Study of Quaternary Ammonium Additives towards High-Rate Zinc Deposition and Dissolution Cycling for Application in Zinc-Based Rechargeable Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Baseline Electrolytes and Working Electrodes
2.3. Electrochemical Measurement
2.4. Physical Characterizations
3. Results and Discussion
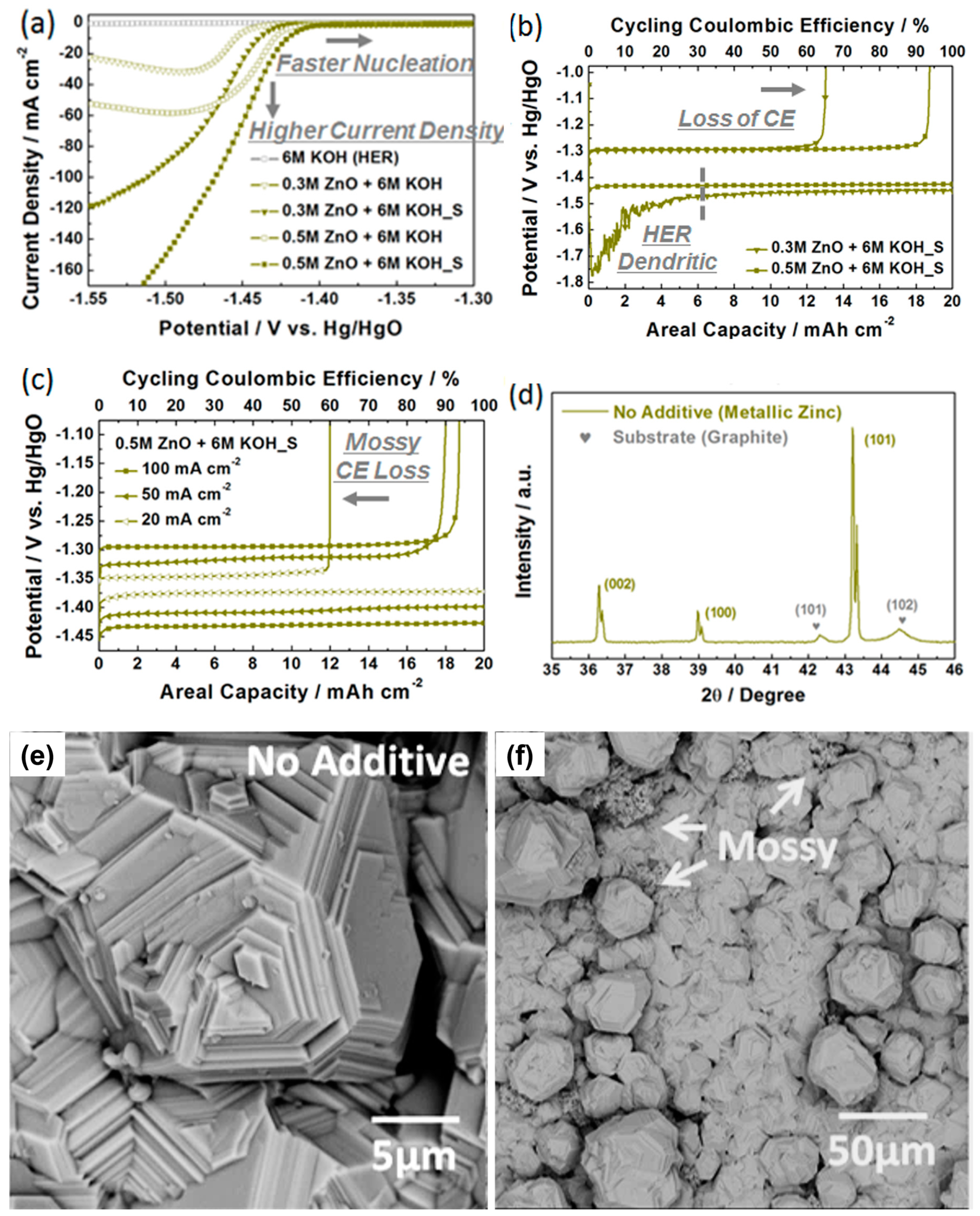
3.1. Determination of Blank Alkaline Electrolytes for Zinc Deposition and Dissolution
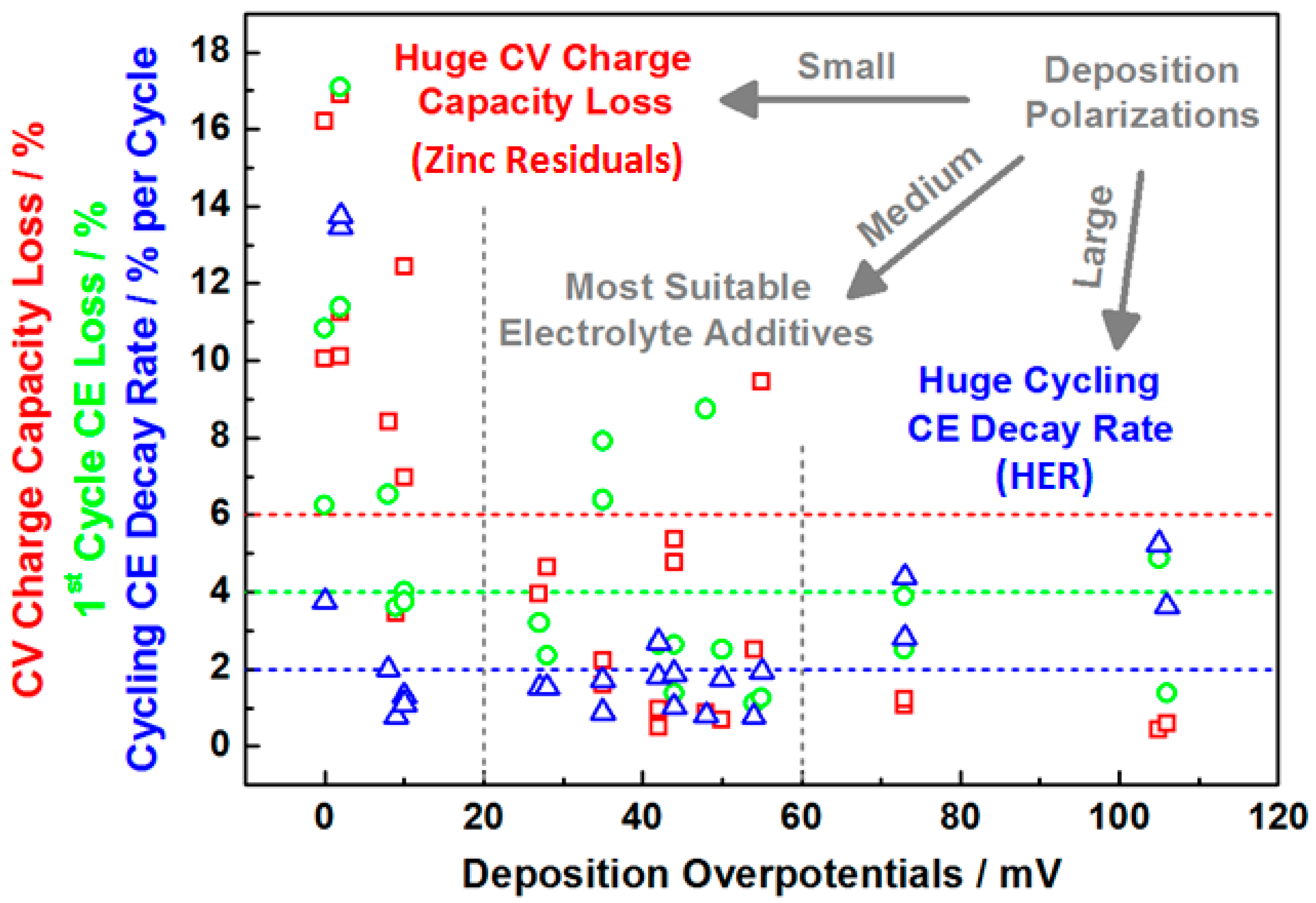
3.2. Screening of Quaternary Ammonium Additives towards High-Rate Cycling
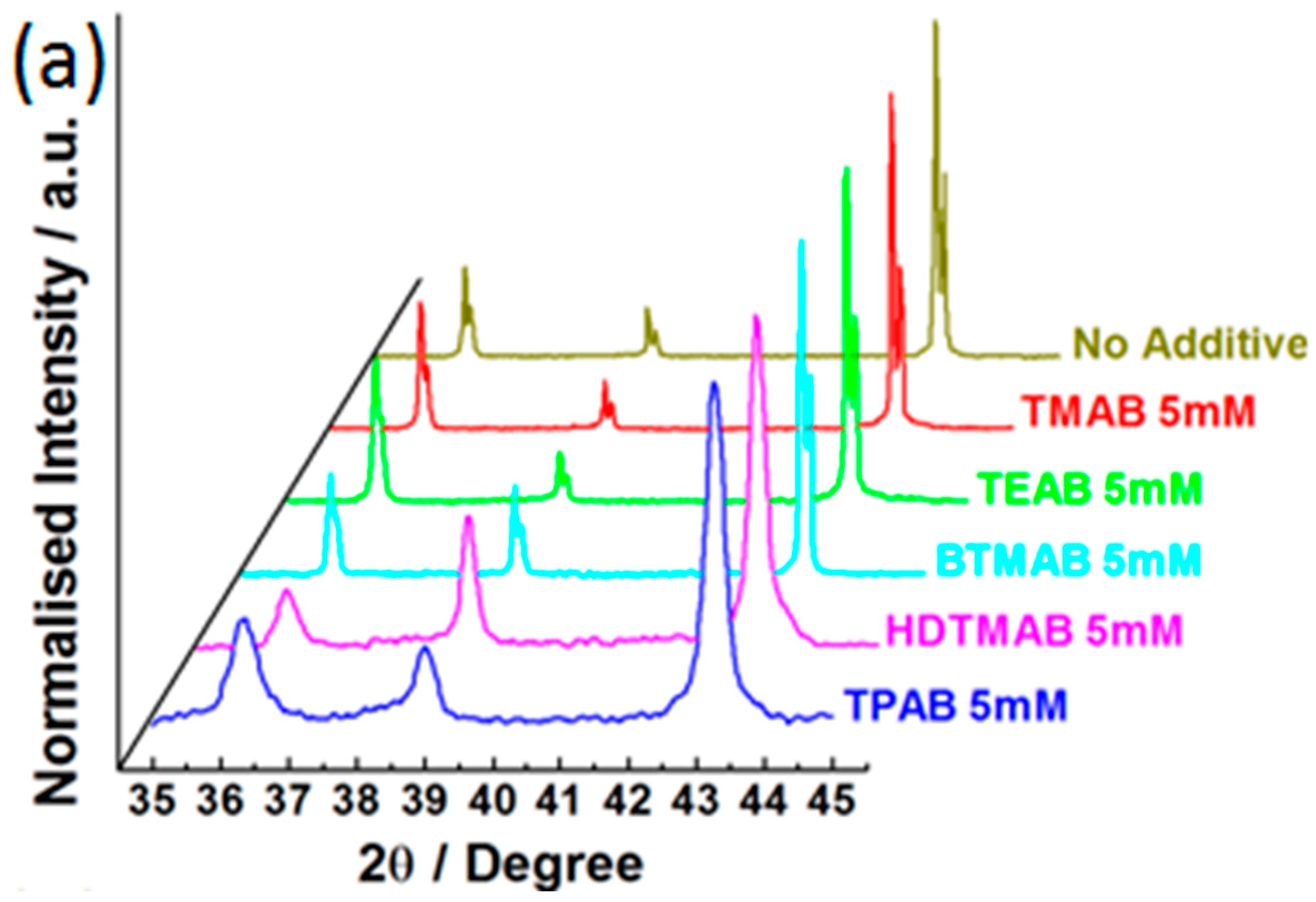
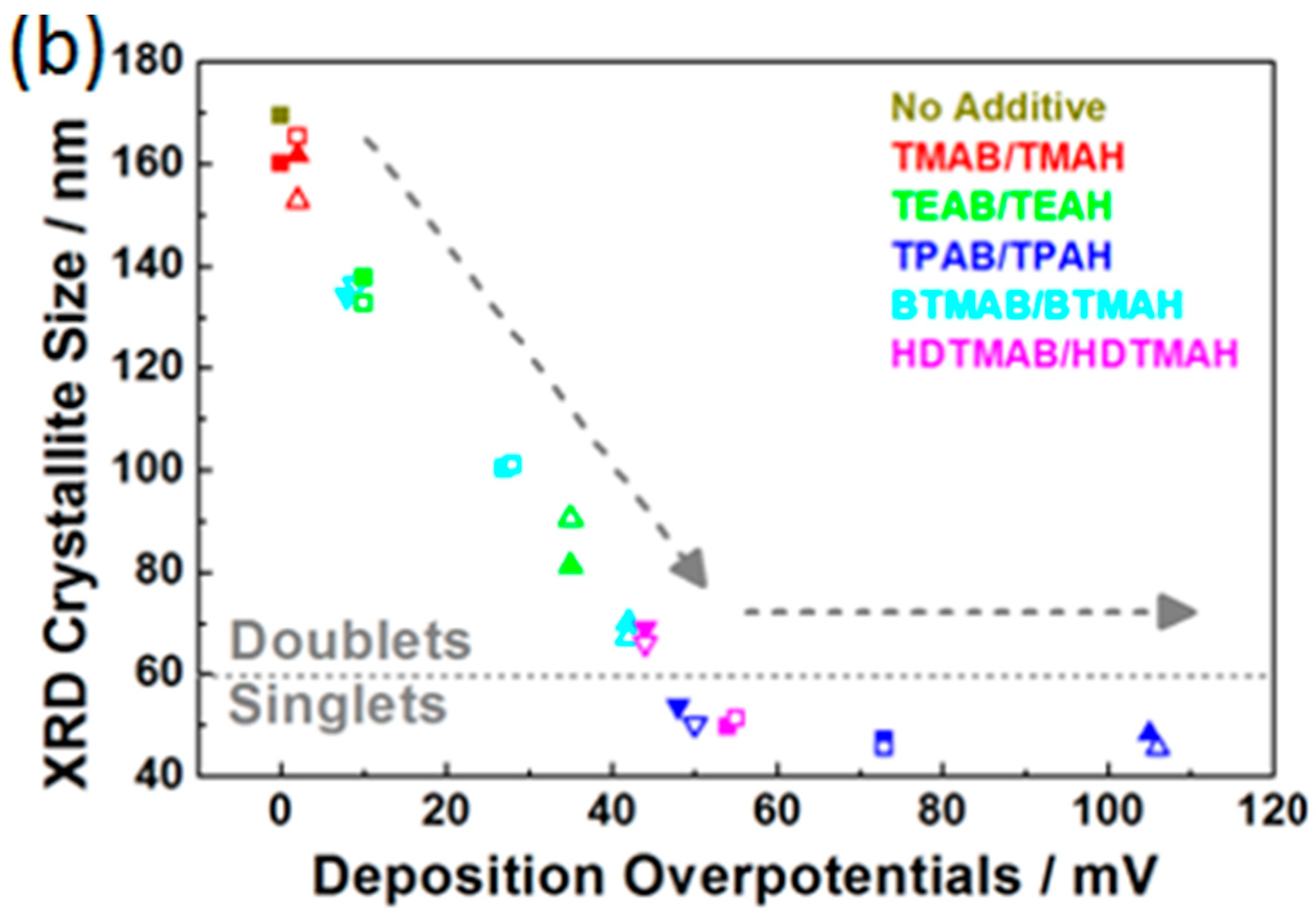
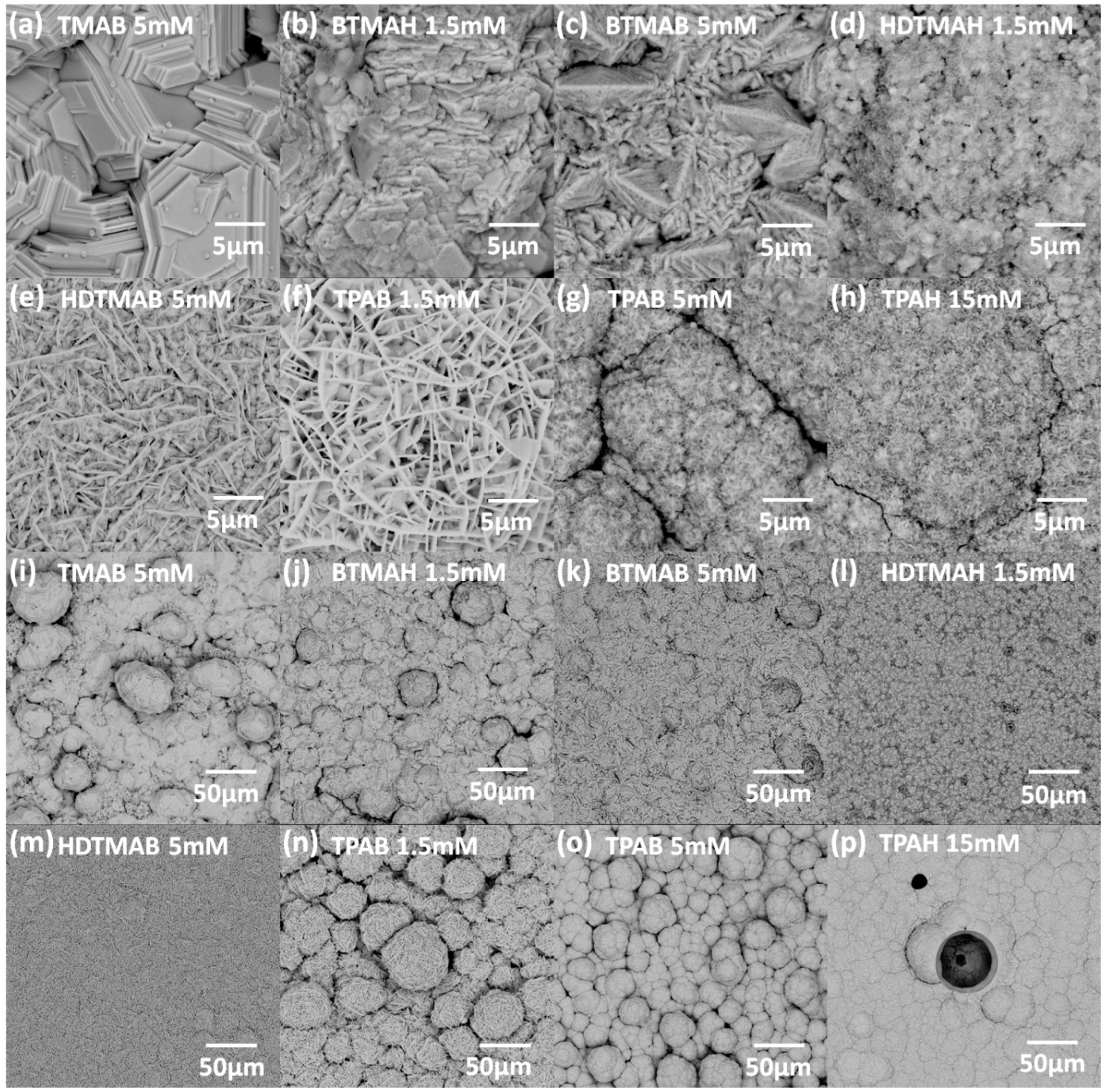
3.3. Influence of QAAs on Crystallinity and Morphology of Zinc Deposits
- (1)
- (2)
- When zinc deposition overpotentials further increase within a range of 20–60 mV, the zinc deposits turn from small crystallized prismatic structures embedded in layered-like agglomerates for 5 mM BTMAB (Figure 6c) to well-distributed agglomerates only for 1.5 mM HDTMAH (Figure 6d), then to long conjunct flakes on top of uniformly distributed small agglomerates for 5 mM HDTMAB (Figure 6e), and eventually to long conjunct flakes only for 1.5 mM TPAB (Figure 6f).
- (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel-3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science 2017, 356, 415. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.F.; Chervin, C.N.; Nelson, E.S.; Rolison, D.R.; Long, J.W. Wiring zinc in three dimensions re-writes battery perfor-mance-dendrite-free cycling. Energy Environ. Sci. 2014, 7, 1117. