Influence of Breathing and Swelling on the Jelly-Roll Case Gap of Cylindrical Lithium-Ion Battery Cells
Abstract
:1. Introduction
- Cell-to-cell variations;
- Differences between cell types;
- Dependence on SOC;
- Dependence on SOH.
2. Experimental Method
2.1. Cell Types and Test Procedure
2.2. Electrical Test Conditions
2.3. CT Scanner and Settings
2.4. Data Analysis
3. Results
3.1. Cell-to-Cell Variation and Influence of Position
3.2. Analysis of Various Cell Types
3.3. Analysis of the Influence of Cyclic Aging on the Jelly-Roll Gap
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | Constant Current |
| CT | Computed Tomography |
| CV | Constant Voltage |
| LFP | Lithium Ferro–Phosphate |
| NNP | Nickel Oxide-Based New Platform |
| SEI | Solid Electrolyte Interphase |
| SOC | State of Charge |
| SOH | State of Health |
References
- Sauerteig, D.; Ivanov, S.; Reinshagen, H.; Bund, A. Reversible and irreversible dilation of lithium-ion battery electrodes investigated by in-situ dilatometry. J. Power Sources 2017, 342, 939–946. [Google Scholar] [CrossRef]
- Sauerteig, D. Implementierung und Parametrierung Eines Physikalischen Simulationsmodells Einer Lithium-Ionen Zelle zur Analyse Elektrochemisch-Mechanischer Wechselwirkungen. Ph.D. Thesis, Technische Universität Ilmenau, Ilmenau, Germany, 2018. [Google Scholar]
- Park, J.; Kalnaus, S.; Han, S.; Lee, Y.K.; Less, G.B.; Dudney, N.J.; Daniel, C.; Sastry, A.M. In situ atomic force microscopy studies on lithium (de)intercalation-induced morphology changes in LixCoO2 micro-machined thin film electrodes. J. Power Sources 2013, 222, 417–425. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.M.; Ahn, S. Battery dimensional changes occurring during charge/discharge cycles—Thin rectangular lithium ion and polymer cells. J. Power Sources 2003, 119–121, 833–837. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Cui, H.; Liu, J.; Huo, H.; Wang, L.; Gao, Y.; He, X. Insight into the electrochemical behaviors of NCM811|SiO–Gr pouch battery through thickness variation. Energy Environ. Mater. 2022. Accepted. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Fritz, H.P. The Electrochemistry of Black Carbons. Angew. Chem. Int. Ed. Eng. 1983, 22, 950–975. [Google Scholar] [CrossRef]
