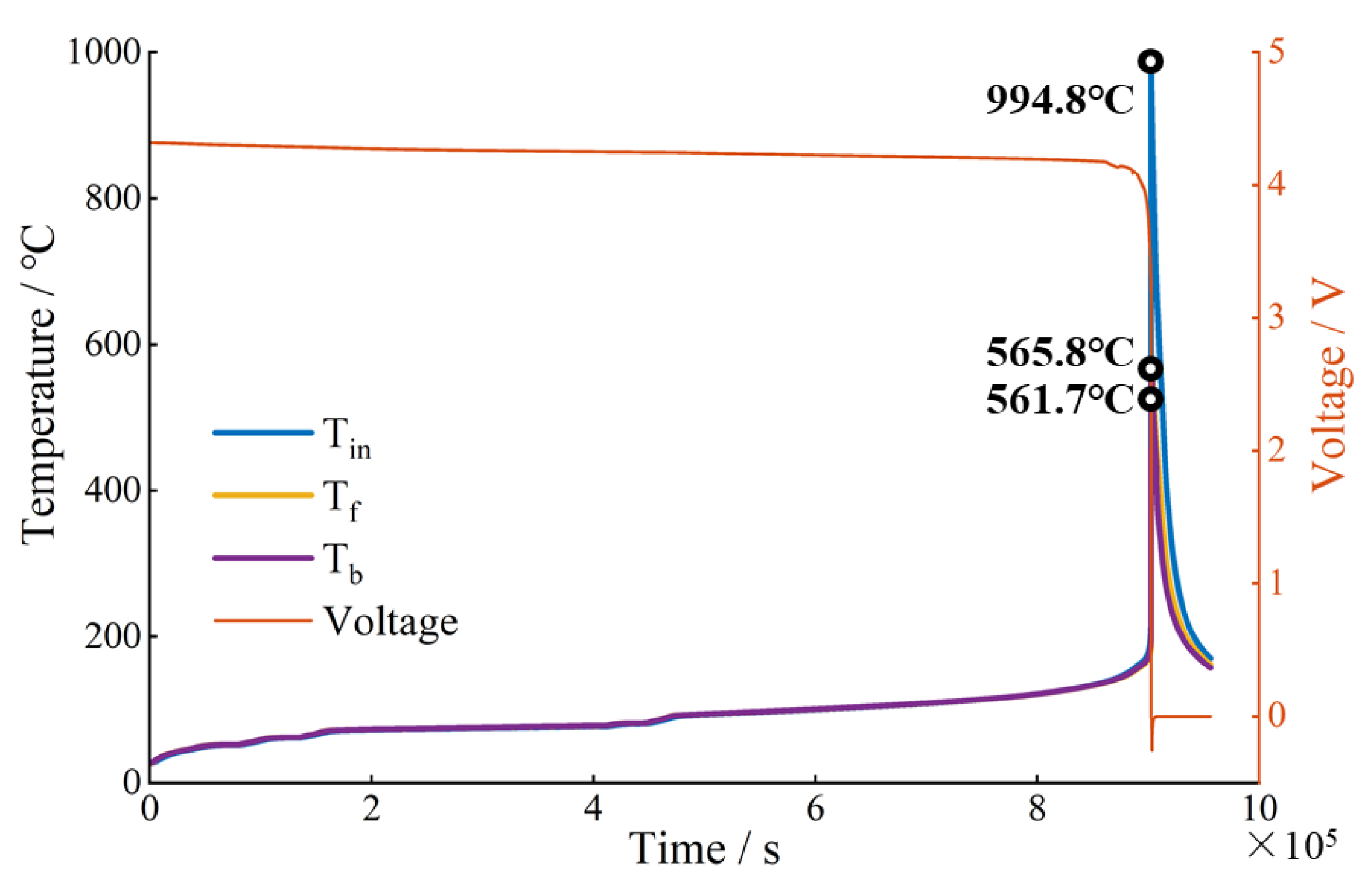
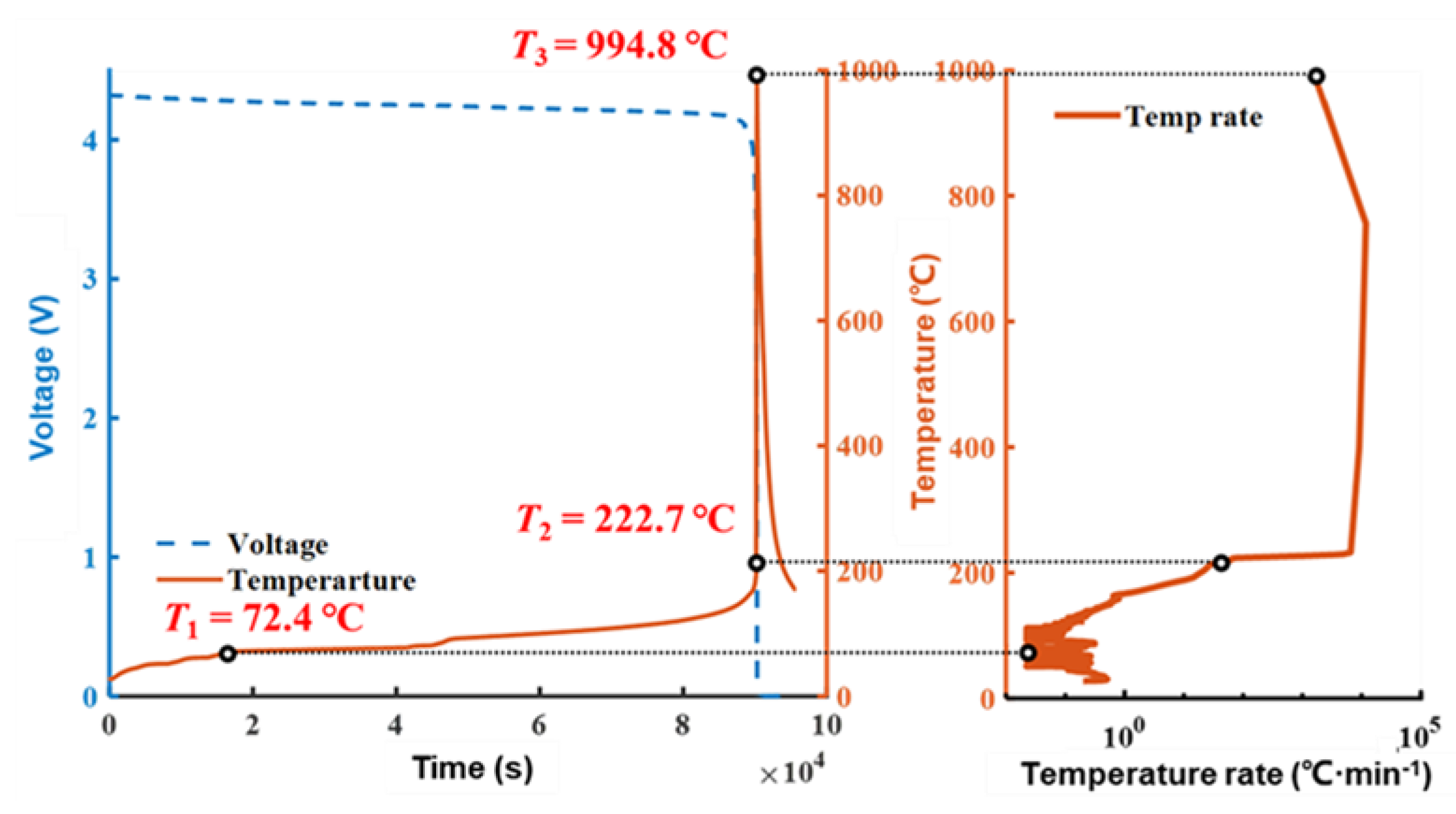
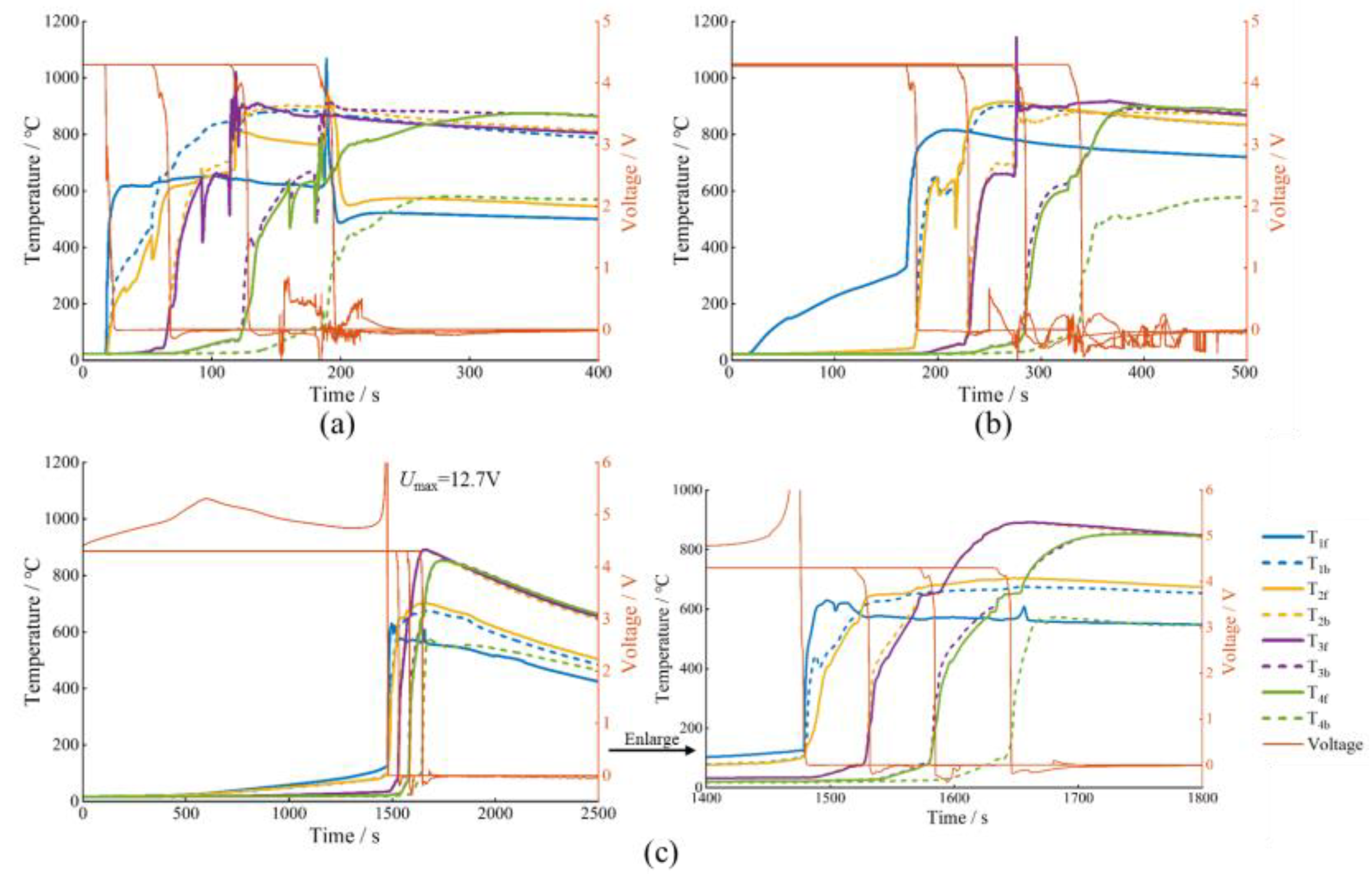
3.1. TR Behavior under EV-ARC Test
3.2. TR Behavior Using Different Abuse Methods
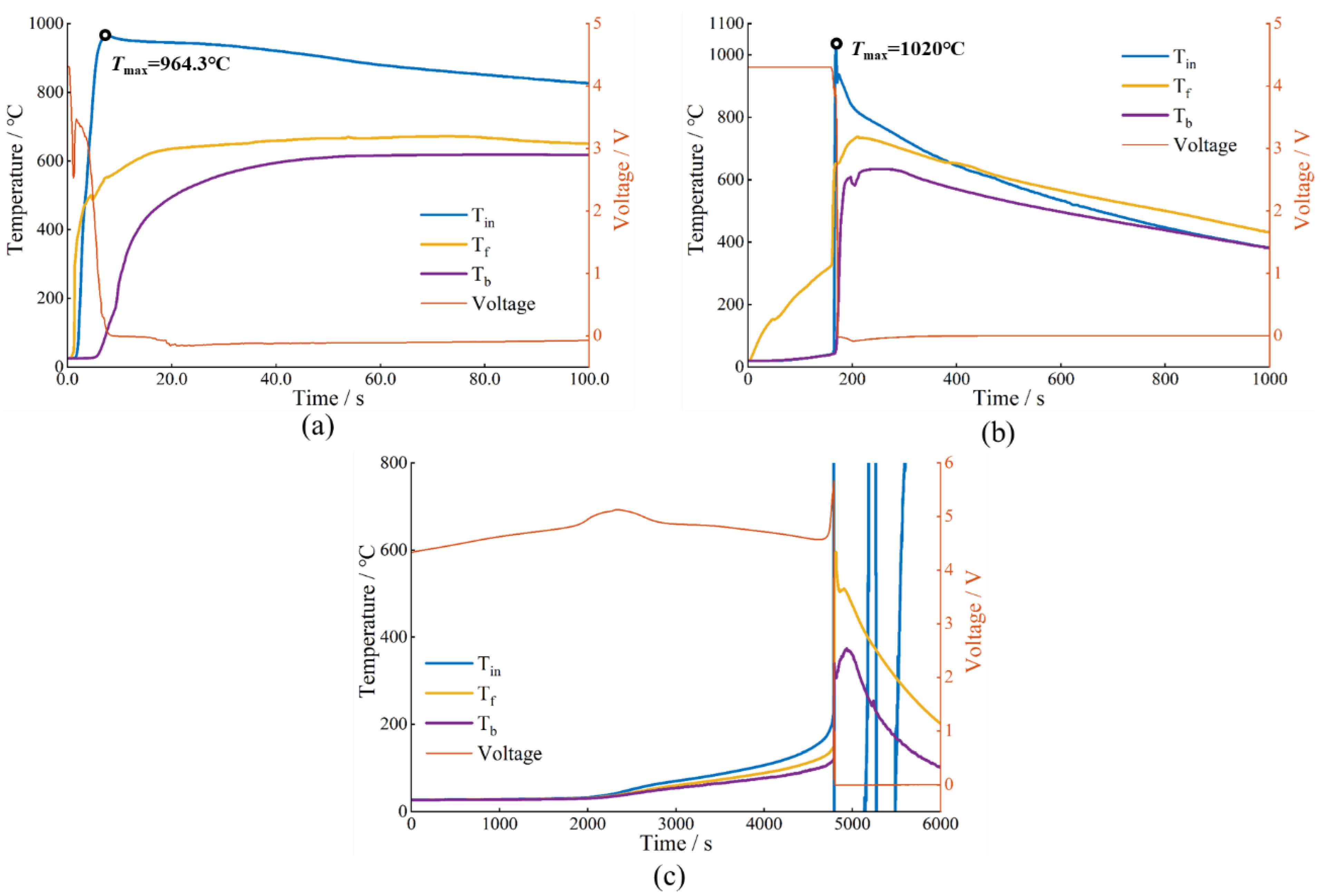
3.2.1. TR Behavior under Nail-Penetration Abuse Test
3.2.2. TR Behavior under Side Heating Abuse Test
3.2.3. TR Behavior under Overcharge Abuse Test
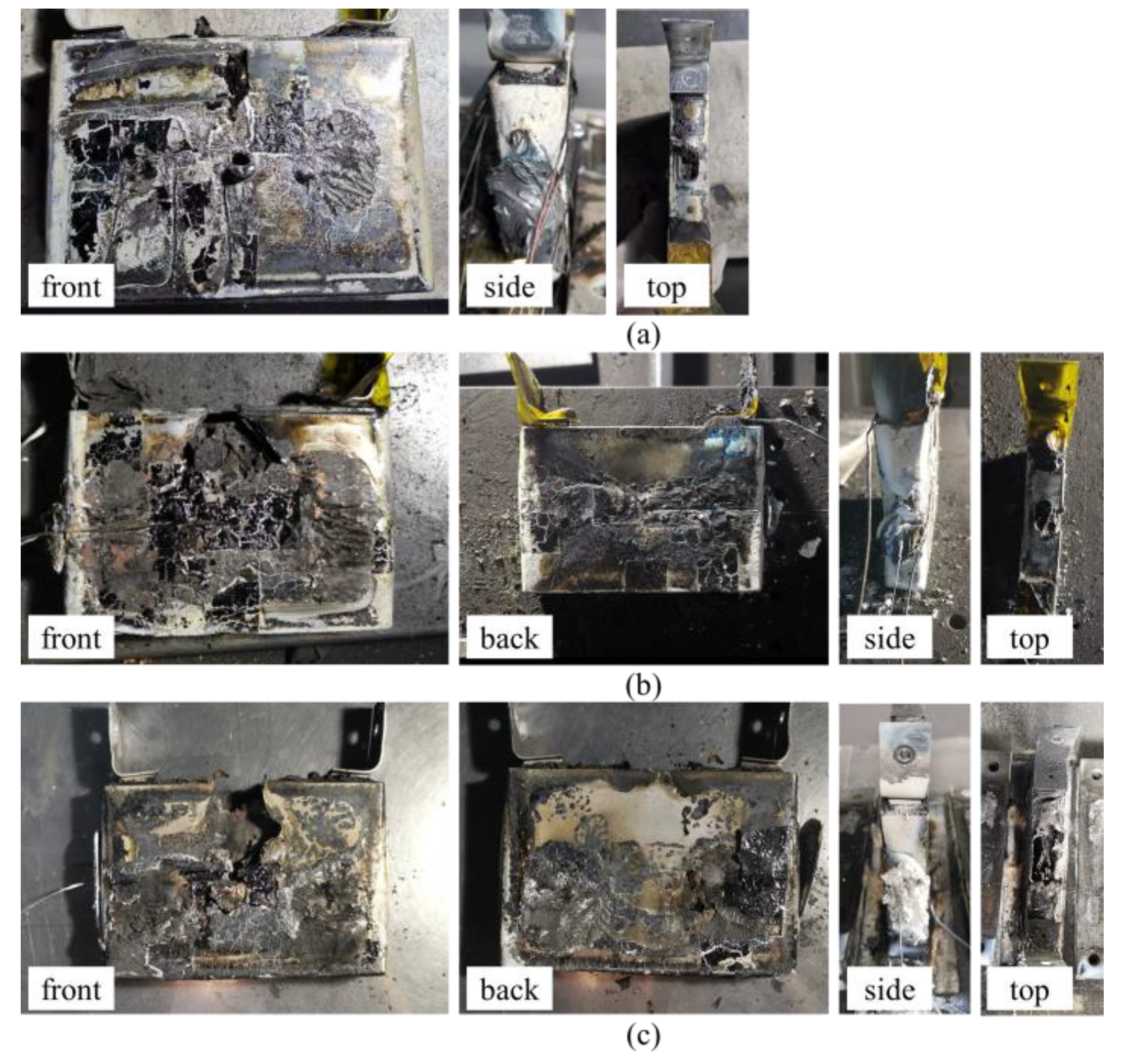
3.2.4. TR Behavior Comparison
3.3. TRP Behavior Using Different Abuse Methods
3.3.1. TRP Behavior under Nail-Penetration Abuse Test
3.3.2. TRP Behavior under Side-Heating Abuse Test
3.3.3. TRP Behavior under Side-Heating Abuse Test
3.3.4. TRP Behavior Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hecht, C.; Victor, K.; Zurmühlen, S.; Sauer, D.U. Electric vehicle route planning using real-world charging infrastructure in Germany. eTransportation 2021, 10, 100143. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H.J. Experimental Analysis of Thermal Runaway in 18650 Cylindrical Li-Ion Cells Using an Accelerating Rate Calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Qian, Y.; Zeng, Z.; Zhang, Y. Simulation and analysis of operating characteristics of power battery for flying car utilization. eTransportation 2021, 8, 100111. [Google Scholar] [CrossRef]
- Thingvad, A.; Andersen, P.B.; Unterluggauer, T.; Træholt, C.; Marinelli, M. Electrification of personal vehicle travels in cities—Quantifying the public charging demand. eTransportation 2021, 9, 100125. [Google Scholar] [CrossRef]
