Carbon Nano-Onion-Encapsulated Ni Nanoparticles for High-Performance Lithium-Ion Capacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Preparation
2.3. LIC Pouch Cells’ Preparation
2.4. Material Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
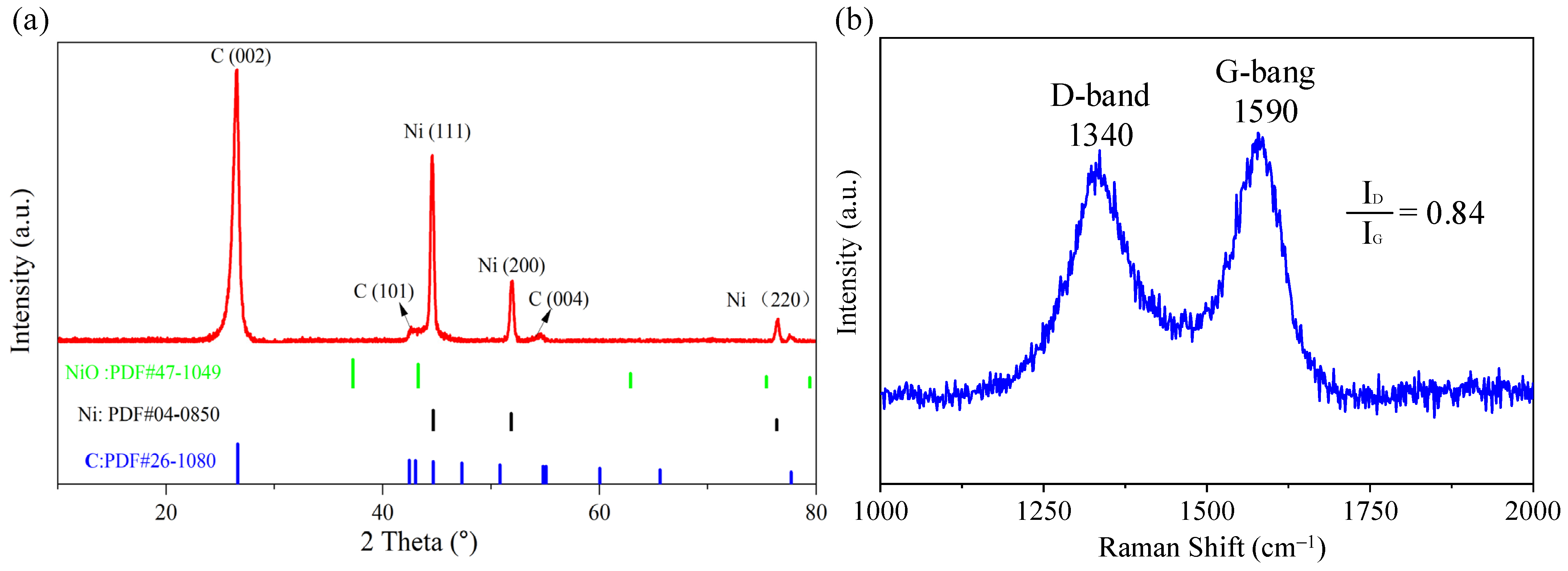
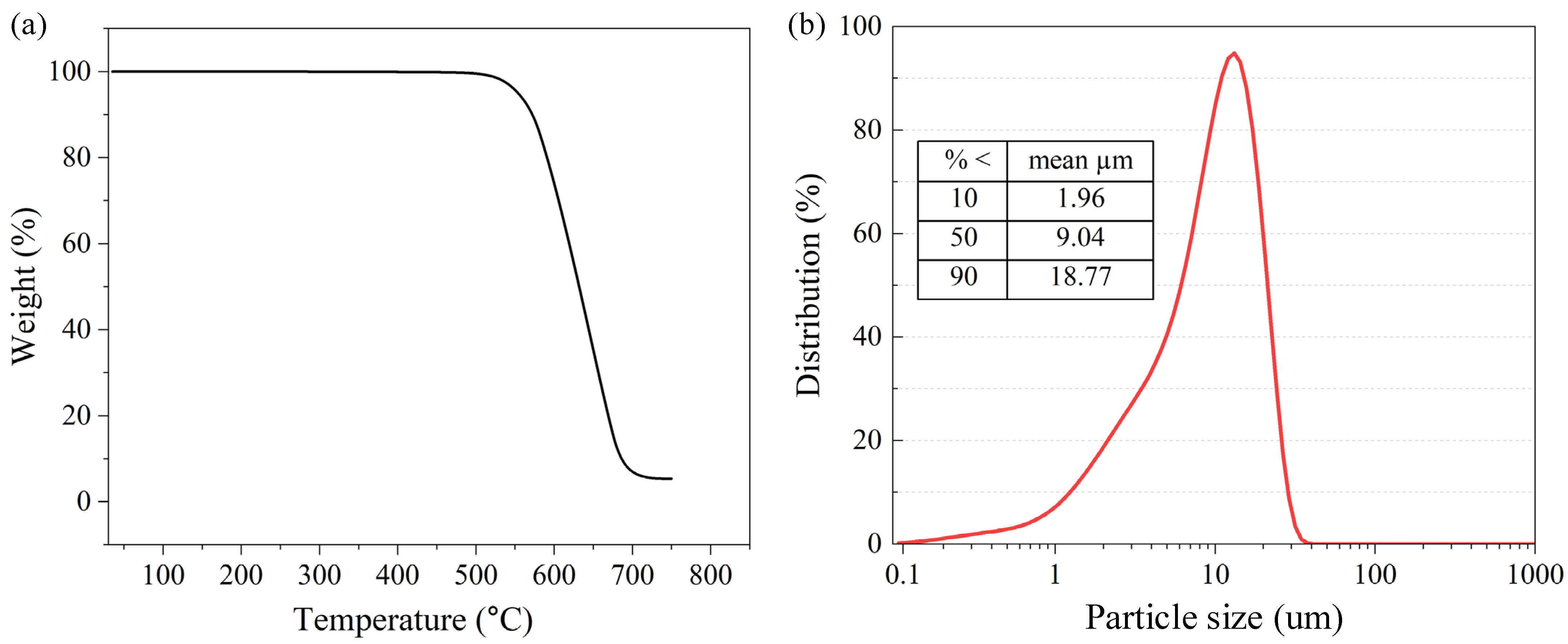
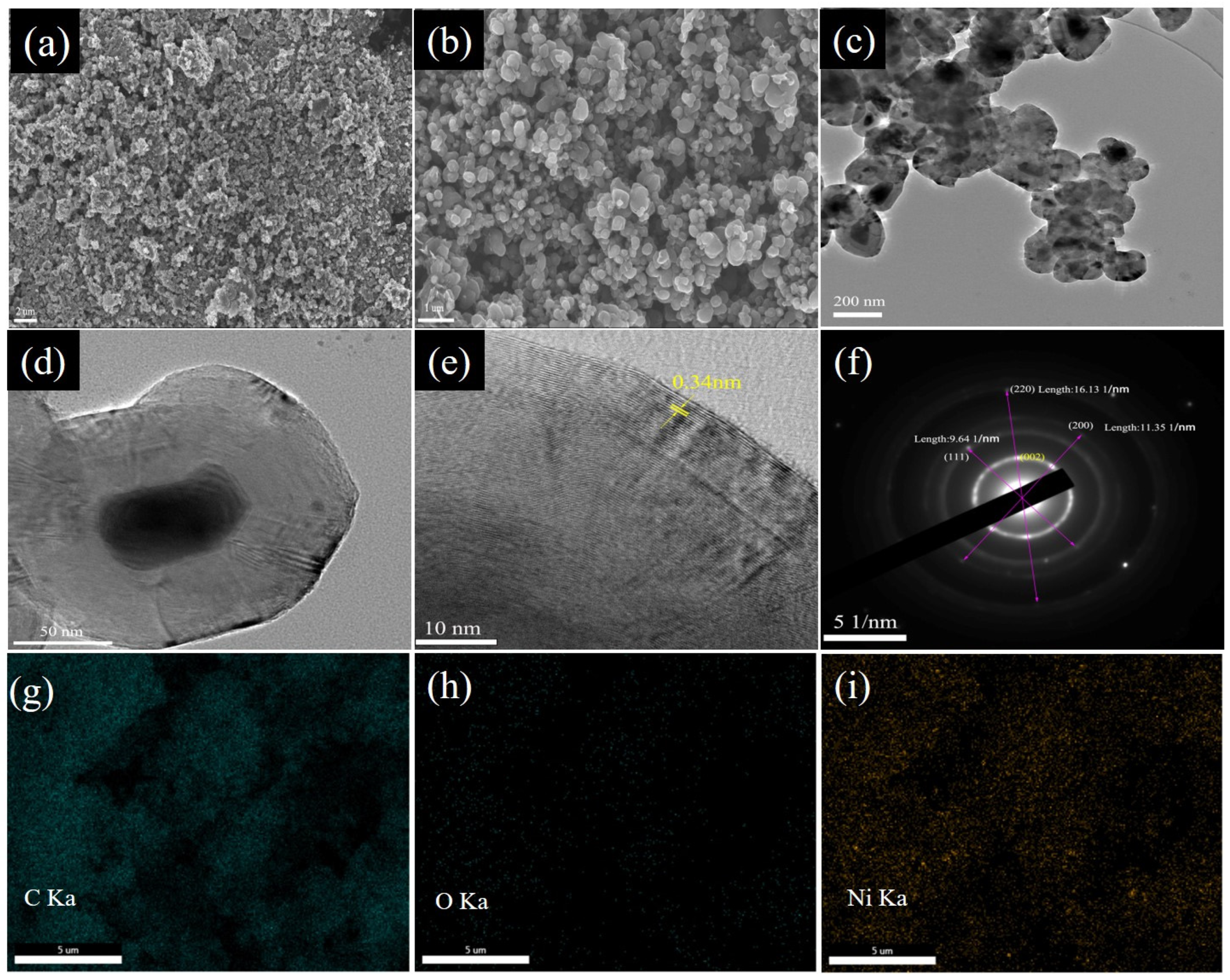
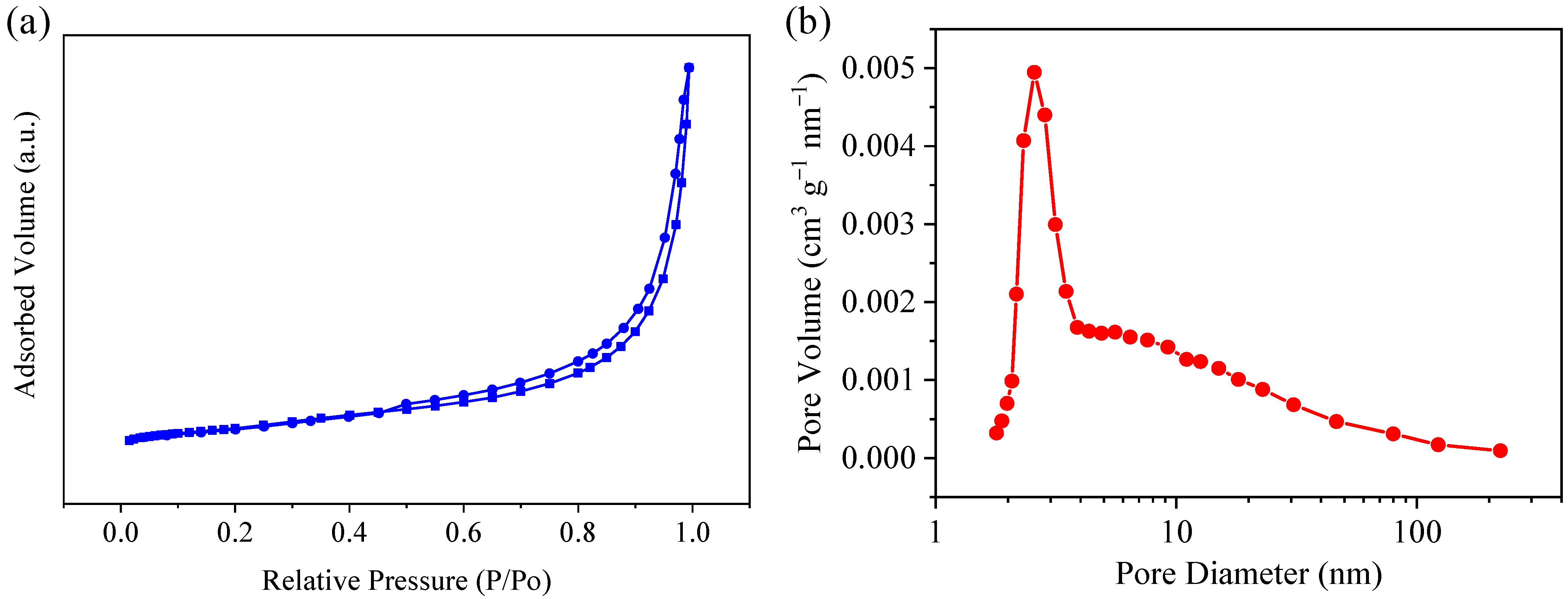
3.1. Morphology and Structure Analysis of Ni@CNOs
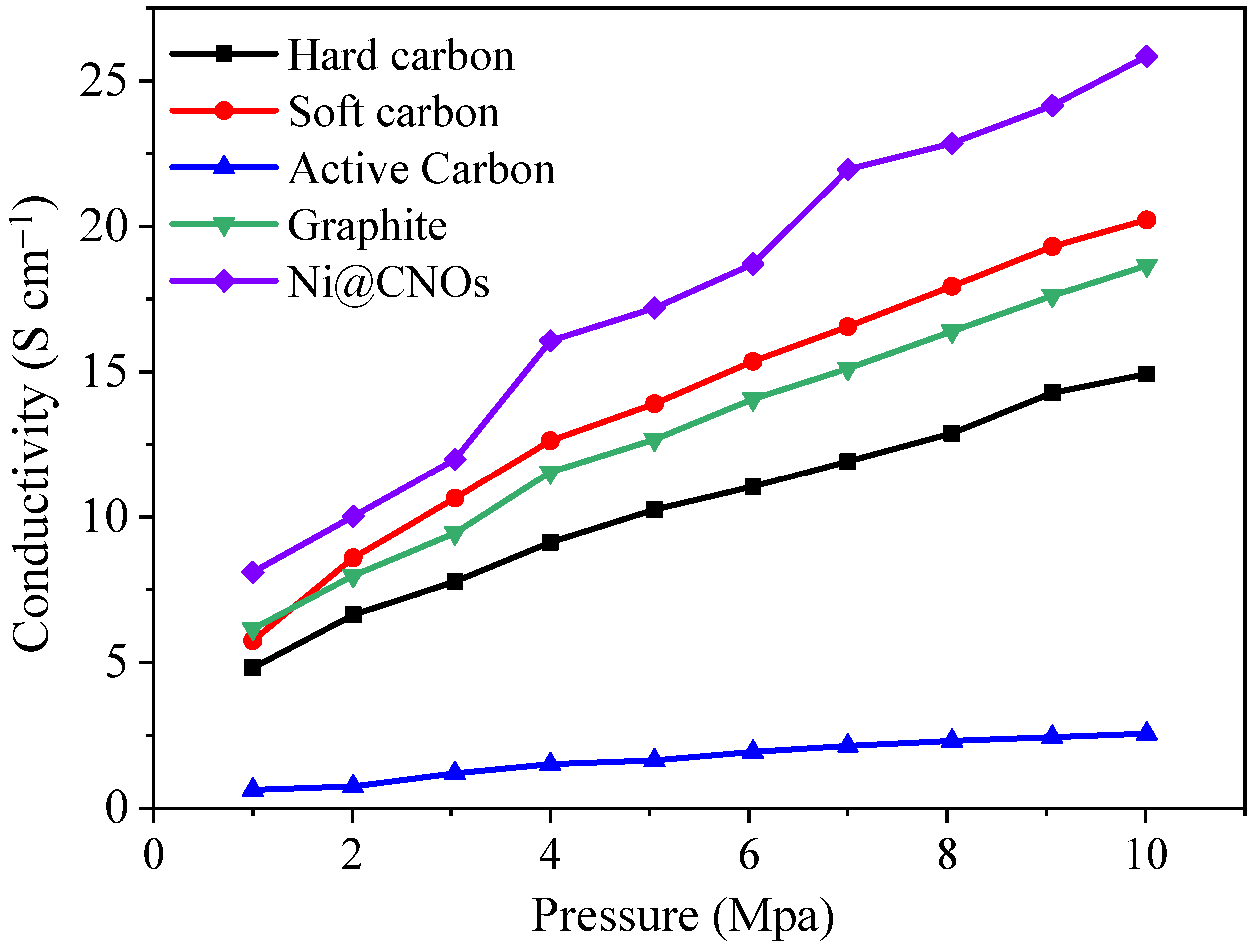
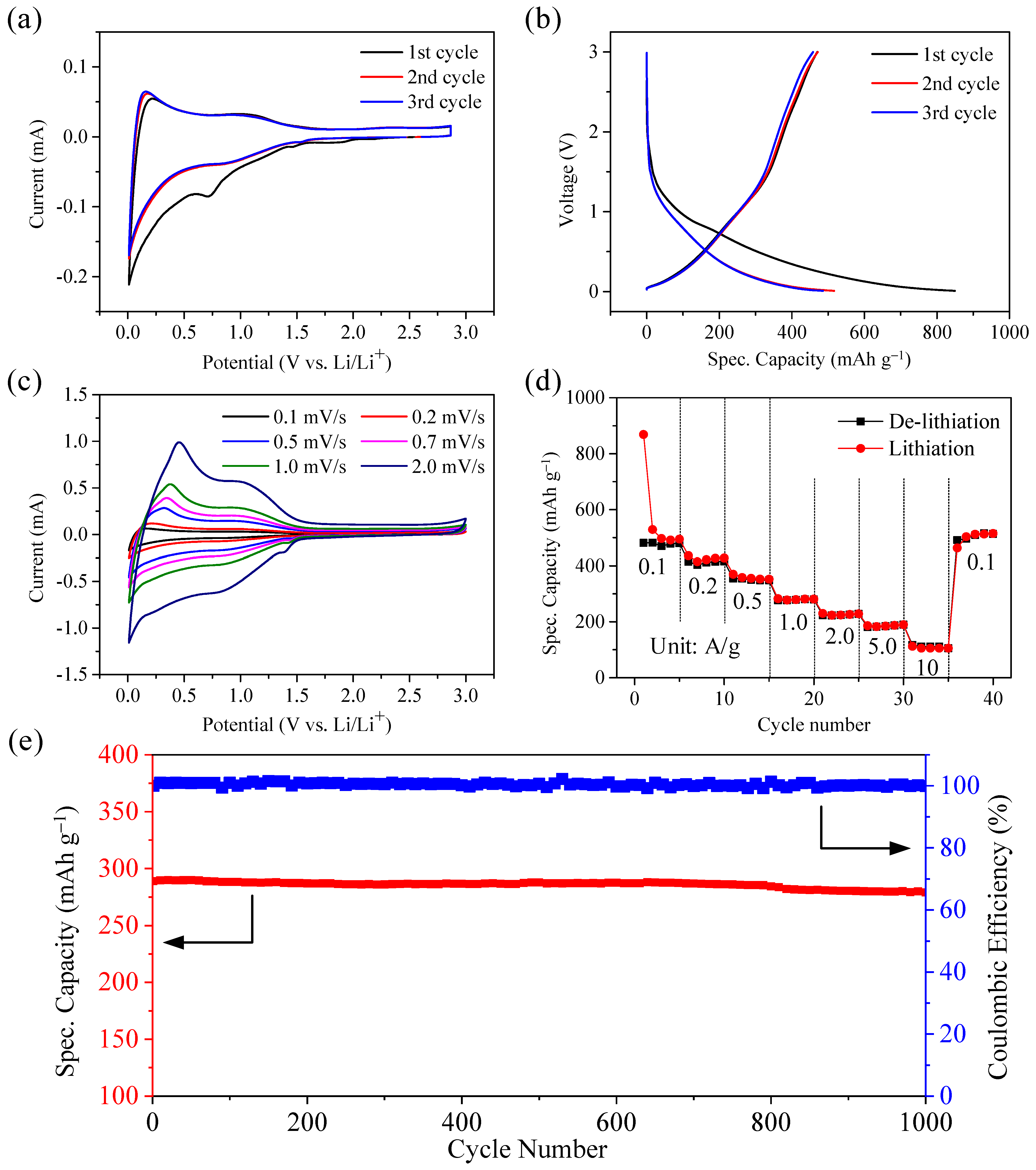
3.2. Electrochemical Performance of Ni@CNOs
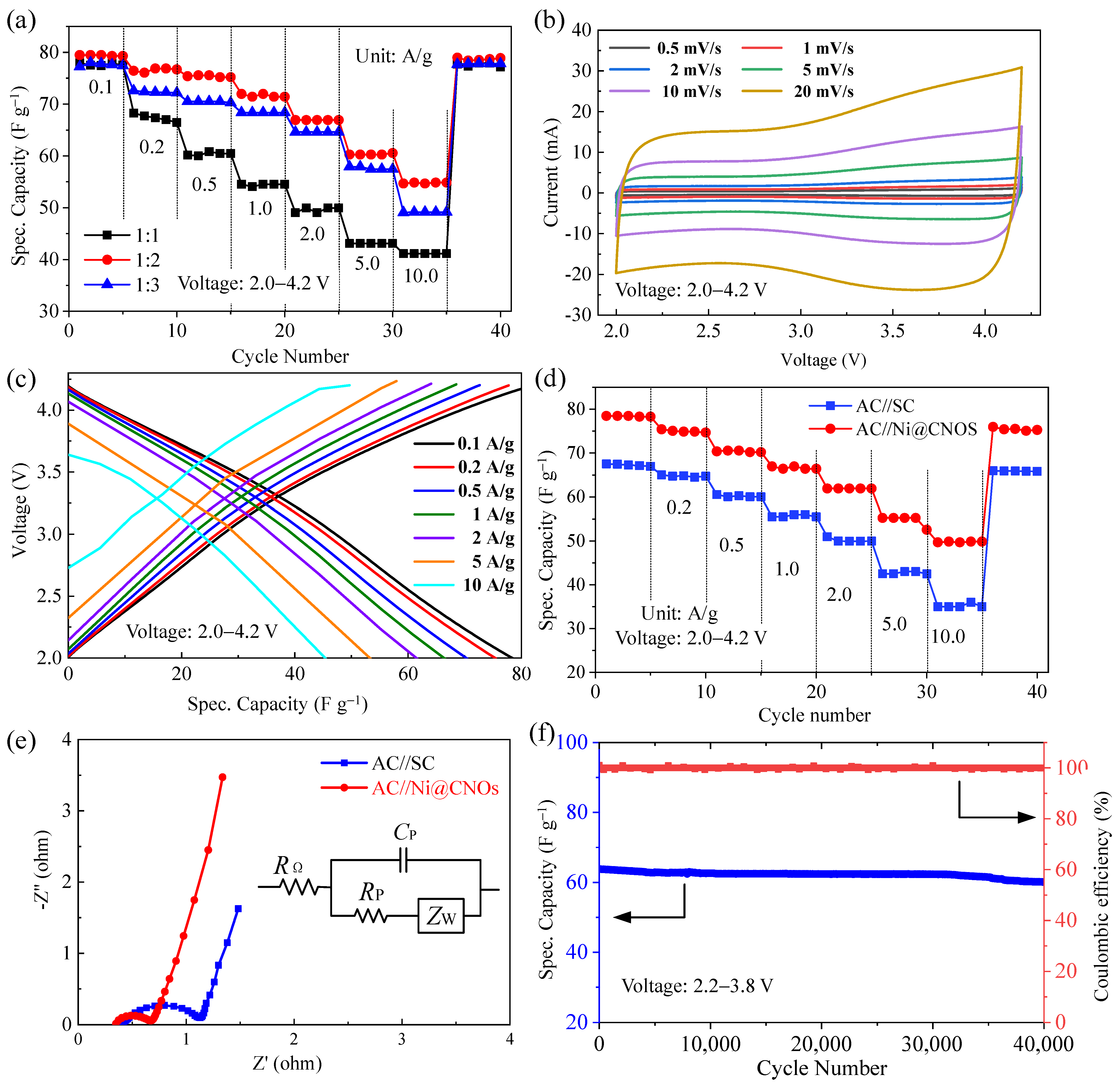
3.3. Performance of LIC Pouch Cells Based on an Ni@CNO Anode and AC (Activated Carbon) Cathode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Ock, I.W.; Lee, J.; Kang, J.K. Metal-organic framework-derived anode and polyaniline chain networked cathode with mesoporous and conductive pathways for high energy density, ultrafast rechargeable, and long-life hybrid capacitors. Adv. Energy Mater. 2020, 10, 2001851. [Google Scholar] [CrossRef]
- Xu, J.; Gao, B.; Huo, K.F.; Chu, P.K. Recent progress in electrode materials for nonaqueous lithium-ion capacitors. J. Nanosci. Nanotechnol. 2020, 20, 2652–2667. [Google Scholar] [CrossRef]
- Zheng, J.P. Theoretical energy density for electrochemical capacitors with intercalation electrodes. J. Electrochem. Soc. 2005, 152, A1864–A1869. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhu, C.R.; Chao, D.L.; Yan, Q.Y.; Fan, H.J. Nonaqueous hybrid lithium-ion and sodium-ion capacitors. Adv. Mater. 2017, 29, 1702093. [Google Scholar] [CrossRef]
- Karimi, D.; Behi, H.; Van Mierlo, J.; Berecibar, M. A comprehensive review of lithium-ion capacitor technology: Theory, development, modeling, thermal management systems, and applications. Molecules 2022, 27, 3119. [Google Scholar] [CrossRef]