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Fu, J.; Li, J.; Park, M.G.; Zhang, Y.; Lui, G.; Chen, Z. Pomegranate-Inspired Design of Highly Active and Durable Bifunctional Electrocatalysts for Rechargeable Metal-Air Batteries. Angew. Chem. Int. Ed. 2016, 55, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Quan, J.; Loh, A.; Chai, J.; Chen, Y.; Tan, C.; Ge, X.; Hor, T.S.A.; Liu, Z.; Zhang, H.; et al. A Robust Hybrid Zn-Battery with Ultralong Cycle Life. Nano Lett. 2017, 17, 156–163. [Google Scholar] [CrossRef]
- Lee, D.U.; Fu, J.; Park, M.G.; Liu, H.; Kashkooli, A.G.; Chen, Z. Self-assembled NiO/Ni(OH)2 nanoflakes as active material for high-power and high-energy hybrid rechargeable battery. Nano Lett. 2016, 16, 1794. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Nie, Z.; Wang, W.; Reed, D.; Liu, J.; McGrail, P.; Sprenkle, V. Metal–organic frameworks as highly active electrocat-alysts for high-energy density, aqueous zinc-polyiodide redox flow batteries. Nano Lett. 2016, 16, 4335. [Google Scholar] [CrossRef]
- Li, B.; Nie, Z.; Vijayakumar, M.; Li, G.; Liu, J.; Sprenkle, V.; Wang, W. Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery. Nat. Commun. 2015, 6, 6303. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Ma, X.; Conforti, K.M.; Kuttler, K.J.; Grunewald, J.B.; Yeager, K.L.; Bazant, M.Z.; Gu, S.; Yan, Y. A zinc–iron redox-flow battery under $100 per kW h of system capital cost. Energy Environ. Sci. 2015, 8, 2941–2945. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, F.; Wang, L.; Li, M.; Wang, Y.; Chen, B.; Zhu, Y.; Fu, L.; Zha, L.; Zhang, L.; et al. An aqueous re-chargeable Zn//Co3O4 battery with high energy density and good cycling behavior. Adv. Mater. 2016, 28, 4904. [Google Scholar] [CrossRef] [PubMed]