- Klink, J.; Hebenbrock, A.; Grabow, J.; Orazov, N.; Nylén, U.; Benger, R.; Beck, H.-P. Comparison of Model-Based and Sensor-Based Detection of Thermal Runaway in Li-Ion Battery Modules for Automotive Application. Batteries 2022, 8, 34. [Google Scholar] [CrossRef]
- Tanim, T.R.; Dufek, E.J.; Walker, L.K.; Ho, C.D.; Hendricks, C.E.; Christophersen, J.P. Advanced diagnostics to evaluate heterogeneity in lithium-ion battery modules. eTransportation 2020, 3, 100045. [Google Scholar] [CrossRef]
- Xiong, R.; Ma, S.; Li, H.; Sun, F.; Li, J. Toward a Safer Battery Management System: A Critical Review on Diagnosis and Prognosis of Battery Short Circuit. iScience 2020, 23, 101010. [Google Scholar] [CrossRef]
- Dixon, J.; Bell, K. Electric vehicles: Battery capacity, charger power, access to charging and the impacts on distribution networks. eTransportation 2020, 4, 100059. [Google Scholar] [CrossRef]
- Qin, P.; Sun, J.; Yang, X.; Wang, Q. Battery thermal management system based on the forced-air convection: A review. eTransportation 2021, 7, 100097. [Google Scholar] [CrossRef]
- Greene, D.L.; Ogden, J.M.; Lin, Z. Challenges in the designing, planning and deployment of hydrogen refueling infrastructure for fuel cell electric vehicles. eTransportation 2020, 6, 100086. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Lin, C.; Yang, X.; Zuo, F. Multi-physics safety model based on structure damage for lithium-ion battery under mechanical abuse. J. Clean. Prod. 2020, 277, 124094. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J. Evaluation of mechanical abuse techniques in lithium ion batteries. J. Power Source 2014, 247, 189–196. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, H.; Qu, Z.; Zhang, J. Recent progress in lithium-ion battery thermal management for a wide range of temperature and abuse conditions. Int. J. Hydrogen Energy 2022, 47, 9428–9459. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Zhang, Y.; Ouyang, M. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse. J. Energy Storage 2019, 24, 100775. [Google Scholar] [CrossRef]
- Oca, L.; Guillet, N.; Tessard, R.; Iraola, U. Lithium-ion capacitor safety assessment under electrical abuse tests based on ultrasound characterization and cell opening. J. Energy Storage 2019, 23, 29–36. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Chen, S.; Zhu, J.; Han, G.; Dai, H. Revealing the Impact of Slight Electrical Abuse on the Thermal Safety Characteristics for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 12858–12870. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Wang, X.; Gao, Y.; Allu, S.; Cakmak, E.; Wang, Z. Internal short circuit and failure mechanisms of lithium-ion pouch cells under mechanical indentation abuse conditions: An experimental study. J. Power Source 2020, 455, 227939. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, L.; Yin, S.; Xu, J. Generalized separator failure criteria for internal short circuit of lithium-ion battery. J. Power Source 2020, 467, 228360. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Ren, D.; Ouyang, M.; Lu, L.; Han, X. Thermal Runaway Triggered by Plated Lithium on the Anode after Fast Charging. ACS Appl. Mater. Interfaces 2019, 11, 46839–46850. [Google Scholar] [CrossRef]
- Cai, W.; Wang, H.; Maleki, H.; Howard, J.; Lara-Curzio, E. Experimental simulation of internal short circuit in Li-ion and Li-ion-polymer cells. J. Power Source 2011, 196, 7779–7783. [Google Scholar] [CrossRef]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, N.; van den Bossche, P.; Boon-Brett, L. A review of international abuse testing standards and regulations for lithium ion batteries in electric and hybrid electric vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, X.; Xu, C.; Jiang, F.; Ouyang, M. Thermal runaway front in failure propagation of long-shape lithium-ion battery. Int. J. Heat Mass Transf. 2022, 182, 121928. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Rui, X.; Wang, S.; Jin, C.; He, L.; Zhang, F.; Wang, Q.; Feng, X. A comparative analysis on thermal runaway behavior of Li (NixCoyMnz) O2 battery with different nickel contents at cell and module level. J. Hazard. Mater. 2020, 393, 122361. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; He, X.; Ouyang, M. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Feng, X.; Xu, C.; He, X.; Wang, L.; Zhang, G.; Ouyang, M. Mechanisms for the evolution of cell variations within a LiNixCoyMnzO2/graphite lithium-ion battery pack caused by temperature non-uniformity. J. Clean. Prod. 2018, 205, 447–462. [Google Scholar] [CrossRef]