- Ma, Y.F.; Chang, H.C.; Zhang, M.; Chen, Y.S. Graphene-based materials for lithium-ion hybrid supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar]
- Zhang, X.H.; Sun, X.Z.; An, Y.B.; Zhang, X.; Li, C.; Zhang, K.L.; Song, S.; Wang, K.; Ma, Y.W. Design of a fast-charge lithium-ion capacitor pack for automated guided vehicle. J. Energy Storage 2022, 48, 104045. [Google Scholar] [CrossRef]
- Omonayo, B.; Annadanesh, S.; Zheng, J.P. Lithium-ion capacitor safety testing for commercial application. Batteries 2019, 5, 74. [Google Scholar]
- Barcellona, S.; Ciccarelli, F.; Iannuzzi, D.; Piegari, L. Overview of lithium-ion capacitor applications based on experimental performances. Electr. Power Compon. Syst. 2016, 44, 1248–1260. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, X.; Xu, Y.N.; Li, C.; Wang, K.; Sun, X.Z.; Su, F.Y.; Chen, C.M.; Liu, F.Y.; Wu, Z.S.; et al. Recent advances of carbon-based materials for high performance lithium-ion capacitors. Batter. Supercaps 2021, 4, 407–428. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, X.; Tong, Q.; Li, H.; Li, J.; Yang, W. Continuously interconnected N-doped porous carbon for high-performance lithium-ion capacitors. Nanoenergy Adv. 2022, 2, 303–315. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Jin, Z.; Qiao, X.; Huang, H.; Chu, X.; Xiong, D.; Zhang, H.; Liu, Y.; Yang, W. Hierarchically divacancy defect building dual-activated porous carbon fibers for high-performance energy-storage devices. Adv. Funct. Mater. 2020, 30, 2002580. [Google Scholar] [CrossRef]