- Winsberg, J.; Janoschka, T.; Morgenstern, S.; Hagemann, T.; Muench, S.; Hauffman, G.; Gohy, J.-F.; Hager, M.D.; Schubert, U.S. Poly(TEMPO)/zinc hybrid-flow battery: A novel, “green,” high voltage, and safe energy storage system. Adv. Mater. 2016, 28, 2238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 2016, 138, 12894. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- He, W.; Zuo, S.; Xu, X.; Zeng, L.; Liu, L.; Zhao, W.; Liu, J. Challenges and strategies of zinc anode for aqueous zinc-ion batteries. Mater. Chem. Front. 2021, 5, 2201–2217. [Google Scholar] [CrossRef]
- Zuo, S.; Liu, J.; He, W.; Osman, S.; Liu, Z.; Xu, X.; Shen, J.; Jiang, W.; Liu, J.; Zeng, Z.; et al. Direct Detection and Visualization of the H+ Reaction Process in a VO2 Cathode for Aqueous Zinc-Ion Batteries. J. Phys. Chem. Lett. 2021, 12, 7076–7084. [Google Scholar] [CrossRef]
- Zuo, S.; Xu, X.; Ji, S.; Wang, Z.; Liu, Z.; Liu, J. Cathodes for aqueous Zn-ion batteries: Materials, mechanisms, and kinetics. Chem. Eur. J. 2021, 27, 830. [Google Scholar] [CrossRef]
- Higashi, S.; Lee, S.W.; Lee, J.; Takechi, K.; Cui, Y. Avoiding short circuits from zinc metal dendrites in anode by backside-plating configuration. Nat. Commun. 2016, 7, 11801. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, L.; Yang, Y.-S.; Wen, Y.-H.; Cao, G.-P.; Wang, X.-D. Preliminary study of single flow zinc–nickel battery. Electrochem. Commun. 2007, 9, 2639–2642. [Google Scholar] [CrossRef]