- Xu, C.; Feng, X.; Huang, W.; Duan, Y.; Chen, T.; Gao, S.; Lu, L.; Jiang, F.; Ouyang, M. Internal temperature detection of thermal runaway in lithium-ion cells tested by extended-volume accelerating rate calorimetry. J. Energy Storage 2020, 31, 101670. [Google Scholar] [CrossRef]
- Jin, C.; Sun, Y.; Wang, H.; Lai, X.; Wang, S.; Chen, S.; Rui, X.; Zheng, Y.; Feng, X.; Wang, H.; et al. Model and experiments to investigate thermal runaway characterization of lithium-ion batteries induced by external heating method. J. Power Source 2021, 504, 230065. [Google Scholar] [CrossRef]
- Lai, X.; Jin, C.; Yi, W.; Han, X.; Feng, X.; Zheng, Y.; Ouyang, M. Mechanism, modeling, detection, and prevention of the internal short circuit in lithium-ion batteries: Recent advances and perspectives. Energy Storage Mater. 2021, 35, 470–499. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Source 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, M.; Han, D.; Xue, J.; Wang, S.; Meng, Y. Thermal runaway features of lithium sulfur pouch cells at various states of charge evaluated by extended volume-accelerating rate calorimetry. J. Power Source 2021, 489, 229503. [Google Scholar] [CrossRef]