- Ohzuku, T.; Iwakoshi, Y.; Sawai, K. Formation of Lithium-Graphite Intercalation Compounds in Nonaqueous Electrolytes and Their Application as a Negative Electrode for a Lithium Ion (Shuttlecock) Cell. J. Electrochem. Soc. 1993, 140, 2490. [Google Scholar] [CrossRef]
- Billaud, D.; Henry, F.X.; Lelaurain, M.; Willmann, P. Revisited structures of dense and dilute stage II lithium-graphite intercalation compounds. J. Phys. Chem. Solids 1995, 57, 775–781. [Google Scholar] [CrossRef]
- Schweidler, S.; de Biasi, L.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Volume Changes of Graphite Anodes Revisited: A Combined Operando X-ray Diffraction and In Situ Pressure Analysis Study. J. Phys. Chem. C 2018, 122, 8829–8835. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Luo, J.; Dai, C.Y.; Wang, Z.; Liu, K.; Mao, W.G.; Fang, D.N.; Chen, X. In-situ measurements of mechanical and volume change of LiCoO2 lithium-ion batteries during repeated charge–discharge cycling by using digital image correlation. Measurement 2016, 94, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.Y.; Siegel, J.B.; Secondo, L.; Kim, S.U.; Samad, N.A.; Qin, J.; Anderson, D.; Garikipati, K.; Knobloch, A.; Epureanu, B.I.; et al. Rate dependence of swelling in lithium-ion cells. J. Power Sources 2014, 267, 197–202. [Google Scholar] [CrossRef]
- Gor, G.Y.; Cannarella, J.; Leng, C.Z.; Vishnyakov, A.; Arnold, C.B. Swelling and softening of lithium-ion battery separators in electrolyte solvents. J. Power Sources 2015, 294, 167–172. [Google Scholar] [CrossRef]
- Qian, J.; Wiener, C.G.; Zhu, Y.; Vogt, B.D. Swelling and plasticization of polymeric binders by Li-containing carbonate electrolytes using quartz crystal microbalance with dissipation. Polymer 2018, 143, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Spingler, F.B.; Patel, Y.; Offer, G.J.; Jossen, A. Localized Swelling Inhomogeneity Detection in Lithium Ion Cells Using Multi-Dimensional Laser Scanning. J. Ofthe Electrochem. Soc. 2019, 166, A27–A34. [Google Scholar] [CrossRef]