- Banerjee, A.; Upadhyay, K.K.; Puthusseri, D.; Aravindan, V.; Madhavi, S.; Ogale, S. MOF-derived crumpled-sheet-assembled perforated carbon cuboids as highly effective cathode active materials for ultra-high energy density Li-ion hybrid. Nanoscale 2014, 6, 4387–4394. [Google Scholar] [CrossRef]
- Stoller, M.D.; Murali, S.; Quarles, N.; Zhu, Y.; Potts, J.R.; Zhu, X.; Ha, H.W.; Ruoff, R.S. Activated graphene as a cathode material for Li-ion hybrid supercapacitors. Phys. Chem. Chem. Phys. 2012, 14, 3388–3391. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, W.H.; Lim, S.Y.; Kim, B.G.; Choi, J.W. Modified graphite and graphene electrodes for high-performance lithium ion hybrid capacitors. Mater. Renew. Sustain. Energy 2014, 3, 22. [Google Scholar] [CrossRef]
- Yi, S.; Wang, L.; Zhang, X.; Li, C.; Liu, W.J.; Wang, K.; Sun, X.Z.; Xu, Y.N.; Yang, Z.X.; Cao, Y.S.; et al. Cationic intermediates assisted self-assembly two-dimensional Ti3C2Tx/rGO hybrid nanoflakes for advanced lithium-ion capacitors. Sci. Bull. 2021, 66, 914. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef]
- Dhand, V.; Yadav, M.; Kim, S.H.; Rhee, K.Y. A comprehensive review on the prospects of multi-functional carbon nano onions as an effective, high- performance energy storage material. Carbon 2021, 175, 534–575. [Google Scholar] [CrossRef]
- Tan, Z.-L.; Wei, J.-X.; Liu, Y.; Zaman, F.U.; Rehman, W.; Hou, L.-R.; Yuan, C.-Z. V2CTx MXene and its derivatives: Synthesis and recent progress in electrochemical energy storage applications. Rare Metals 2022, 41, 775. [Google Scholar] [CrossRef]
- Sun, X.Z.; Geng, L.B.; Yi, S.; Li, C.; An, Y.B.; Zhang, X.H.; Zhang, X.; Wang, K.; Ma, Y.W. Effects of carbon black on the electrochemical performances of SiOx anode for lithium-ion capacitors. J. Power Source 2021, 499, 229936. [Google Scholar] [CrossRef]