- Mainar, A.R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Blázquez, J.A. Alkaline aqueous electrolytes for secondary zinc-air batteries: An overview. Int. J. Energy Res. 2016, 40, 1032–1049. [Google Scholar] [CrossRef]
- Bass, K.; Mitchell, P.; Wilcox, G.; Smith, J. Methods for the reduction of shape change and dendritic growth in zinc-based secondary cells. J. Power Sources 1991, 35, 333–351. [Google Scholar] [CrossRef]
- Turney, D.E.; Gallaway, J.W.; Yadav, G.G.; Ramirez, R.; Nyce, M.; Banerjee, S.; Chen-Wiegart, Y.-C.K.; Wang, J.; D’Ambrose, M.J.; Kolhekar, S.; et al. Rechargeable Zinc Alkaline Anodes for Long-Cycle Energy Storage. Chem. Mater. 2017, 29, 4819–4832. [Google Scholar] [CrossRef]
- Sun, K.E.K.; Hoang, T.K.A.; Doan, T.N.L.; Yu, Y.; Zhu, X.; Tian, Y.; Chen, P. Suppression of Dendrite Formation and Corrosion on Zinc Anode of Secondary Aqueous Batteries. ACS Appl. Mater. Interfaces 2017, 9, 9681–9687. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Z.; Tian, Z.; Zhang, K.; Li, J.; Lai, Y. Effects of Carboxymethyl Cellulose on the Electrochemical Characteristics and Dendrite Growth of Zinc in Alkaline Solution. J. Electrochem. Soc. 2016, 163, A1836–A1840. [Google Scholar] [CrossRef]
- Song, Y.; Hu, J.; Tang, J.; Gu, W.; He, L.; Ji, X. Real-Time X-ray Imaging Reveals Interfacial Growth, Suppression, and Dissolution of Zinc Dendrites Dependent on Anions of Ionic Liquid Additives for Rechargeable Battery Applications. ACS Appl. Mater. Interfaces 2016, 8, 32031–32040. [Google Scholar] [CrossRef]
- Trudgeon, D.P.; Qiu, K.; Li, X.; Mallick, T.; Taiwo, O.O.; Chakrabarti, B.; Yufit, V.; Brandon, N.P.; Crevillen-Garcia, D.; Shah, A. Screening of effective electrolyte additives for zinc-based redox flow battery systems. J. Power Sources 2019, 412, 44–54. [Google Scholar] [CrossRef]