- Willenberg, L.; Dechent, P.; Fuchs, G.; Teuber, M.; Eckert, M.; Graff, M.; Kürten, N.; Sauer, D.U.; Figgemeier, E. The Development of Jelly Roll Deformation in 18650 Lithium-Ion Batteries at Low State of Charge. J. Electrochem. Soc. 2020, 167, 120502. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Llewellyn, A.V.; Leach, A.S.; Kok, M.D.R.; Tan, C.; Jervis, R.; Brett, D.J.L.; Shearing, P.R. Resolving Li-Ion Battery Electrode Particles Using Rapid Lab-Based X-Ray Nano-Computed Tomography for High-Throughput Quantification. Adv. Sci. 2020, 7, 2000362. [Google Scholar] [CrossRef]
- Le Houx, J.; Kramer, D. X-ray tomography for lithium ion battery electrode characterisation—A review. Energy Rep. 2021, 7, 9–14. [Google Scholar] [CrossRef]
- Boyce, A.M.; Martínez-Pañeda, E.; Wade, A.; Zhang, Y.S.; Bailey, J.J.; Heenan, T.M.; Brett, D.J.; Shearing, P.R. Cracking predictions of lithium-ion battery electrodes by X-ray computed tomography and modelling. J. Power Sources 2022, 526, 231119. [Google Scholar] [CrossRef]
- Li, W.; Crompton, K.R.; Hacker, C.; Ostanek, J.K. Comparison of Current Interrupt Device and Vent Design for 18650 Format Lithium-ion Battery Caps. J. Energy Storage 2020, 32, 101890. [Google Scholar] [CrossRef]
- Berg, P.; Spielbauer, M.; Tillinger, M.; Merkel, M.; Schoenfuss, M.; Bohlen, O.; Jossen, A. Durability of lithium-ion 18650 cells under random vibration load with respect to the inner cell design. J. Energy Storage 2020, 31, 101499. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Y.; Zhao, Z.; Zou, Y.; Luo, D. Investigation of the swelling failure of lithium-ion battery packs at low temperatures using 2D/3D X-ray computed tomography. Electrochim. Acta 2019, 305, 65–71. [Google Scholar] [CrossRef]
- Louli, A.J.; Eldesoky, A.; Weber, R.; Genovese, M.; Coon, M.; deGooyer, J.; Deng, Z.; White, R.T.; Lee, J.; Rodgers, T.; et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Waldmann, T.; Gorse, S.; Samtleben, T.; Schneider, G.; Knoblauch, V.; Wohlfahrt-Mehrens, M. A Mechanical Aging Mechanism in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1742–A1747. [Google Scholar] [CrossRef]