- Akintola, T.; Shellikeri, A.; Akintola, T.; Zheng, J.P. The influence of Li4Ti5O12 preparation method on lithium-ion capacitor performance. Batteries 2021, 7, 33. [Google Scholar] [CrossRef]
- An, Y.B.; Liu, T.Y.; Li, C.; Zhang, X.; Hu, T.; Sun, X.Z.; Wang, K.; Wang, C.D.; Ma, Y.W. A general route for the mass production of graphene-enhanced carbon composites toward practical pouch lithium-ion capacitors. J. Mater. Chem. A 2021, 9, 15654. [Google Scholar] [CrossRef]
- Portet, C.; Yushin, G.; Gogotsi, Y. Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotube in electrical double layer capacitors. Carbon 2007, 45, 2511–2518. [Google Scholar] [CrossRef]
- Chen, J.; Yang, B.; Li, H.; Ma, P.; Lang, J.; Yan, X. Candle soot: Onion-like carbon, an advanced anode material for a potassium-ion hybrid capacitor. J. Mater. Chem. A 2019, 7, 9247–9252. [Google Scholar] [CrossRef]
- Jäckel, N.; Weingarth, D.; Zeiger, M.; Aslan, M.; Grobelsek, I.; Presser, V. Comparison of carbon onions and carbon blacks as conductive additives for carbon supercapacitors in organic electrolytes. J. Power Source 2014, 272, 1122–1133. [Google Scholar] [CrossRef]
- Shi, K.Y.; Liu, J.W.; Chen, R. Nitrogen-Doped nano-carbon onion rings for energy storage in Lithium-ion capacitors. J. Energy Storage 2020, 31, 101609. [Google Scholar] [CrossRef]
- Permana, A.D.C.; Ding, L.; Gonzalez-Martinez, I.G.; Hantusch, M.; Nielsch, K.; Mikhailova, D.; Omar, A. Comparative study of onion-like carbons prepared from different synthesis routes towards li-ion capacitor application. Batteries 2022, 8, 160. [Google Scholar] [CrossRef]
- Permana, A.D.C.; Omar, A.; Gonzalez-Martinez, L.G.; Oswald, S.; Giebeler, L.; Nielsch, K.; Mikhailova, D. MOF-derived onion-like carbon with superior surface area and porosity for high performance lithium-ion capacitors. Batter. Supercaps 2022, 5, e202100353. [Google Scholar]