- Trudgeon, D.P.; Li, X. The effect of electrolyte and additive concentration on zinc–nickel flow cell performance. Electrochim. Acta 2021, 367, 137479. [Google Scholar] [CrossRef]
- Trudgeon, D.P.; Loh, A.; Ullah, H.; Li, X.; Yufit, V.; Brandon, N.; Liu, M.; Kong, L. The influence of zinc electrode substrate, electrolyte flow rate and current density on zinc-nickel flow cell performance. Electrochim. Acta 2021, 373, 137890. [Google Scholar] [CrossRef]
- Oniciu, L.; Mureşan, L. Some fundamental aspects of levelling and brightening in metal electrodeposition. J. Appl. Electrochem. 1991, 21, 565. [Google Scholar] [CrossRef]
- Wang, R.Y.; Kirk, D.W.; Zhang, G.X. Effects of Deposition Conditions on the Morphology of Zinc Deposits from Alkaline Zincate Solutions. J. Electrochem. Soc. 2006, 153, C357–C364. [Google Scholar] [CrossRef]
- Wilcox, G.; Mitchell, P. Electrolyte additives for zinc-anoded secondary cells II. Quaternary ammonium compounds. J. Power Sources 1990, 32, 31–41. [Google Scholar] [CrossRef]
- Lan, C.J.; Lee, C.Y.; Chin, T.S. Tetra-alkyl ammonium hydroxides as inhibitors of Zn dendrite in Zn-based secondary batteries. Electrochim. Acta 2007, 52, 5407. [Google Scholar]
- Pletcher, D.; Zhou, H.; Kear, G.; Low, C.T.J.; Walsh, F.C.; Wills, R.G.A. A novel flow battery—A lead-acid battery based on an electrolyte with soluble lead(II): V. studies of the lead negative electrode. J. Power Sources 2008, 180, 621. [Google Scholar] [CrossRef]