- Mei, W.; Duan, Q.; Zhao, C.; Lu, W.; Sun, J.; Wang, Q. Three-dimensional layered electrochemical-thermal model for a lithium-ion pouch cell Part II. The effect of units number on the performance under adiabatic condition during the discharge. Int. J. Heat Mass Transf. 2020, 148, 119082. [Google Scholar] [CrossRef]
- Vendra, C.M.; Shelke, A.V.; Buston, J.E.; Gill, J.; Howard, D.; Read, E.; Abaza, A.; Cooper, B.; Wen, J.X. Numerical and experimental characterisation of high energy density 21,700 lithium-ion battery fires. Process Saf. Environ. Prot. 2022, 160, 153–165. [Google Scholar] [CrossRef]
- Greve, L.; Fehrenbach, C. Mechanical testing and macro-mechanical finite element simulation of the deformation, fracture, and short circuit initiation of cylindrical Lithium ion battery cells. J. Power Source 2012, 214, 377–385. [Google Scholar] [CrossRef]
- Lai, X.; Zheng, Y.; Zhou, L.; Gao, W. Electrical behavior of overdischarge-induced internal short circuit in lithium-ion cells. Electrochim. Acta 2018, 278, 245–254. [Google Scholar] [CrossRef]
- Ramasamy, R.P.; White, R.E.; Popov, B.N. Calendar life performance of pouch lithium-ion cells. J. Power Source 2005, 141, 298–306. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. Thermal stability of LiPF6/EC+DEC electrolyte with charged electrodes for lithium ion batteries. Thermochim. Acta 2005, 437, 12–16. [Google Scholar] [CrossRef]