- Pfrang, A.; Kersys, A.; Kriston, A.; Sauer, D.U.; Rahe, C.; Käbitz, S.; Figgemeier, E. Geometrical Inhomogeneities as Cause of Mechanical Failure in Commercial 18650 Lithium Ion Cells. J. Electrochem. Soc. 2019, 166, A3745–A3752. [Google Scholar] [CrossRef]
- Carter, R.; Klein, E.J.; Atkinson, R.W.; Love, C.T. Mechanical collapse as primary degradation mode in mandrel-free 18650 Li-ion cells operated at 0 °C. J. Power Sources 2019, 437, 226820. [Google Scholar] [CrossRef]
- Brand, M.J.; Schuster, S.F.; Bach, T.; Fleder, E.; Stelz, M.; Gläser, S.; Müller, J.; Sextl, G.; Jossen, A. Effects of vibrations and shocks on lithium-ion cells. J. Power Sources 2015, 288, 62–69. [Google Scholar] [CrossRef]
- Sahraei, E.; Kahn, M.; Meier, J.; Wierzbicki, T. Modelling of cracks developed in lithium-ion cells under mechanical loading. RSC Adv. 2015, 5, 80369–80380. [Google Scholar] [CrossRef]
- Zhu, J.; Li, W.; Wierzbicki, T.; Xia, Y.; Harding, J. Deformation and failure of lithium-ion batteries treated as a discrete layered structure. Int. J. Plast. 2019, 121, 293–311. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Hunt, I.; Mason, T.J.; Millichamp, J.; Di Michiel, M.; Offer, G.J.; Hinds, G.; et al. In-operando high-speed tomography of lithium-ion batteries during thermal runaway. Nat. Commun. 2015, 6, 6924. [Google Scholar] [CrossRef] [Green Version]
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Bailey, J.J.; Malik, R.; Vo, N.T.; Magdysyuk, O.V.; et al. Characterising thermal runaway within lithium-ion cells by inducing and monitoring internal short circuits. Energy Environ. Sci. 2017, 10, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Pham, M.T.; Darst, J.J.; Walker, W.Q.; Heenan, T.M.; Patel, D.; Iacoviello, F.; Rack, A.; Olbinado, M.P.; Hinds, G.; Brett, D.J.; et al. Prevention of lithium-ion battery thermal runaway using polymer-substrate current collectors. Cell Rep. Phys. Sci. 2021, 2, 100360. [Google Scholar] [CrossRef]
- Waldmann, T.; Scurtu, R.G.; Richter, K.; Wohlfahrt-Mehrens, M. 18650 vs. 21700 Li-ion cells—A direct comparison of electrochemical, thermal, and geometrical properties. J. Power Sources 2020, 472, 228614. [Google Scholar] [CrossRef]