- Aref, A.R.; Chen, S.W.; Rajagopalan, R.; Randall, C. Bimodal porous carbon cathode and prelithiated coalesced carbon onion anode for ultrahigh power energy efficient lithium ion capacitors. Carbon 2019, 152, 89–97. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Zhao, H.T.; Ruan, C.; Wang, Y.J.; Li, Z.J.; Lian, Y.F. The arc-discharged Ni-cored carbon onions with enhanced microwave absorption performances. Mater. Lett. 2020, 265, 127408. [Google Scholar] [CrossRef]
- Hechmann, A.; Fromm, O.; Rodehorst, U.; Münster, P.; Winter, M.; Placke, T. New Insights into Electrochemical Anion Intercalation into Carbonaceous Materials for Dual-Ion Batteries: Impact of Graphitization Degree. Carbon 2018, 131, 201–212. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, K.; Xu, N.Y.; Zhang, D.X.; Peng, F.Q.; Li, N.S.; Zhang, X.; Sun, Z.X.; Ma, W.Y. Dehalogenation produces graphene wrapped carbon cages as fast-kinetics and large-capacity anode for lithium-ion capacitors. Carbon 2023, 202, 175–185. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, Z.X.; Ma, W.Y. High-power and long-life lithium-ion capacitors constructed from N-doped hierarchical carbon nanolayer cathode and mesoporous graphene anode. Carbon 2018, 140, 237–248. [Google Scholar] [CrossRef]
- Chen, C.J.; Wang, Z.G.; Miao, L.; Cai, J.; Peng, L.F.; Huang, Y.Y.; Zhang, L.N.; Xie, J. Nitrogen-rich hard carbon as a highly durable anode for high-power potassium-ion batteries. Energy Storage Mater. 2017, 8, 161–168. [Google Scholar] [CrossRef]
