Conductive Metal–Organic Frameworks for Rechargeable Lithium Batteries
Abstract
:1. Introduction
2. Conduction Mechanism
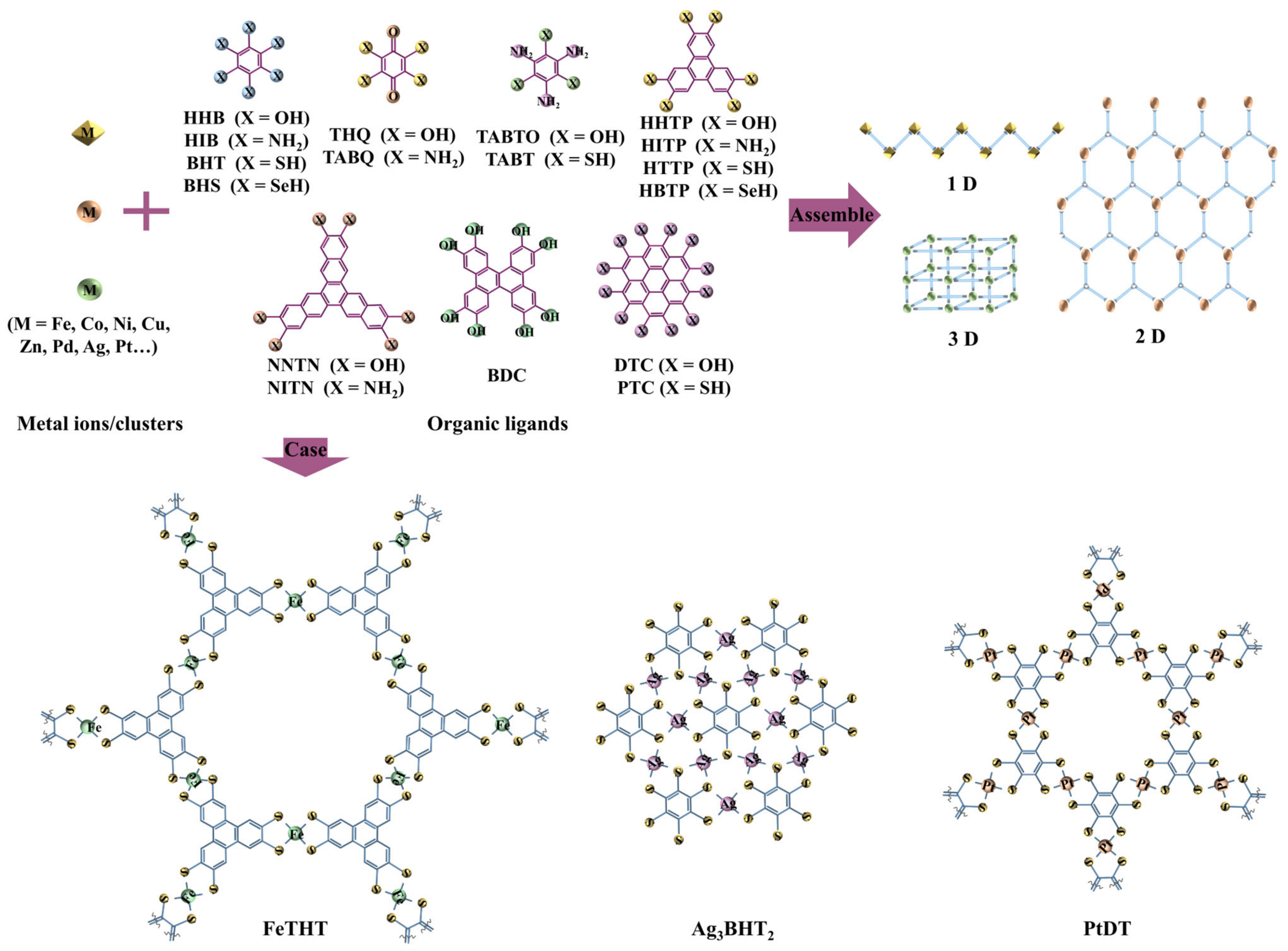
3. Synthetic Methodologies
3.1. Hydro-/Solvothermal Reactions
3.2. Interface-Assisted Synthesis
3.3. Layer-by-Layer (LBL) Self-Assembly
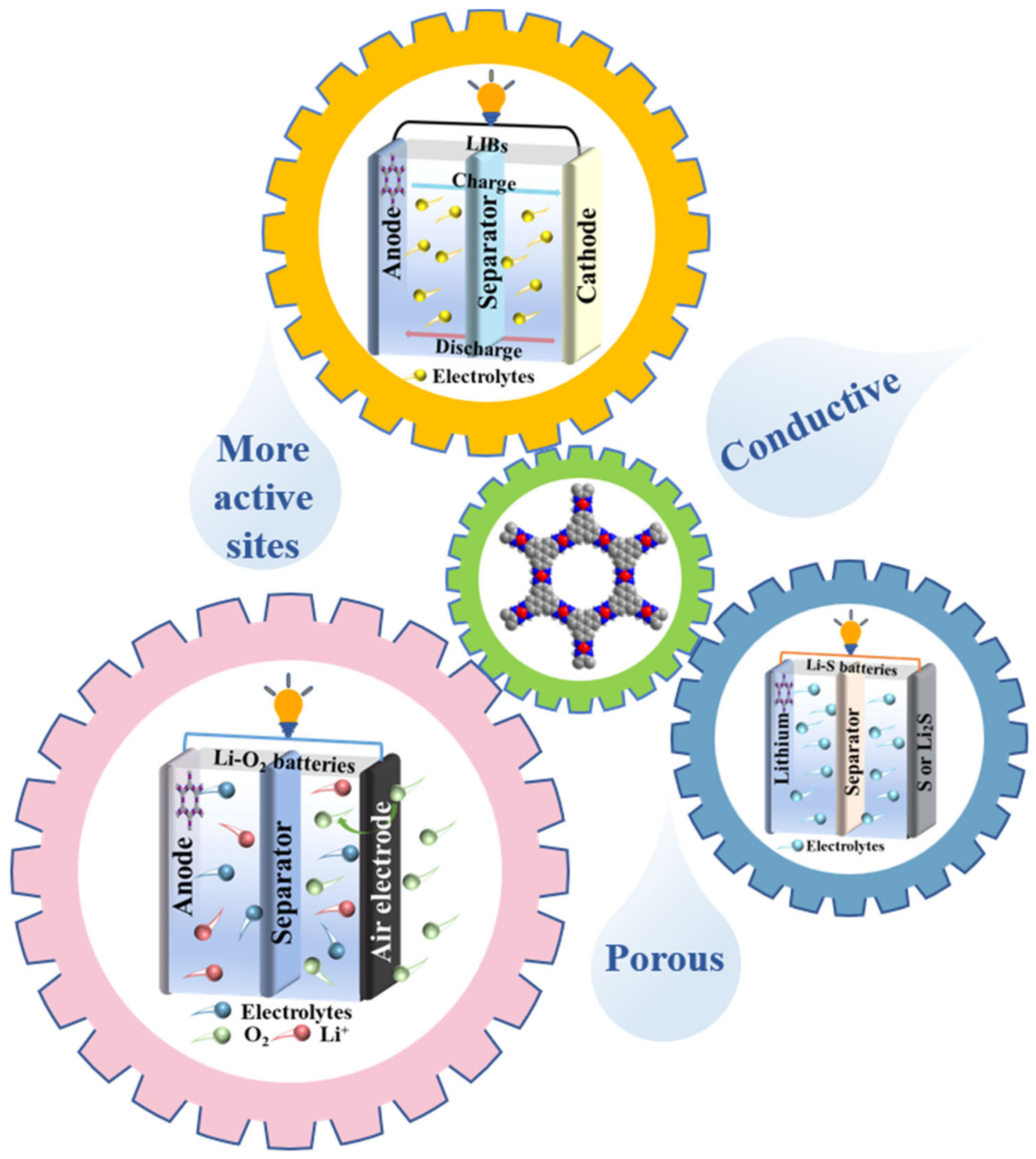
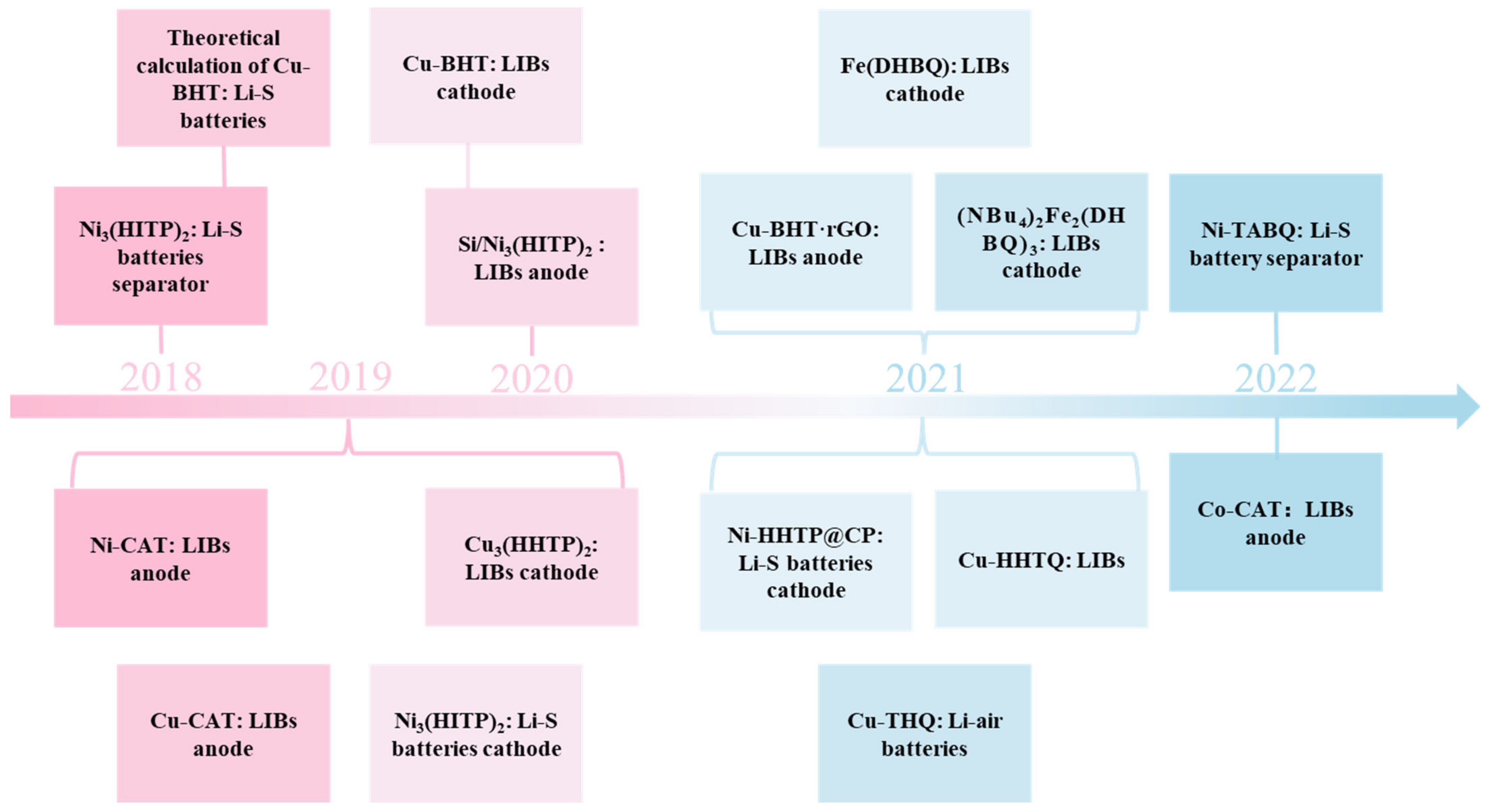
4. Application of c-MOFs in LIBs
4.1. LIBs Anode
4.2. LIBs Cathode
5. Application of c-MOFs in Li–S Batteries and Li–Air Batteries
5.1. Li–S Batteries
5.1.1. Li–S Batteries Separators
5.1.2. Li–S Batteries Cathode
5.2. Li–Air Batteries
6. Theoretical Calculation
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Liu, J.; Liu, J.; Ouyang, L.; Hu, R.; Wang, H.; Yang, L.; Zhu, M. A General Metal-Organic Framework (MOF)-Derived Selenidation Strategy for In Situ Carbon-Encapsulated Metal Selenides as High-Rate Anodes for Na-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1870108. [Google Scholar] [CrossRef]
- Shen, K.; Xu, X.; Tang, Y. Recent progress of magnetic field application in lithium-based batteries. Nano Energy 2022, 92, 106703. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Xu, X.; Zhang, L.; Hu, R.; Liu, J.; Yang, L.; Zhu, M. Metal–Organic Framework-Derived NiSb Alloy Embedded in Carbon Hollow Spheres as Superior Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2017, 9, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, D.; Luo, J.; Xiang, Y. First-principles study of ZIF-8 as anode for Na and K ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130802. [Google Scholar] [CrossRef]
- Nasser, O.A.; Petranikova, M. Review of Achieved Purities after Li-Ion Batteries Hydrometallurgical Treatment and Impurities Effects on the Cathode Performance. Batteries 2021, 7, 60. [Google Scholar] [CrossRef]