- Heenan, T.M.; Tan, C.; Hack, J.; Brett, D.J.; Shearing, P.R. Developments in X-ray tomography characterization for electrochemical devices. Mater. Today 2019, 31, 69–85. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Jnawali, A.; Kok, M.D.R.; Tranter, T.G.; Tan, C.; Dimitrijevic, A.; Jervis, R.; Brett, D.J.L.; Shearing, P.R. An Advanced Microstructural and Electrochemical Datasheet on 18650 Li-Ion Batteries with Nickel-Rich NMC811 Cathodes and Graphite-Silicon Anodes. J. Electrochem. Soc. 2020, 167, 140530. [Google Scholar] [CrossRef]
- Wu, Y.; Saxena, S.; Xing, Y.; Wang, Y.; Li, C.; Yung, W.; Pecht, M. Analysis of Manufacturing-Induced Defects and Structural Deformations in Lithium-Ion Batteries Using Computed Tomography. Energies 2018, 11, 925. [Google Scholar] [CrossRef] [Green Version]
- Kok, M.D.R.; Robinson, J.B.; Weaving, J.S.; Jnawali, A.; Pham, M.; Iacoviello, F.; Brett, D.J.L.; Shearing, P.R. Virtual unrolling of spirally-wound lithium-ion cells for correlative degradation studies and predictive fault detection. Sustain. Energy Fuels 2019, 3, 2972–2976. [Google Scholar] [CrossRef] [Green Version]
- Ziesche, R.F.; Arlt, T.; Finegan, D.P.; Heenan, T.M.M.; Tengattini, A.; Baum, D.; Kardjilov, N.; Markötter, H.; Manke, I.; Kockelmann, W.; et al. 4D imaging of lithium-batteries using correlative neutron and X-ray tomography with a virtual unrolling technique. Nat. Commun. 2020, 11, 777. [Google Scholar] [CrossRef] [Green Version]
- Blazek, P.; Westenberger, P.; Erker, S.; Brinek, A.; Zikmund, T.; Rettenwander, D.; Wagner, N.P.; Keckes, J.; Kaiser, J.; Kazda, T.; et al. Axially and radially inhomogeneous swelling in commercial 18650 Li-ion battery cells. J. Energy Storage 2022, 52, 104563. [Google Scholar] [CrossRef]
- Tranter, T.G.; Timms, R.; Heenan, T.M.M.; Marquis, S.G.; Sulzer, V.; Jnawali, A.; Kok, M.D.R.; Please, C.P.; Chapman, S.J.; Shearing, P.R.; et al. Probing Heterogeneity in Li-Ion Batteries with Coupled Multiscale Models of Electrochemistry and Thermal Transport using Tomographic Domains. J. Electrochem. Soc. 2020, 167, 110538. [Google Scholar] [CrossRef]