- Yue, Y.; Juarez-Robles, D.; Mukherjee, P.; Liang, H. Superhierarchical Nickel–Vanadia Nanocomposites for Lithium Storage. ACS Appl. Energy Mater. 2018, 1, 2056–2066. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.Y.; Du, F.H.; Shen, X.P.; Ji, Z.Y.; Zhou, H.B.; Yuan, A.H. In-situ construction of nano-sized Ni-NiO-MoO2 heterostructures on holey reduced graphene oxide nanosheets as high-capacity lithium-ion battery anodes. J. Alloys Compd. 2022, 926, 166847. [Google Scholar] [CrossRef]
- Jin, L.M.; Guo, X.; Shen, C.; Qin, N.; Zheng, J.S.; Wu, Q.; Zhang, C.M.; Zheng, J.P. A universal matching approach for high power-density and high cycling-stability lithium ion capacitor. J. Power Source 2019, 441, 227211. [Google Scholar] [CrossRef]
- Naderi, R.; Shellikeri, A.; Hagen, M.; Cao, W.; Zheng, J.P. The influence of anode/cathode capacity ratio on cycle life and potential variations of lithium-ion capacitors. J. Electrochem. Soc. 2019, 166, A2610–A2617. [Google Scholar] [CrossRef]
- Yu, X.L.; Zhan, C.Z.; Lv, R.T.; Bai, Y.; Lin, Y.X.; Huang, Z.H.; Shen, W.C.; Qiu, X.P.; Kang, F.Y. Ultrahigh-rate and high-density lithium-ion capacitors through hybriding nitrogen-enriched hierarchical porous carbon cathode with prelithiated microcrystalline graphite anode. Nano Energy 2015, 5, 43–53. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, W.H.; Ryon, M.H.; Jin, J.K.; Kim, J.Y.; Choi, J.W. Functionalized graphene for high performance lithium ion capacitors. ChemSusChem 2012, 5, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kin, J.S.; Lim, Y.G.; Lee, J.G.; Kim, Y.J. Effect of carbon types on the electrochemical properties of negative electrodes for Li-ion capacitors. J. Power Source 2011, 196, 10490–10495. [Google Scholar] [CrossRef]
- Cao, W.J.; Zheng, J.P. Li-ion capacitors with carbon cathode and hard carbon/stabilized lithium metal powder anode electrodes. J. Power Source 2012, 213, 180–185. [Google Scholar] [CrossRef]
