Influence of Switching on the Aging of High Power Lithium-Ion Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Reference Tests
3.2. Switching Tests at 50 Hz
3.2.1. Capacity and Internal Resistance
3.2.2. Differential Voltage Analysis
3.2.3. EIS
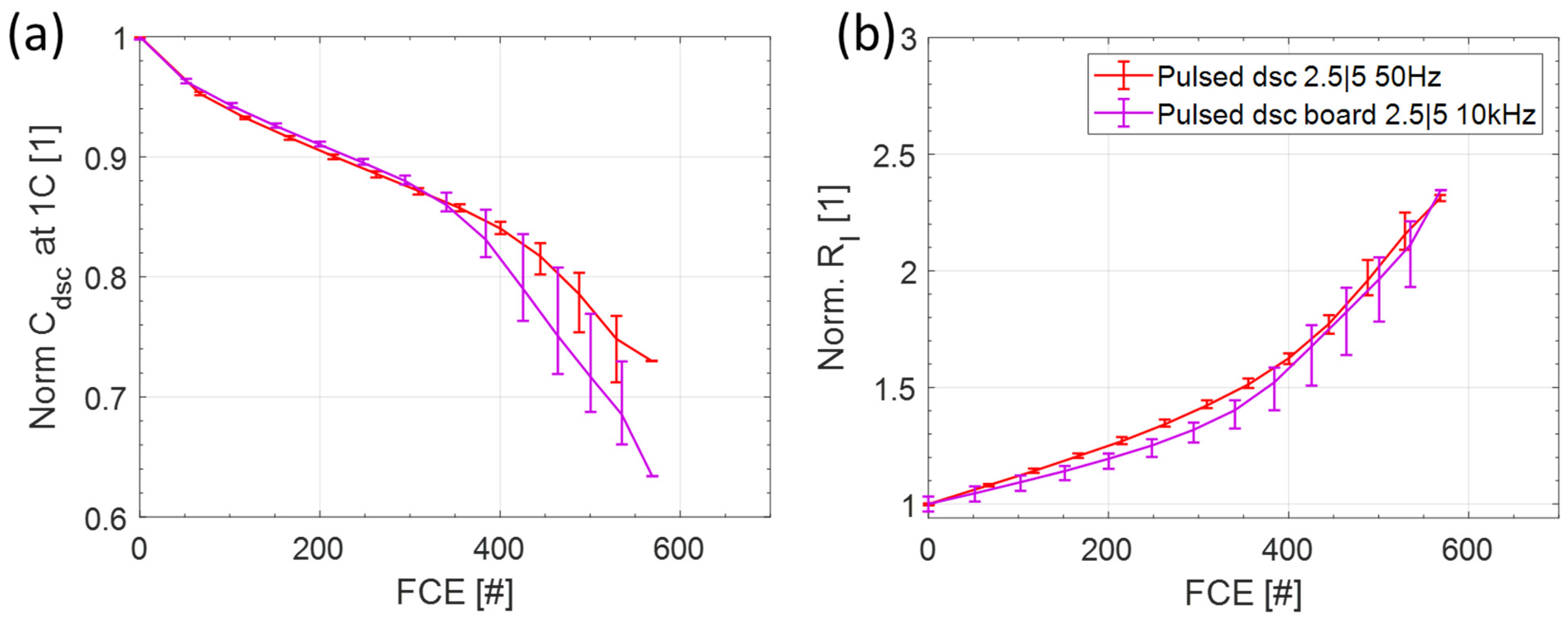
3.3. Comparison of the Switching Frequency
3.3.1. Capacity and Internal Resistance
3.3.2. Differential Voltage Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Hosainy, A.; Hamed, H.A.; Azazi, H.Z.; El-Kholy, E.E. A review of multilevel inverter topologies, control techniques, and applications. In Proceedings of the 2017 Nineteenth International Middle East Power Systems Conference (MEPCON), Cairo, Egypt, 19–21 December 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1265–1275, ISBN 978-1-5386-0990-3. [Google Scholar]
- Hasan, N.S.; Rosmin, N.; Osman, D.A.A.; Hatib, A. Reviews on multilevel converter and modulation techniques. Renew. Sustain. Energy Rev. 2017, 80, 163–174. [Google Scholar] [CrossRef]
- Hanzl, C.; Hartmann, C.; Hölzle, M.; Liebhart, B.; Schmid, M.; Endisch, C. Current Commutation in a Switched Lithium-Ion Cell used in Cascaded Half-Bridge Multilevel Inverters. IET Power Electron 2021, 14, 1073–1088. [Google Scholar] [CrossRef]
- Korth Pereira Ferraz, P.; Schmidt, R.; Kober, D.; Kowal, J. A high frequency model for predicting the behavior of lithium-ion batteries connected to fast switching power electronics. J. Energy Storage 2018, 18, 40–49. [Google Scholar] [CrossRef]
- Korte, C.; Specht, E.; Hiller, M.; Goetz, S. Efficiency evaluation of MMSPC/CHB topologies for automotive applications. In Proceedings of the 2017 IEEE 12th International Conference on Power Electronics and Drive Systems (PEDS), Honolulu, HI, USA, 12–15 December 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 324–330, ISBN 978-1-5090-2364-6. [Google Scholar]
- Bughneda, A.; Salem, M.; Richelli, A.; Ishak, D.; Alatai, S. Review of Multilevel Inverters for PV Energy System Applications. Energies 2021, 14, 1585. [Google Scholar] [CrossRef]
- Jiang, X.; Doumbia, M.L. Comparative Study of Grid-Connected Multilevel Inverters for High Power Phtovoltaic Systems. In Proceedings of the 2019 IEEE 7th International Conference on Smart Energy Grid Engineering (SEGE), Oshawa, ON, Canada, 12–14 August 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 184–190, ISBN 978-1-7281-2440-7. [Google Scholar]
- Komsiyska, L.; Buchberger, T.; Diehl, S.; Ehrensberger, M.; Hanzl, C.; Hartmann, C.; Hölzle, M.; Kleiner, J.; Lewerenz, M.; Liebhart, B.; et al. Critical Review of Intelligent Battery Systems: Challenges, Implementation, and Potential for Electric Vehicles. Energies 2021, 14, 5989. [Google Scholar] [CrossRef]