- Hazza, A.; Pletcher, D.; Wills, R. A novel flow battery—A lead acid battery based on an electrolyte with soluble lead(II): IV. the influence of additives. J. Power Sources 2005, 149, 103. [Google Scholar] [CrossRef]
- Sonneveld, P.; Visscher, W.; Barendrecht, E. Nucleation and growth of zinc on a glassy carbon electrode from a zincate solution. Electrochim. Acta 1992, 37, 1199–1205. [Google Scholar] [CrossRef]
- Ito, Y.; Wei, X.; Desai, D.; Steingart, D.; Banerjee, S. An indicator of zinc morphology transition in flowing alkaline electrolyte. J. Power Sources 2012, 211, 119–128. [Google Scholar] [CrossRef]
- Desai, D.; Wei, X.; Steingart, D.A.; Banerjee, S. Electrodeposition of preferentially oriented zinc for flow-assisted alkaline batteries. J. Power Sources 2014, 256, 145–152. [Google Scholar] [CrossRef]
- Mai, N.T.; Thuy, T.T.; Mott, D.M.; Maenosono, S. Chemical synthesis of blue-emitting metallic zinc nano-hexagons. CrystEngComm 2013, 15, 6606–6610. [Google Scholar] [CrossRef]
- Otani, T.; Fukunaka, Y.; Homma, T. Effect of lead and tin additives on surface morphology evolution of electrodeposited zinc. Electrochim. Acta 2017, 242, 364–372. [Google Scholar] [CrossRef]
- Iwashita, N.; Park, C.R.; Fujimoto, H.; Shiraishi, M.; Inagaki, M. Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon 2004, 42, 701. [Google Scholar] [CrossRef]
- Leung, P.; Ponce-De-León, C.; Recio, F.; Herrasti, P.; Walsh, F.C. Corrosion of the zinc negative electrode of zinc–cerium hybrid redox flow batteries in methanesulfonic acid. J. Appl. Electrochem. 2014, 44, 1025–1035. [Google Scholar] [CrossRef]