- Du, H.P.; Yang, H.; Huang, C.S.; He, J.J.; Liu, H.B.; Li, Y.L. Graphdiyne applied for lithium-ion capacitors displaying high power and energy densities. Nano Energy 2016, 22, 615–622. [Google Scholar] [CrossRef]
- Yang, Z.W.; Guo, H.J.; Li, X.H.; Wang, Z.X.; Wang, J.X.; Wang, Y.S.; Yan, Z.L.; Zhang, D.C. Graphitic carbon balanced between high plateau capacity and high rate capability for lithium ion capacitors. J. Mater. Chem. A 2017, 5, 15302–15309. [Google Scholar] [CrossRef]
- Han, X.Q.; Han, P.X.; Yao, J.H.; Zhang, S.; Cao, X.Y.; Xiong, J.W.; Zhang, J.N.; Cui, G.L. Nitrogen-doped carbonized polyimide microsphere as a novel anode material for high performance lithium-ion capacitors. Electrochim. Acta 2016, 196, 603–610. [Google Scholar] [CrossRef]
- Schroeder, M.; Winter, M.; Passerini, S.; Balducci, A. On the cycling stability of lithium-ion capacitors containing soft carbon as anodic material. J. Power Source 2013, 238, 388–394. [Google Scholar] [CrossRef]
- Ren, J.J.; Su, L.W.; Qin, X.; Yang, M.; Wei, J.P.; Zhou, Z.; Shen, P.W. Pre-lithiated graphene nanosheets as negative electrode materials for Li-ion capacitors with high power and energy density. J. Power Source 2014, 264, 108–113. [Google Scholar] [CrossRef]
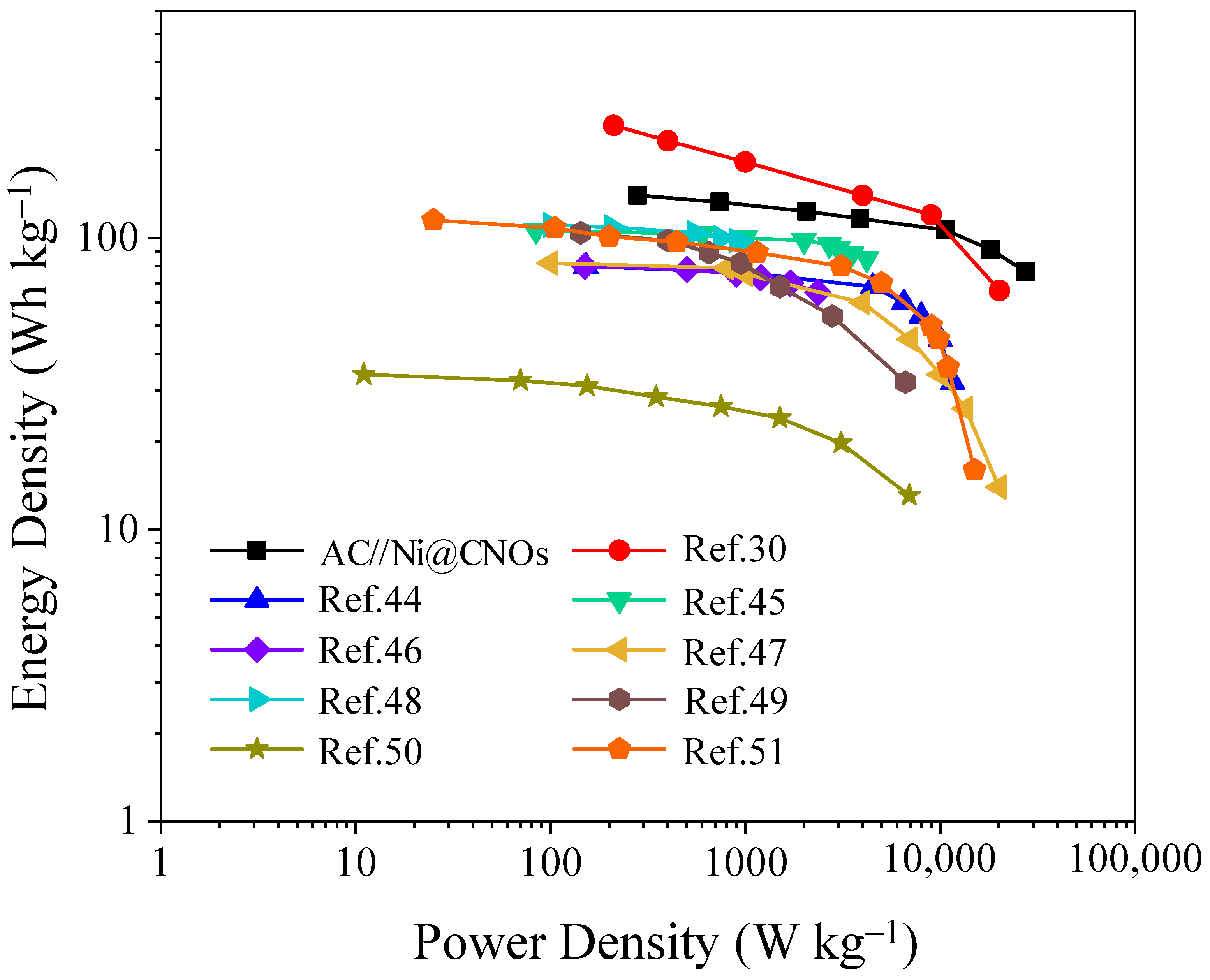
| Ref | No. Anode//Cathode | Working Potential Range (vs. Li/Li+) | Max. Energy Density Wh kg−1/Power Density W kg−1 | Max. Power Density W kg−1/Energy Density Wh kg−1 | Cyclability Cycles @ Current Density A g−1 |
|---|---|---|---|---|---|
| This work | Ni@CNOs//AC | 2.0–4.2 | 140.1/275 | 27,000/76.6 | 40,000 cycles (94.09%) @ 2 A g−1 in the voltage range of 2.2–3.8 V |
| [30] | OLC-B//AC | 2.0–4.0 | 243 @ 211 | 20,149 @ 66 | 10,000 cycles (78%) |
| [44] | Graphite//functionalized graphene | 2.0–4.0 | 106/84 | 4200/85 | 100% over 1000 cycles |