- Suzanowicz, A.M.; Mei, C.W.; Mandal, B.K. Approaches to Combat the Polysulfide Shuttle Phenomenon in Li–S Battery Technology. Batteries 2022, 8, 45. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, S. The applications of semiconductor materials in air batteries. Chin. Chem. Lett. 2021, 32, 3277–3287. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, S.; Huang, J. Germanium-modified silicon as anodes in Si-Ge air batteries with enhanced properties. J. Phys. Chem. Solids 2021, 157, e110226. [Google Scholar] [CrossRef]
- Wang, K.; Pei, S.; He, Z.; Huang, L.-A.; Zhu, S.; Guo, J.; Shao, H.; Wang, J. Synthesis of a novel porous silicon microsphere@carbon core-shell composite via in situ MOF coating for lithium ion battery anodes. Chem. Eng. J. 2019, 356, 272–281. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, P.; Yan, X.; Gou, H.; Zhang, S.; Shi, S. Facile One-Step Solution-Phase Route to Synthesize Hollow Nanoporous CuxO Microcages on 3D Copper Foam for Superior Li Storage. ACS Sustain. Chem. Eng. 2021, 9, 4363–4370. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, S.; Huang, J. Adsorption and diffusion of lithium and sodium on the silicon nanowire with substrate for energy storage application: A first principles study. Mater. Chem. Phys. 2020, 253, 123243. [Google Scholar] [CrossRef]
- Collins, G.A.; Geaney, H.; Ryan, K.M. Alternative anodes for low temperature lithium-ion batteries. J. Mater. Chem. A 2021, 9, 14172–14213. [Google Scholar] [CrossRef]
- Hou, J.; Ma, X.; Fu, J.; Vanaphuti, P.; Yao, Z.; Liu, Y.; Yang, Z.; Wang, Y. A green closed-loop process for selective recycling of lithium from spent lithium-ion batteries. Green Chem. 2022, 24, 7049–7060. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, X.; Wen, J.; Wang, C.; Ma, X.; Yang, Y.; Huang, G.; Ye, H.