- Hemmerling, J.; Guhathakurta, J.; Dettinger, F.; Fill, A.; Birke, K.P. Non-Uniform Circumferential Expansion of Cylindrical Li-Ion Cells—The Potato Effect. Batteries 2021, 7, 61. [Google Scholar] [CrossRef]
- Gaitonde, A.; Nimmagadda, A.; Marconnet, A. Measurement of interfacial thermal conductance in Lithium ion batteries. J. Power Sources 2017, 343, 431–436. [Google Scholar] [CrossRef]
- Spielbauer, M.; Soellner, J.; Berg, P.; Koch, K.; Keil, P.; Rosenmüller, C.; Bohlen, O.; Jossen, A. Experimental investigation of the impact of mechanical deformation on aging, safety and electrical behavior of 18650 lithium-ion battery cells. J. Energy Storage 2022, 55, 105564. [Google Scholar] [CrossRef]
- Lithium Werks. APR18650M1B 18650 Data Sheet; Lithium Werks: Austin, TX, USA, 2019. [Google Scholar]
- Sturm, J.; Rheinfeld, A.; Zilberman, I.; Spingler, F.B.; Kosch, S.; Frie, F.; Jossen, A. Modeling and simulation of inhomogeneities in a 18650 nickel-rich, silicon-graphite lithium-ion cell during fast charging. J. Power Sources 2019, 412, 204–223. [Google Scholar] [CrossRef] [Green Version]
- Panasonic. NCR18650B Data Sheet; Panasonic: Tokyo, Japan, 2022. [Google Scholar]
- Zhang, L.; Mu, Z.; Gao, X. Coupling Analysis and Performance Study of Commercial 18650 Lithium-Ion Batteries under Conditions of Temperature and Vibration. Energies 2018, 11, 2856. [Google Scholar] [CrossRef] [Green Version]
- Samsung. INR18650-25R Data Sheet; Samsung: Seoul, Korea, 2013. [Google Scholar]
- E-One Moli Energy. Molicell INR21700-P42A Data Sheet; E-One Moli Energy: Taiwan, China, 2019. [Google Scholar]
- Wohlfahrt-Mehrens, M.; Vogler, C.; Garche, J. Aging mechanisms of lithium cathode materials. J. Power Sources 2004, 127, 58–64. [Google Scholar] [CrossRef]
- Uddin, K.; Perera, S.; Widanage, W.; Somerville, L.; Marco, J. Characterising Lithium-Ion Battery Degradation through the Identification and Tracking of Electrochemical Battery Model Parameters. Batteries 2016, 2, 13. [Google Scholar] [CrossRef]
- Fath, J.P.; Dragicevic, D.; Bittel, L.; Nuhic, A.; Sieg, J.; Hahn, S.; Alsheimer, L.; Spier, B.; Wetzel, T. Quantification of aging mechanisms and inhomogeneity in cycled lithium-ion cells by differential voltage analysis. J. Energy Storage 2019, 25, 100813. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Lu, B.; Ning, C.; Shi, D.; Zhao, Y.; Zhang, J. Review on electrode-level fracture in lithium-ion batteries*. Chin. Phys. B 2020, 29, 026201. [Google Scholar] [CrossRef]