- Uno, M.; Tanaka, K. Influence of High-Frequency Charge–Discharge Cycling Induced by Cell Voltage Equalizers on the Life Performance of Lithium-Ion Cells. IEEE Trans. Veh. Technol. 2011, 60, 1505–1515. [Google Scholar] [CrossRef]
- Chang, F.; Roemer, F.; Lienkamp, M. Influence of Current Ripples in Cascaded Multilevel Topologies on the Aging of Lithium Batteries. IEEE Trans. Power Electron 2020, 35, 11879–11890. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, X.; Tomaszewska, A.; Chen, H.; Wei, Y.; Zhu, H.; Li, Y.; Cui, Z.; Huang, J.; Du, J.; et al. Lithium-ion batteries under pulsed current operation to stabilize future grids. Cell Rep. Phys. Sci. 2022, 3, 100708. [Google Scholar] [CrossRef]
- Wong, D.; Shrestha, B.; Wetz, D.A.; Heinzel, J.M. Impact of high rate discharge on the aging of lithium nickel cobalt aluminum oxide batteries. J. Power Source 2015, 280, 363–372. [Google Scholar] [CrossRef]
- Soares, R.; Bessman, A.; Wallmark, O.; Lindbergh, G.; Svens, P. An Experimental Setup with Alternating Current Capability for Evaluating Large Lithium-Ion Battery Cells. Batteries 2018, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Namuduri, C.; Kollmeyer, P. Onboard unidirectional automotive G2V battery charger using sine charging and its effect on li-ion batteries. In Proceedings of the 2015 IEEE Energy Conversion Congress and Exposition (ECCE), Montreal, QC, Canada, 20–24 September 2015; pp. 6299–6305. [Google Scholar]
- Korth Pereira Ferraz, P.; Kowal, J. A Comparative Study on the Influence of DC/DC-Converter Induced High Frequency Current Ripple on Lithium-Ion Batteries. Sustainability 2019, 11, 6050. [Google Scholar] [CrossRef] [Green Version]
- Lain, M.J.; Brandon, J.; Kendrick, E. Design Strategies for High Power vs. High Energy Lithium Ion Cells. Batteries 2019, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Keil, P.; Schuter, S.F.; Wilhelm, J.; Travi, J.; Hauser, A.; Katl, R.C.; Jossen, A. Calendar Aging of Lithium-Ion Batteries: I. Impact of the Graphite Anode on Capacity Fade. J. Electrochem. Soc. 2016, 163, A1872–A1880. [Google Scholar] [CrossRef]
- Gyenes, B.; Stevens, D.A.; Chevrier, V.L.; Dahn, J.R. Understanding Anomalous Behavior in Coulombic Efficiency Measurements on Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A278–A283. [Google Scholar] [CrossRef]
- Lewerenz, M.; Fuchs, G.; Becker, L.; Sauer, D.U. Irreversible calendar aging and quantification of the reversible capacity loss caused by anode overhang. J. Energy Storage 2018, 18, 149–159. [Google Scholar] [CrossRef]
- Cui, D.; Wang, J.; Sun, A.; Song, H.; Wei, W. Anomalously Faster Deterioration of LiNi0.8Co0.15Al0.05O2/Graphite High-Energy 18650 Cells at 1.5 C than 2.0 C. Scanning 2018, 2018, 2593780. [Google Scholar] [CrossRef] [Green Version]
- Safari, M.; Delacourt, C. Aging of a Commercial Graphite/LiFePO4 Cell. J. Electrochem. Soc. 2011, 158, A1123. [Google Scholar] [CrossRef]