-M.; Xu, S. Research progress on high-temperature resistant polymer separators for lithium-ion batteries. Energy Storage Mater. 2022, 51, 638–659. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, X.-Q.; Huang, J.-Q.; Liu, Q.; Zhang, Q. Lithium-Anode Protection in Lithium-Sulfur Batteries. Trends Chem. 2019, 1, 693–704. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Qiao, F.; Wang, J.; Li, H.; Eichel, R.-A.; Notten, P.H.L. Sulfur Reduction Reaction in Lithium-Sulfur Batteries: Mechanisms, Catalysts, and Characterization. Adv. Energy Mater. 2022, 2202094. [Google Scholar] [CrossRef]
- Ko, S.; Yoo, Y.; Choi, J.; Lim, H.-D.; Park, C.B.; Lee, M. Discovery of organic catalysts boosting lithium carbonate decomposition toward ambient air operational lithium-air batteries. J. Mater. Chem. A 2022, 10, 20464–20472. [Google Scholar] [CrossRef]
- Wu, Z.; Tian, Y.; Chen, H.; Wang, L.; Qian, S.; Wu, T.; Zhang, S.; Lu, J. Evolving aprotic Li–air batteries. Chem. Soc. Rev. 2022, 51, 8045–8101. [Google Scholar] [CrossRef]
- Wada, K.; Sakaushi, K.; Sasaki, S.; Nishihara, H. Multielectron-Transfer-based Rechargeable Energy Storage of Two-Dimensional Coordination Frameworks with Non-Innocent Ligands. Angew. Chem. Int. Ed. 2018, 57, 8886–8890. [Google Scholar] [CrossRef]
- Liang, Z.; Qu, c.; Guo, w.; Zou, R.; Xu, Q. Pristine Metal-Organic Frameworks and their Composites for Energy Storage and Conversion. Adv. Mater. 2018, 30, 1702891. [Google Scholar] [CrossRef]
- Huang, W.; Huang, S.; Chen, G.; Ouyang, G. Biocatalytic Metal-Organic Frameworks: Promising Materials for Biosensing. ChemBioChem 2022, 23, 202100567. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, Z.; Davoodian, N.; Taghdisi, S.M.; Abnous, K. Metal organic frameworks as advanced functional materials for aptasensor design. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 276, 121251. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qu, X.; Liu, C.; Xu, Q.; Tu, K. Metal-Organic Frameworks and Their Composites Towards Biomedical Applications. Front. Mol. Biosci. 2021, 8, 805228. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Han, M.; Liang, J.; Zhang, M.; Bai, X.; Yue, T.; Gao, Z. Evaluating the changes in phytochemical composition, hypoglycemic effect, and influence on mice intestinal microbiota of fermented apple juice. Food Res. Int. 2022, 155, 110998. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Fan, R.; Zhang, J.; Fang, X.; Sun, T.; Zhu, K.; Zhou, X.; Xu, Y.; Yang, Y. Sequentially epitaxial growth multi-guest encapsulation strategy in MOF-on-MOF platform: Biogenic amine detection and systematic white light adjustment. Chem. Eng. J. 2022, 436, 135236. [Google Scholar] [CrossRef]
- Tarasi, S.; Ramazani, A.; Morsali, A.; Hu, M.-L.; Ghafghazi, S.; Tarasi, R.; Ahmadi, Y. Drug Delivery Using Hydrophilic Metal-Organic Frameworks (MOFs): Effect of Structure Properties of MOFs on Biological Behavior of Carriers. Inorg. Chem. 2022, 61, 13125–13132. [Google Scholar] [CrossRef]
- Peng, X.; Chen, L.; Li, Y. Ordered macroporous MOF-based materials for catalysis. Mol. Catal. 2022, 529, 112568. [Google Scholar] [CrossRef]
- Wang, T.-S.; Liu, X.; Zhao, X.; He, P.; Nan, C.-W.; Fan, L.-Z. Regulating Uniform Li Plating/Stripping via Dual-Conductive Metal-Organic Frameworks for High-Rate Lithium Metal Batteries. Adv. Funct. Mater. 2020, 30, 2000786. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Dai, Y.; Qu, W.; Xing, Y.; Ye, Y.; Wen, Z.; Guo, C.; Wu, F.; Chen, R. A Li+ conductive metal organic framework electrolyte boosts the high-temperature performance of dendrite-free lithium batteries. J. Mater. Chem. A 2019, 7, 9530–9536. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Chen, J.; Li, X.; Sun, J.; Zhu, J.; Wang, X.; Fu, Y. Recent development and applications of electrical conductive MOFs. Nanoscale 2021, 13, 485–509. [Google Scholar] [CrossRef]