- Venegas, F.G.; Petit, M.; Perez, Y. Plug-in behavior of electric vehicles users: Insights from a large-scale trial and impacts for grid integration studies. eTransportation 2021, 10, 100131. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, J.-N.; Lee, D.J.; Kim, W.-K.; Jeong, Y.K.; Choi, J.W.; Park, J.-K.; Lee, Y.M. Effects of lithium salts on thermal stabilities of lithium alkyl carbonates in SEI layer. Electrochim. Acta 2012, 83, 259–263. [Google Scholar] [CrossRef]
- Wang, H.; Tang, A.; Huang, K. Oxygen Evolution in Overcharged LixNi1/3Co1/3Mn1/3O2 Electrode and Its Thermal Analysis Kinetics. Chin. J. Chem. 2011, 29, 1583–1588. [Google Scholar] [CrossRef]
- Zhao, W.; Rohde, M.; Mohsin, I.U.; Ziebert, C.; Du, Y.; Seifert, H.J. Combined Thermal Runaway Investigation of Coin Cells with an Accelerating Rate Calorimeter and a Tian-Calvet Calorimeter. Batteries 2022, 8, 15. [Google Scholar] [CrossRef]
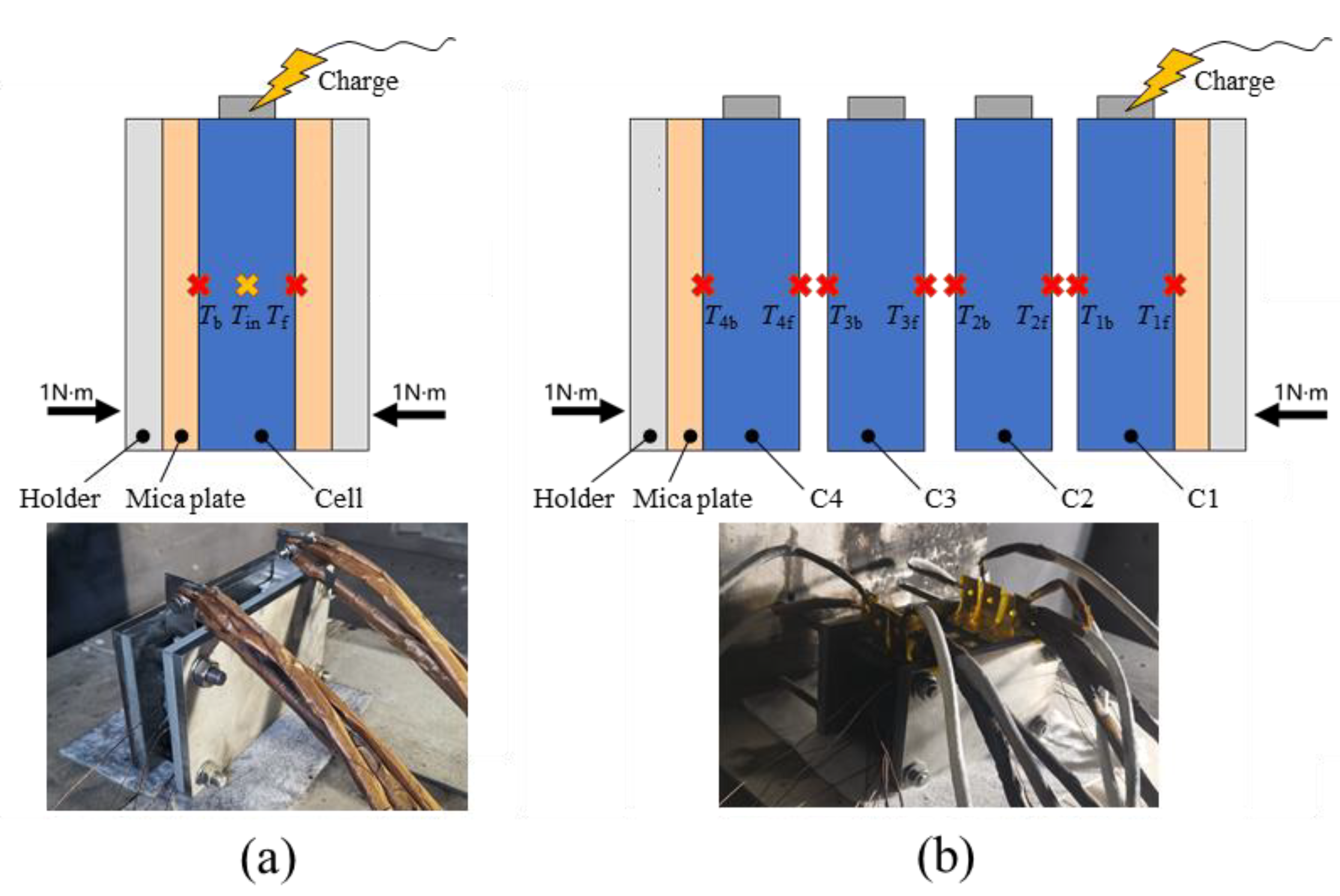




| Length (mm) | Width (mm) | Height (mm) | Capacity (Ah) | Weight (g) |
|---|---|---|---|---|
| 108 | 102 | 27 | 60 | 930 |
| Characteristics | Tmax (℃) | Mloss (g) | Mloss Rate (%) | TR Energy (J) | Extra Energy (J) | TR Trigger Time (s) | Time of Ejection & Fire (s) |
|---|---|---|---|---|---|---|---|
| EV-ARC | 994.8 | 272.3 | 32.4 | 7.19 × 105 | None | Over 90,000 | - |
| Nail penetration | 964.3 | 331.3 | 35.6 | 6.96 × 105 | None | 1 | 6 |
| Side heating | 1020 | 372.1 | 39.9 | 7.39 × 105 | 2.00 × 104 | 180 | 136 |
| Overcharge | None | 601.1 | 64.3 | 1.17 × 106 | 4.48 × 105 | 4700 | 165 |
| Abuse Method | t1–2 (s) | t2–3 (s) | t3–4 (s) | TRP Time (s) |
|---|---|---|---|---|