- Lewerenz, M.; Rahe, C.; Fuchs, G.; Endisch, C.; Sauer, D.U. Evaluation of shallow cycling on two types of uncompressed automotive Li (Ni1/3Mn1/3Co1/3)O2-Graphite pouch cells. J. Electrochem. Soc. 2020, 30, 10159. [Google Scholar] [CrossRef]
- Lewerenz, M.; Dechent, P.; Sauer, D.U. Investigation of capacity recovery during rest period at different states-of-charge after cycle life test for prismatic Li(Ni1/3Mn1/3Co1/3)O2-graphite cells. J. Energy Storage 2019, 21, 680–690. [Google Scholar] [CrossRef]
- Epding, B.; Rumberg, B.; Jahnke, H.; Stradtmann, I.; Kwade, A. Investigation of significant capacity recovery effects due to long rest periods during high current cyclic aging tests in automotive lithium ion cells and their influence on lifetime. J. Energy Storage 2019, 22, 249–256. [Google Scholar] [CrossRef]
- Spingler, F.B.; Naumann, M.; Jossen, A. Capacity Recovery Effect in Commercial LiFePO4/Graphite Cells. J. Electrochem. Soc. 2020, 167, 40526. [Google Scholar] [CrossRef]
- Lewerenz, M.; Warnecke, A.; Sauer, D.U. Post-mortem analysis on LiFePO4|Graphite cells describing the evolution & composition of covering layer on anode and their impact on cell performance. J. Power Source 2017, 369, 122–132. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Source 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Baghdadi, I.; Briat, O.; Delétage, J.-Y.; Gyan, P.; Vinassa, J.-M. Lithium battery aging model based on Dakin’s degradation approach. J. Power Source 2016, 325, 273–285. [Google Scholar] [CrossRef]
- Benavente-Araoz, F.; Varini, M.; Lundblad, A.; Cabrera, S.; Lindbergh, G. Effect of Partial Cycling of NCA/Graphite Cylindrical Cells in Different SOC Intervals. J. Electrochem. Soc. 2020, 167, 40529. [Google Scholar] [CrossRef]
- Bloom, I.; Christophersen, J.P.; Abraham, D.P.; Gering, K.L. Differential voltage analyses of high-power lithium-ion cells. J. Power Source 2006, 157, 537–542. [Google Scholar] [CrossRef]
- Watanabe, S.; Hosokawa, T.; Morigaki, K.; Kinoshita, M.; Nakura, K. Prevention of the Micro Cracks Generation in LiNiCoAlO2 Cathode by the Restriction of ΔDOD. ECS Trans. 2012, 41, 65–74. [Google Scholar] [CrossRef]
- Abraham, D.P.; Reynolds, E.M.; Sammann, E.; Jansen, A.N.; Dees, D.W. Aging characteristics of high-power lithium-ion cells with LiNi0.8Co0.15Al0.05O2 and Li4/3Ti5/3O4 electrodes. Electrochim. Acta 2005, 51, 502–510. [Google Scholar] [CrossRef]
- Deshpande, R.D.; Ridgway, P.; Fu, Y.; Zhang, W.; Cai, J.; Battaglia, V. The Limited Effect of VC in Graphite/NMC Cells. J. Electrochem. Soc. 2015, 162, A330–A338. [Google Scholar] [CrossRef] [Green Version]
- Zilberman, I.; Ludwig, S.; Schiller, M.; Jossen, A. Online aging determination in lithium-ion battery module with forced temperature gradient. J. Energy Storage 2020, 28, 101170. [Google Scholar] [CrossRef]