- Mu, X.; Wang, W.; Sun, C.; Wang, J.; Wang, C.; Knez, M. Recent Progress on Conductive Metal-Organic Framework Films. Adv. Mater. Interfaces 2021, 8, 2002151. [Google Scholar] [CrossRef]
- Zhu, B.; Wen, D.; Liang, Z.; Zou, R. Conductive metal-organic frameworks for electrochemical energy conversion and storage. Coord. Chem. Rev. 2021, 446, 214119. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Lu, Y.; Liu, D.; Zhang, J.; Hao, D.; Zhang, X.; Xiong, L.; Huang, J. Sensitive humidity sensors based on ionically conductive metal-organic frameworks for breath monitoring and non-contact sensing. Appl. Mater. Today 2022, 26, 101391. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Y.; Zhu, P.; Du, L.; Chen, W.; Wu, C. Electrochemically Activated Conductive Ni-Based MOFs for Non-enzymatic Sensors Toward Long-Term Glucose Monitoring. Front. Chem. 2020, 8, 602752. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Ye, R.; Huang, D.; Wang, Y.; Chen, S. Fabrication of a novel electrochemical sensor using conductive MOF Cu–CAT anchored on reduced graphene oxide for BPA detection. J. Appl. Electrochem. 2022, 52, 1617–1628. [Google Scholar] [CrossRef]
- Niu, L.; Wu, T.; Chen, M.; Yang, L.; Yang, J.; Wang, Z.; Kornyshev, A.A.; Jiang, H.; Bi, S.; Feng, G. Conductive Metal-Organic Frameworks for Supercapacitors. Adv. Mater. 2022, 2200999. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Ma, Z.-L.; Yan, L.-T.; Zhao, X.-B.; Xue, Y.-Y.; Huo, J.-M.; Yuan, X.; Li, S.-N.; Zhai, Q.-G. Competitive Coordination-Oriented Monodispersed Ruthenium Sites in Conductive MOF/LDH Hetero-Nanotree Catalysts for Efficient Overall Water Splitting in Alkaline Media. Adv. Mater. 2022, 34, 2107488. [Google Scholar] [CrossRef]