| Nail penetration | 44.2 | 59.2 | 63.5 | 177.8 |
| Side heating | 47.5 | 54.8 | 55.2 | 158.5 |
| Overcharge | 37.2 | 48.3 | 51.3 | 146.6 |
| Trigger Method | Cell | Mloss (g) | Mloss Rate (%) |
|---|---|---|---|
| Nail penetration | C1 | 451.9 | 48.53 |
| C2 | 295.4 | 31.82 | |
| C3 | 309.0 | 33.20 | |
| C4 | 323.6 | 34.79 | |
| Side heating | C1 | 425.9 | 45.89 |
| C2 | 323.5 | 34.92 | |
| C3 | 310.7 | 33.46 | |
| C4 | 301.9 | 32.71 | |
| Overcharge | C1 | 655.5 | 70.16 |
| C2 | 316.7 | 33.90 | |
| C3 | 314.9 | 33.70 | |
| C4 | 279.5 | 29.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Zhang, M.; Zhu, L.; Chen, H.; Huang, W.; Yao, J.; Yuan, Z.; Xu, C.; Feng, X. Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods. Batteries 2022, 8, 201. https://doi.org/10.3390/batteries8110201
Wei D, Zhang M, Zhu L, Chen H, Huang W, Yao J, Yuan Z, Xu C, Feng X. Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods. Batteries. 2022; 8(11):201. https://doi.org/10.3390/batteries8110201
Chicago/Turabian StyleWei, Dan, Mengqi Zhang, Linpei Zhu, Hu Chen, Wensheng Huang, Jian Yao, Zhuchen Yuan, Chengshan Xu, and Xuning Feng. 2022. "Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods" Batteries 8, no. 11: 201. https://doi.org/10.3390/batteries8110201
APA StyleWei, D., Zhang, M., Zhu, L., Chen, H., Huang, W., Yao, J., Yuan, Z., Xu, C., & Feng, X. (2022). Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods. Batteries, 8(11), 201. https://doi.org/10.3390/batteries8110201