- Foster, J.M.; Huang, X.; Jiang, M.; Chapman, S.J.; Protas, B.; Richardson, G. Causes of binder damage in porous battery electrodes and strategies to prevent it. J. Power Sources 2017, 350, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Lin, L.; Bi, X.; Tian, L.; Yang, K.; Liu, J.; Li, M.; Chen, Z.; Lu, J.; Amine, K.; et al. In situ quantification of interphasial chemistry in Li-ion battery. Nat. Nanotechnol. 2019, 14, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Pathan, T.S.; Rashid, M.; Walker, M.; Widanage, W.D.; Kendrick, E. Active formation of Li-ion batteries and its effect on cycle life. J. Phys. Energy 2019, 1, 044003. [Google Scholar] [CrossRef]
- Günter, F.J.; Burgstaller, C.; Konwitschny, F.; Reinhart, G. Influence of the Electrolyte Quantity on Lithium-Ion Cells. J. Electrochem. Soc. 2019, 166, A1709–A1714. [Google Scholar] [CrossRef]
- Weydanz, W.J.; Reisenweber, H.; Gottschalk, A.; Schulz, M.; Knoche, T.; Reinhart, G.; Masuch, M.; Franke, J.; Gilles, R. Visualization of electrolyte filling process and influence of vacuum during filling for hard case prismatic lithium ion cells by neutron imaging to optimize the production process. J. Power Sources 2018, 380, 126–134. [Google Scholar] [CrossRef]
- Lautenschlaeger, M.P.; Prifling, B.; Kellers, B.; Weinmiller, J.; Danner, T.; Schmidt, V.; Latz, A. Understanding Electrolyte Filling of Lithium–Ion Battery Electrodes on the Pore Scale Using the Lattice Boltzmann Method. Batter. Supercaps 2022, 5, e202200090. [Google Scholar] [CrossRef]


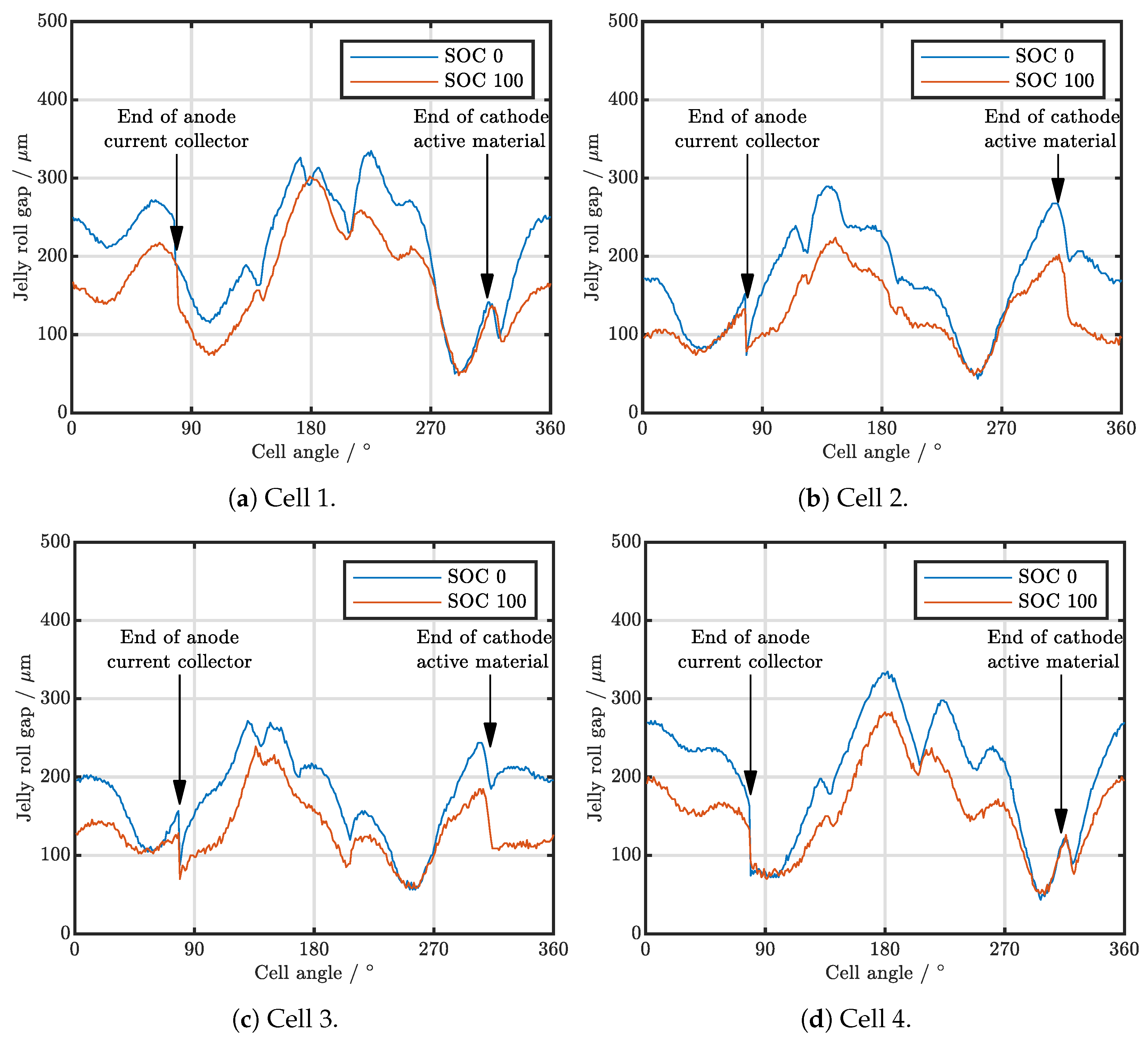
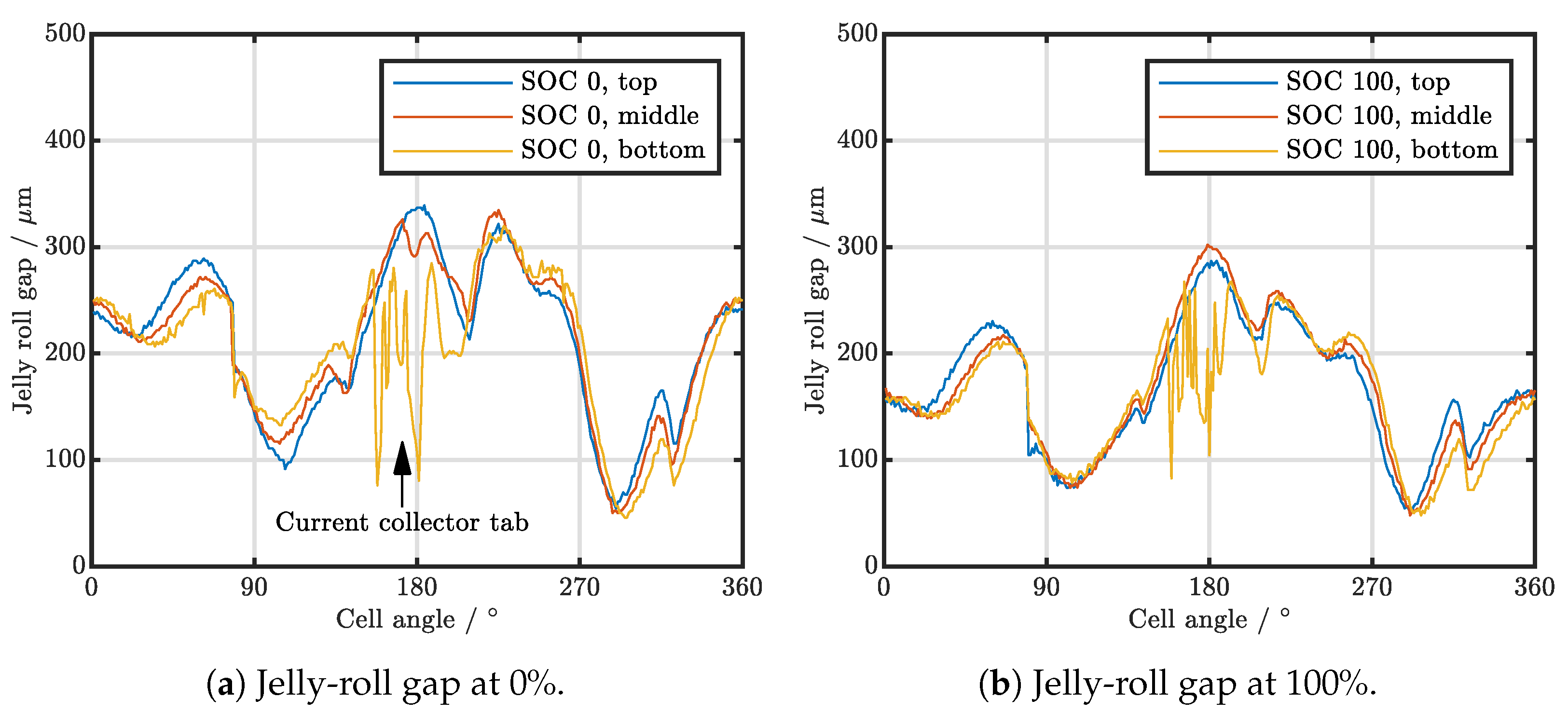
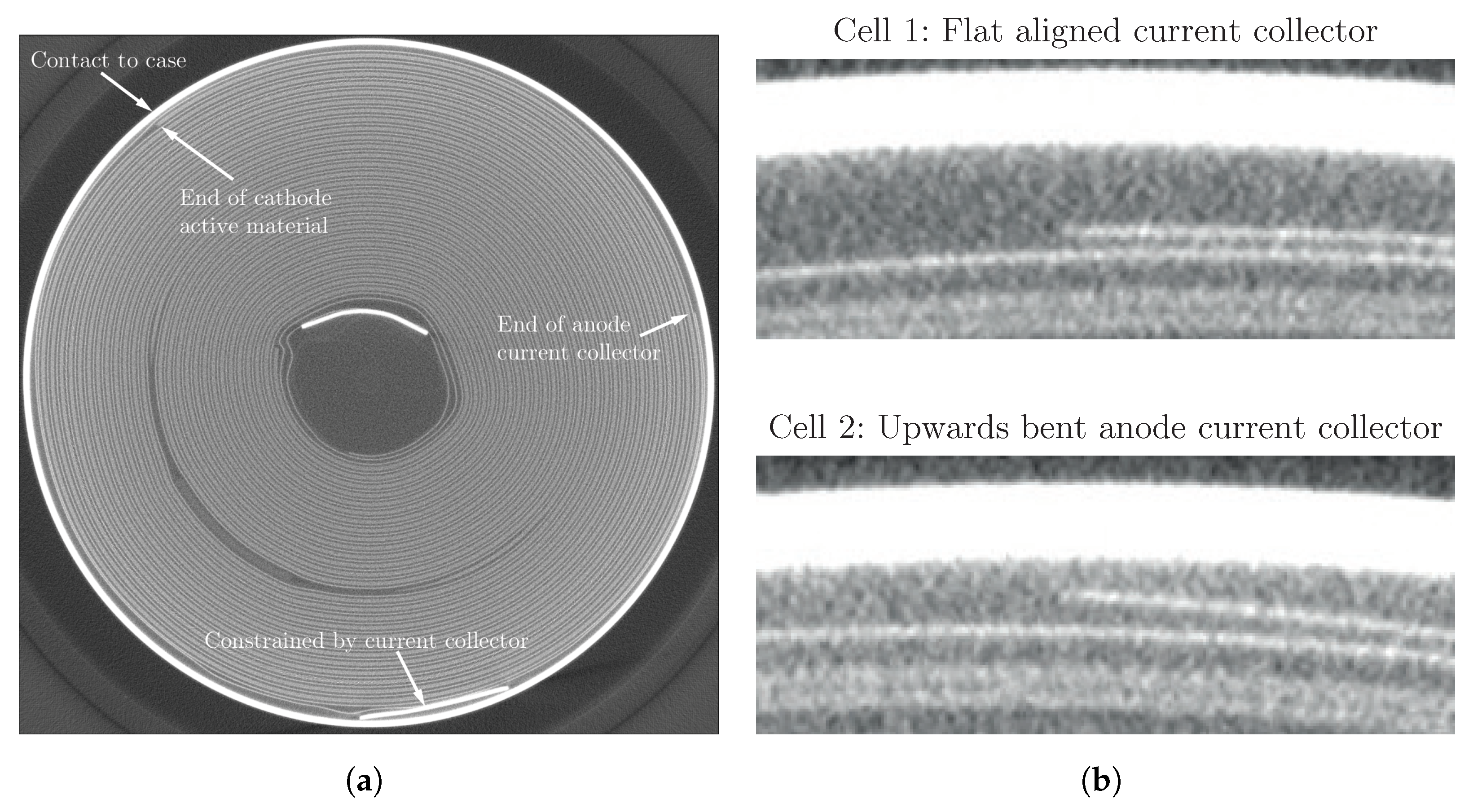
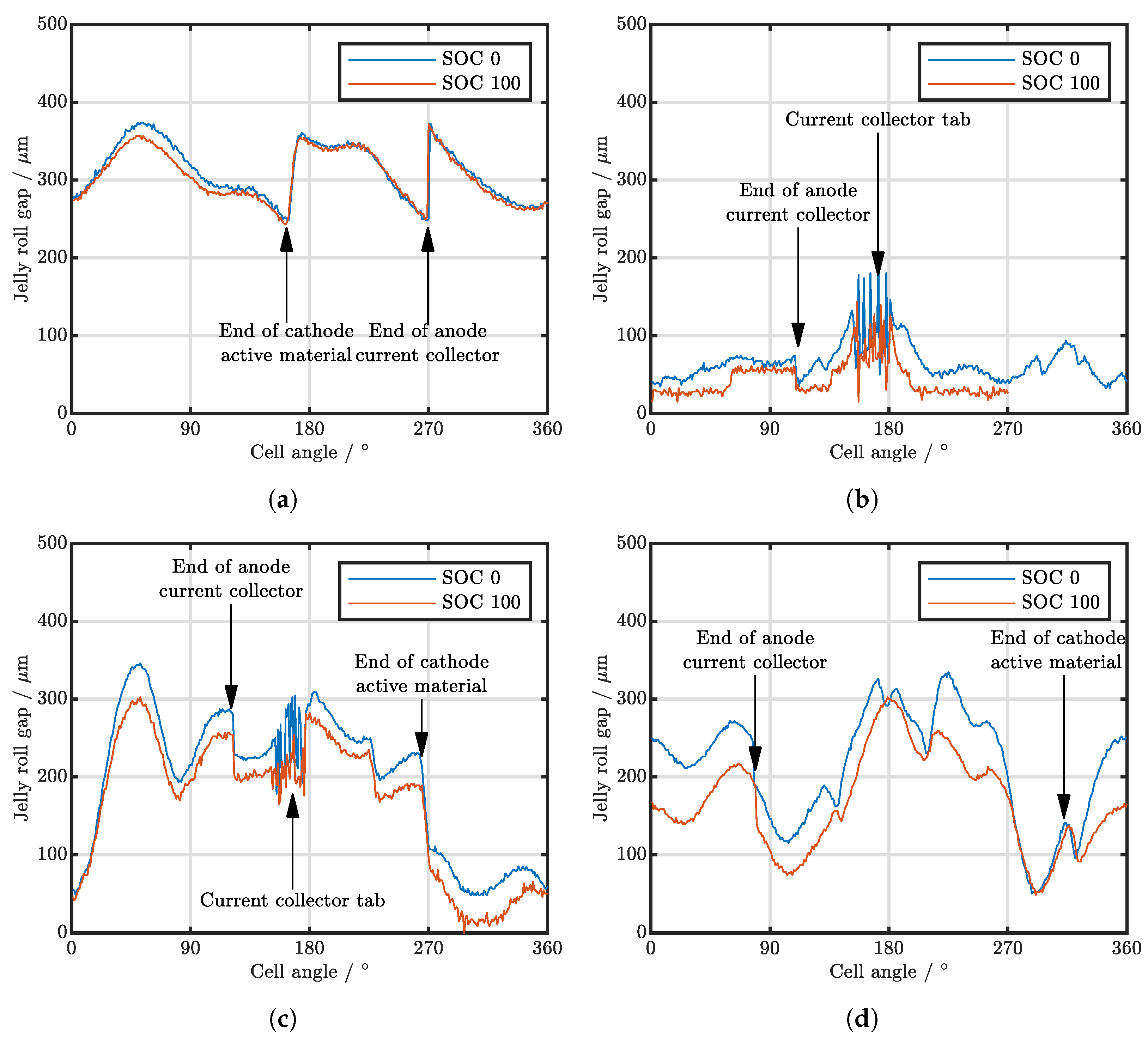
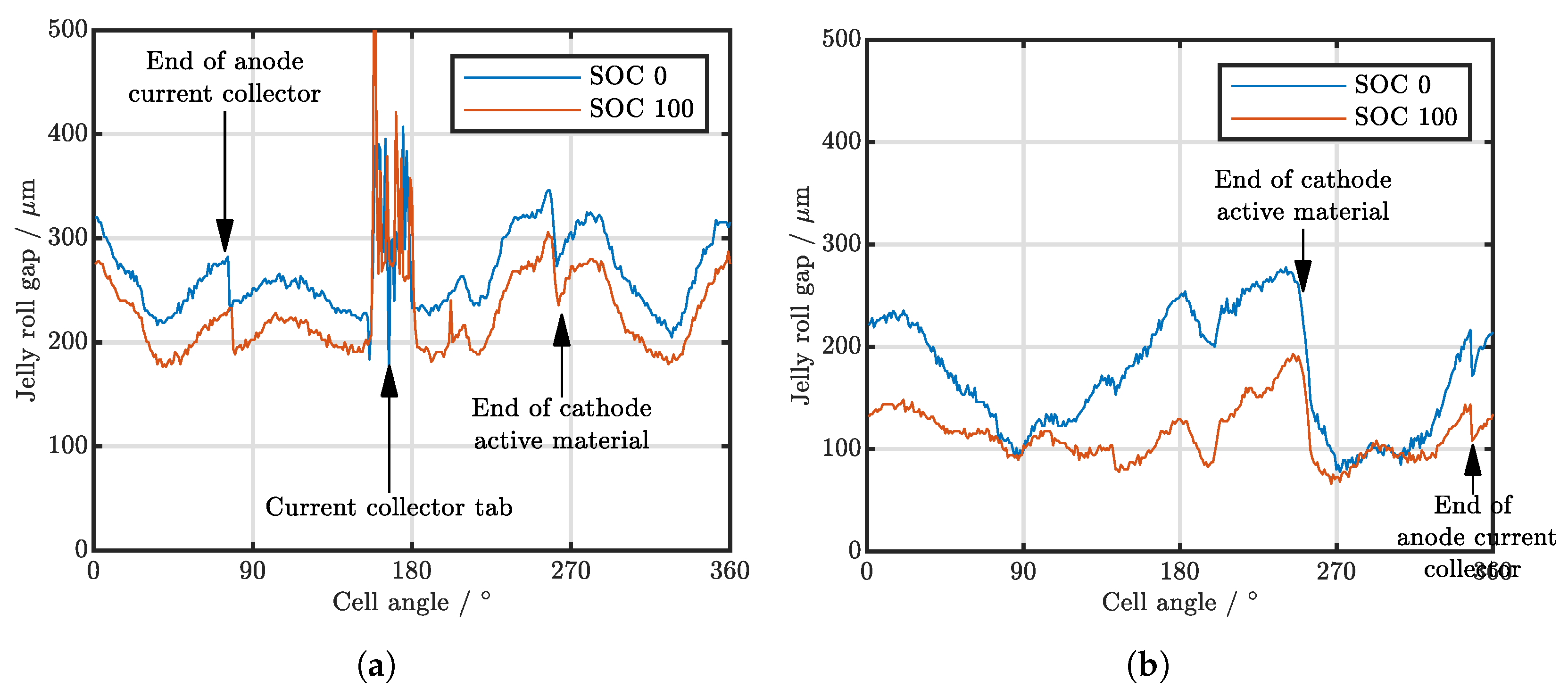
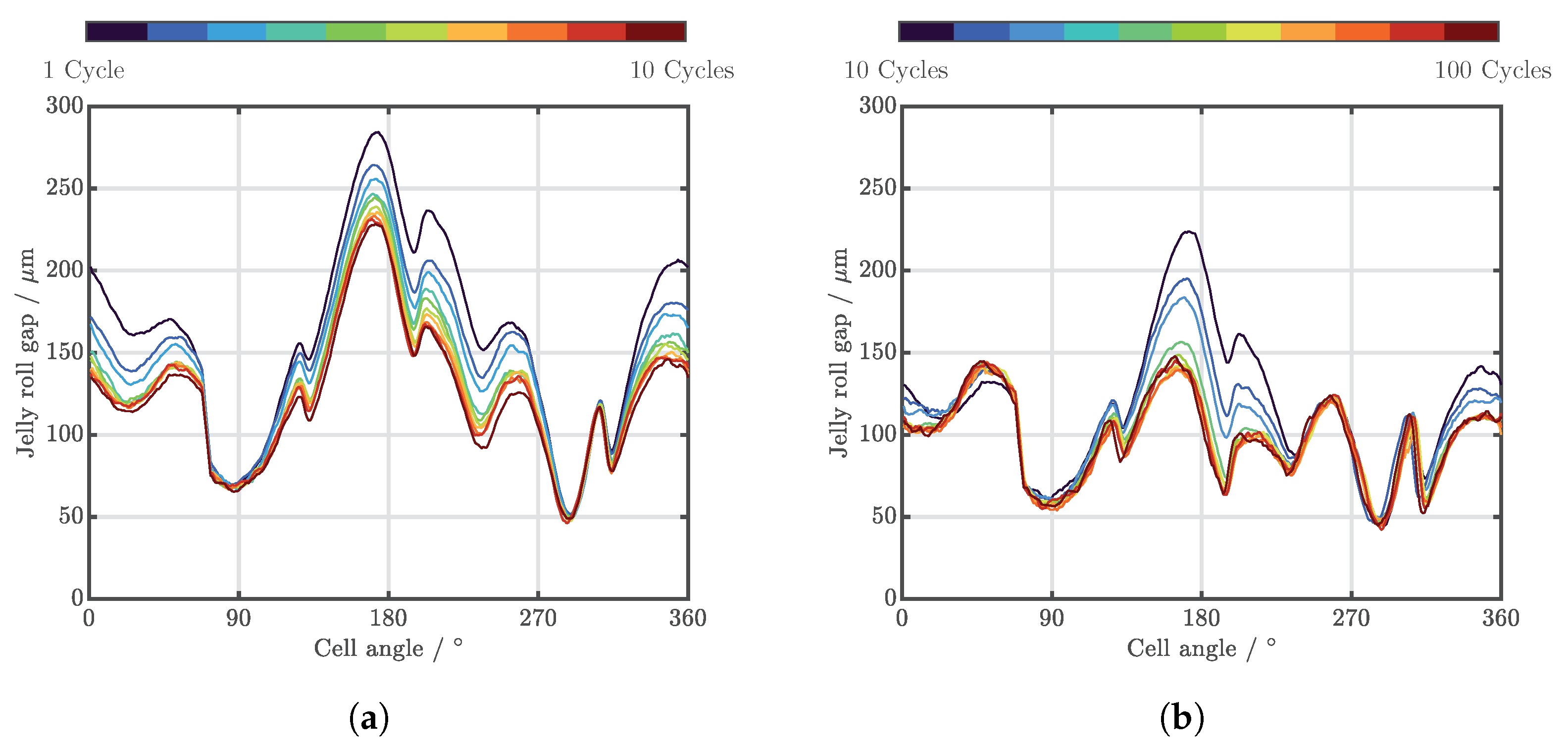
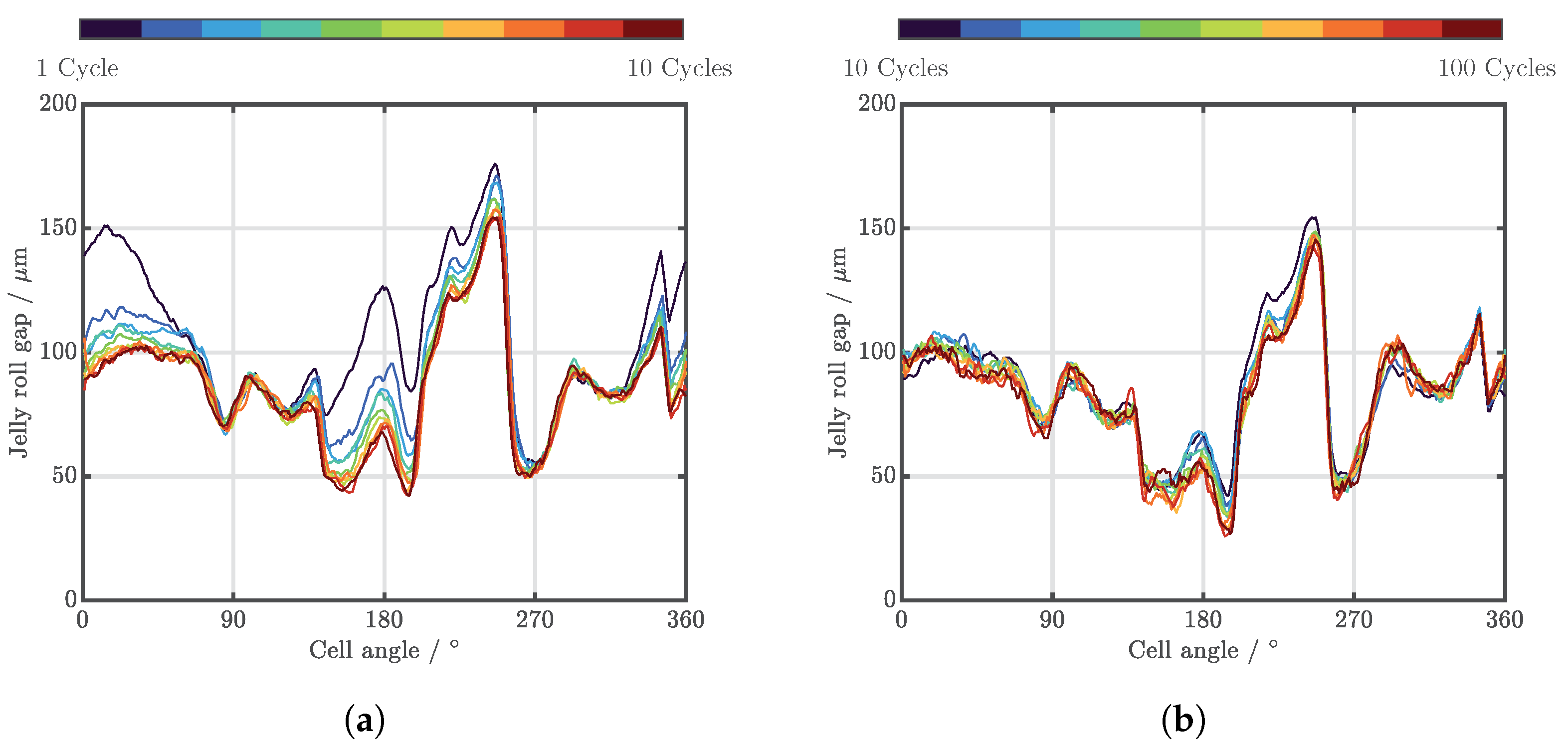
| Cell Type | Capacity | Max. Charge Current | Max. Discharge Current | Chemistry |
|---|---|---|---|---|
| A123 APR18650M1B | Ah | 5 | 30 | LFP/C [45] |