- Ito, Y.; Nyce, M.; Plivelich, R.; Klein, M.; Banerjee, S. Gas evolution in a flow-assisted zinc–nickel oxide battery. J. Power Sources 2011, 196, 6583–6587. [Google Scholar] [CrossRef]


| Additive Name | Short Name | Cation Molecular Formula | Cation Structure |
|---|---|---|---|
| Tetramethylammonium bromide | TMAB | [CH3]N+[CH3]3 | 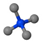 |
| Tetramethylammonium hydroxide | TMAH | ||
| Tetraethylammonium bromide | TEAB | [CH3CH2]N+[CH2CH3]3 | 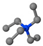 |
| Tetraethylammonium hydroxide | TEAH | ||
| Tetrapropylammonium bromide | TPAB | [CH3(CH2)2]N+[(CH2)2CH3]3 |  |
| Tetrapropylammonium hydroxide | TPAH | ||
| Benzyltrimethylammonium bromide | BTMAB | [C6H5CH2]N+[CH3]3 | 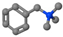 |
| Benzyltrimethylammonium hydroxide | BTMAH | ||
| Hexadecyltrimethylammonium bromide | HDTMAB | [CH3(CH2)15]N+[CH3]3 | 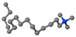 |
| Hexadecyltrimethylammonium hydroxide | HDTMAH |
| CV Capacity Loss (%)/1st Cycle CE Loss (%)/Cycling CE Decay Rate (% per Cycle) | |||
|---|---|---|---|
| No Additive | 10.0/6.3/3.8 | ||
| Additives | 1.5 mM | 5 mM | 15 mM |
| TMAB | NA | 16.2/10.8/22.8 | 10.1/11.4/13.5 |
| TMAH | NA | 11.3/11.4/21.3 | 16.9/17.1/13.8 |
| TEAB | NA | 7.0/4.0/1.3 | 1.6/7.9/0.9 |
| TEAH | NA | 12.5/3.8/1.1 | 2.2/6.4/1.7 |
| TPAB | 0.9/8.8/0.8 | 1.1/3.9/2.8 | 0.4/4.9/5.3 |
| TPAH | 0.7/2.5/1.7 | 1.2/2.5/4.4 | 0.6/1.4/3.6 |
| BTMAB | 8.4/6.5/2.0 | 4.0/3.2/1.5 | 0.7/2.6/2.7 |
| BTMAH | 3.5/3.6/0.8 | 4.6/2.4/1.5 | 1.0/2.6/1.8 |
| HDTMAB | 4.8/2.6/1.9 | 2.5/1.1/0.8 | NA |
| HDTMAH | 5.4/1.4/1.0 | 9.44/1.3/1.9 | NA |
| XRD Crystallite Size/nm | |||