- Westerhoff, U.; Kurbach, K.; Lienesch, F.; Kurrat, M. Analysis of Lithium-Ion Battery Models Based on Electrochemical Impedance Spectroscopy. Energy Technol. 2016, 4, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. The effects of pulse charging on cycling characteristics of commercial lithium-ion batteries. Electrochem. Solid-State Lett. 2001, 102, 302–309. [Google Scholar] [CrossRef]
- Bloom, I.; Walker, L.K.; Basco, J.K.; Abraham, D.P.; Christophersen, J.P.; Ho, C.D. Differential voltage analyses of high-power lithium-ion cells. 4. Cells containing NMC. J. Power Source 2010, 195, 877–882. [Google Scholar] [CrossRef]
- Lee, S.; Cho, W.; Do, V.; Choi, W. Effects of Pulse Current Charging on the Aging Performance of Commercial Cylindrical Lithium Ion Batteries. Appl. Sci. 2021, 11, 4918. [Google Scholar] [CrossRef]
- Weber, R.; Louli, A.J.; Plucknett, K.P.; Dahn, J.R. Resistance Growth in Lithium-Ion Pouch Cells with LiNi0.80Co0.15Al0.05O2 Positive Electrodes and Proposed Mechanism for Voltage Dependent Charge-Transfer Resistance. J. Electrochem. Soc. 2019, 166, A1779–A1784. [Google Scholar] [CrossRef]










| Producer | Samsung |
| Cell type | INR18650-25R |
| Cathode | NCA: Li(Ni0.8Co0.15Al0.05)O2 + NMC: Li(Ni0.6Mn0.2Co0.2)O2 |
| Anode | Graphite + Silicon |
| Nom. Capacity at 0.5 C | 2.5 Ah |
| Max. discharge voltage | 2.5 V |
| Max. charge voltage | 4.2 V |
| Max. discharge current | 20 A |
| Energy density | 216 Wh/kg |
| Storage voltage (SOC) before test | 3.52 V (20%) |
| Test-Name | # Test Cells | Imax [A] | Iavg [A] | ||
|---|---|---|---|---|---|
| cha | dsc | cha | dsc | ||
| Ref 1 | 2 | 2.5 | 5 | 2.5 | 5 |
| Ref 2 | 2 | 2.5 | 2.5 | 2.5 | 2.5 |
| Ref 3 | 1 | 1.25 | 2.5 | 1.25 | 2.5 |
| Ref 4 | 2 | 1.25 | 5 | 1.25 | 5 |
| Pulsed cha—50 Hz | 3 | 2.5 | 5 | 1.25 | 5 |
| Pulsed cha/dsc—50 Hz | 3 | 2.5 | 5 | 1.25 | 2.5 |
| Pulsed dsc—50 Hz | 3 | 2.5 | 5 | 2.5 | 2.5 |
| Pulsed dsc—10 kHz | 3 | 2.5 | 5 | 2.5 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngaleu, G.W.; Theiler, M.; Straßer, X.; Hanzl, C.; Komsiyska, L.; Endisch, C.; Lewerenz, M. Influence of Switching on the Aging of High Power Lithium-Ion Cells. Batteries 2022, 8, 33. https://doi.org/10.3390/batteries8040033
Ngaleu GW, Theiler M, Straßer X, Hanzl C, Komsiyska L, Endisch C, Lewerenz M. Influence of Switching on the Aging of High Power Lithium-Ion Cells. Batteries. 2022; 8(4):33. https://doi.org/10.3390/batteries8040033
Chicago/Turabian StyleNgaleu, Guy Williams, Michael Theiler, Xenia Straßer, Christian Hanzl, Lidiya Komsiyska, Christian Endisch, and Meinert Lewerenz. 2022. "Influence of Switching on the Aging of High Power Lithium-Ion Cells" Batteries 8, no. 4: 33. https://doi.org/10.3390/batteries8040033