| [45] | Hard carbon//activated carbon | 1.4–4.3 | 80/150 | 2350/65 | 83% over 10,000 cycles |
| [46] | Hard carbon//activated carbon | 2.0–4.0 | 82/100 | 20,000/14 | 97% over 600 cycles |
| [47] | Graphdiyne//AC | 2.0–4.0 | 110.7/100.3 | 1000.4/95.1 | 1000 cycles @ 0.2 A g−1 |
| [48] | Sisal fiber-derived graphitic carbon//Sisal fiber AC | 2.0–4.0 | 104/143 | 6628/32 | 3000 cycles @ 1 A g−1 |
| [49] | N-doped hard carbon//activated carbon | 2.0–4.0 | 28.5/348 | 6940/13.1 | 97% over 5000 cycles |
| [50] | Soft carbon//activated carbon | 0–4.4 | 115/25 | 15,000/16 | 63% over 15,000 cycles |
| [51] | Graphene//activated carbon | 2.0–4.0 | 95/27 | 222.2/61.7 | 74% over 300 cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, K.; Zhang, W.; Zhang, X.; Wang, L.; An, Y.; Sun, X.; Li, C.; Wang, K.; Ma, Y. Carbon Nano-Onion-Encapsulated Ni Nanoparticles for High-Performance Lithium-Ion Capacitors. Batteries 2023, 9, 102. https://doi.org/10.3390/batteries9020102
Zhang X, Zhang K, Zhang W, Zhang X, Wang L, An Y, Sun X, Li C, Wang K, Ma Y. Carbon Nano-Onion-Encapsulated Ni Nanoparticles for High-Performance Lithium-Ion Capacitors. Batteries. 2023; 9(2):102. https://doi.org/10.3390/batteries9020102
Chicago/Turabian StyleZhang, Xiaohu, Keliang Zhang, Weike Zhang, Xiong Zhang, Lei Wang, Yabin An, Xianzhong Sun, Chen Li, Kai Wang, and Yanwei Ma. 2023. "Carbon Nano-Onion-Encapsulated Ni Nanoparticles for High-Performance Lithium-Ion Capacitors" Batteries 9, no. 2: 102. https://doi.org/10.3390/batteries9020102
APA StyleZhang, X., Zhang, K., Zhang, W., Zhang, X., Wang, L., An, Y., Sun, X., Li, C., Wang, K., & Ma, Y. (2023). Carbon Nano-Onion-Encapsulated Ni Nanoparticles for High-Performance Lithium-Ion Capacitors. Batteries, 9(2), 102. https://doi.org/10.3390/batteries9020102