|---|---|---|---|
| No Additive | 169 | ||
| Additives | 1.5 mM | 5 mM | 15 mM |
| TMAB | NA | 160 | 162 |
| TMAH | NA | 165 | 153 |
| TEAB | NA | 138 | 81 |
| TEAH | NA | 133 | 91 |
| TPAB | 54 | 47 | 48 |
| TPAH | 50 | 46 | 46 |
| BTMAB | 138 | 100 | 70 |
| BTMAH | 136 | 101 | 67 |
| HDTMAB | 69 | 50 | NA |
| HDTMAH | 66 | 51 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, K.; Trudgeon, D.; Li, X.; Yufit, V.; Chakrabarti, B.; Brandon, N.; Shah, A. Study of Quaternary Ammonium Additives towards High-Rate Zinc Deposition and Dissolution Cycling for Application in Zinc-Based Rechargeable Batteries. Batteries 2022, 8, 106. https://doi.org/10.3390/batteries8090106
Qiu K, Trudgeon D, Li X, Yufit V, Chakrabarti B, Brandon N, Shah A. Study of Quaternary Ammonium Additives towards High-Rate Zinc Deposition and Dissolution Cycling for Application in Zinc-Based Rechargeable Batteries. Batteries. 2022; 8(9):106. https://doi.org/10.3390/batteries8090106
Chicago/Turabian StyleQiu, Kaipei, David Trudgeon, Xiaohong Li, Vladimir Yufit, Barun Chakrabarti, Nigel Brandon, and Akeel Shah. 2022. "Study of Quaternary Ammonium Additives towards High-Rate Zinc Deposition and Dissolution Cycling for Application in Zinc-Based Rechargeable Batteries" Batteries 8, no. 9: 106. https://doi.org/10.3390/batteries8090106
APA StyleQiu, K., Trudgeon, D., Li, X., Yufit, V., Chakrabarti, B., Brandon, N., & Shah, A. (2022). Study of Quaternary Ammonium Additives towards High-Rate Zinc Deposition and Dissolution Cycling for Application in Zinc-Based Rechargeable Batteries. Batteries, 8(9), 106. https://doi.org/10.3390/batteries8090106