| LG INR18650MJ1 | Ah | 10 | NMC811/SiC [36,46] | |
| Murata/Sony US18650VTC4 | Ah | 4 | 30 | - |
| PAN NCR18650B | Ah | NNP [47] | ||
| PAN NCR18650PF | Ah | 2 | NCA/C [48] | |
| Samsung INR18650-25RM | Ah | 4 | 20 | NCA/C [49] |
| Molicell INR21700-P42A | Ah | 6 | 30 to 45 | - [50] |
| Samsung INR21700-40T | Ah | 6 | 35 to 45 | - |
| Cell Type | Min SOC 0 in | Max SOC 0 in | Mean SOC 0 in | Min SOC 100 in | Max SOC 100 in | Mean SOC 100 in |
|---|---|---|---|---|---|---|
| A123 APR18650M1B | 248 | 374 | 311 | 243 | 372 | 306 |
| LG INR18650MJ1 | 33 | 200 | 65 | |||
| Muarata/Sony US18650VTC4 | 48 | 346 | 196 | 9 | 302 | 167 |
| Samsung INR18650-25RM cell 1 | 50 | 335 | 214 | 48 | 302 | 168 |
| Samsung INR18650-25RM cell 2 | 43 | 289 | 171 | 47 | 224 | 126 |
| Samsung INR18650-25RM cell 3 | 56 | 271 | 176 | 59 | 239 | 130 |
| Samsung INR18650-25RM cell 4 | 43 | 335 | 203 | 50 | 283 | 157 |
| Molicell INR21700-P42A | 204 | 345 | 263 | 176 | 305 | 222 |
| Samsung INR21700-40T | 78 | 278 | 173 | 66 | 193 | 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spielbauer, M.; Steinhardt, M.; Singer, J.; Aufschläger, A.; Bohlen, O.; Jossen, A. Influence of Breathing and Swelling on the Jelly-Roll Case Gap of Cylindrical Lithium-Ion Battery Cells. Batteries 2023, 9, 6. https://doi.org/10.3390/batteries9010006
Spielbauer M, Steinhardt M, Singer J, Aufschläger A, Bohlen O, Jossen A. Influence of Breathing and Swelling on the Jelly-Roll Case Gap of Cylindrical Lithium-Ion Battery Cells. Batteries. 2023; 9(1):6. https://doi.org/10.3390/batteries9010006
Chicago/Turabian StyleSpielbauer, Markus, Marco Steinhardt, Jan Singer, Andreas Aufschläger, Oliver Bohlen, and Andreas Jossen. 2023. "Influence of Breathing and Swelling on the Jelly-Roll Case Gap of Cylindrical Lithium-Ion Battery Cells" Batteries 9, no. 1: 6. https://doi.org/10.3390/batteries9010006
APA StyleSpielbauer, M., Steinhardt, M., Singer, J., Aufschläger, A., Bohlen, O., & Jossen, A. (2023). Influence of Breathing and Swelling on the Jelly-Roll Case Gap of Cylindrical Lithium-Ion Battery Cells. Batteries, 9(1), 6. https://doi.org/10.3390/batteries9010006