- Meng, H.; Han, Y.; Zhou, C.; Jiang, Q.; Shi, X.; Zhan, C.; Zhang, R. Conductive Metal–Organic Frameworks: Design, Synthesis, and Applications. Small Methods 2020, 4, 2000396. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Q.; Ni, Y.; Shang, L.; Zhang, X.; Yan, Z.; Zhao, Q.; Chen, J. Rational design and synthesis of two-dimensional conjugated metal-organic polymers for electrocatalysis applications. Chem 2022, 8, 1822–1854. [Google Scholar] [CrossRef]
- Li, W.-H.; Deng, W.-H.; Wang, G.-E.; Xu, G. Conductive MOFs. EnergyChem 2020, 2, 100029. [Google Scholar] [CrossRef]
- Deng, X.; Hu, J.-Y.; Luo, J.; Liao, W.-M.; He, J. Conductive Metal-Organic Frameworks: Mechanisms, Design Strategies and Recent Advances. Top. Curr. Chem. 2020, 378, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jin, L.; Zhang, R.; Bai, Y.; Zhu, R.; Pang, H. Recent advances in the development of electronically and ionically conductive metal-organic frameworks. Coord. Chem. Rev. 2021, 439, 213915. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, G.-H.; Yang, X.-J.; Lim, D.-W.; Bao, S.-S.; Donoshita, M.; Wu, L.Q.; Kitagawa, H.; Zheng, L.-M. Anhydrous Superprotonic Conductivity of a Uranyl-Based MOF from Ambient Temperature to 110 °C. ACS Mater. Lett. 2021, 3, 744–751. [Google Scholar] [CrossRef]
- Tang, H.; Lv, X.; Du, J.; Liu, Y.; Liu, J.; Guo, L.; Zheng, X.; Hao, H.; Liu, Z. Improving proton conductivity of metal organic framework materials by reducing crystallinity. Appl. Organomet. Chem. 2022, 36, e6777. [Google Scholar] [CrossRef]
- Ko, M.; Mendecki, L.; Mirica, K.A. Conductive two-dimensional metal-organic frameworks as multifunctional materials. Chem. Commun. 2018, 54, 7873–7891. [Google Scholar] [CrossRef]
- Thanasekaran, P.; Su, C.-H.; Liu, Y.-H.; Lu, K.-L. Weak interactions in conducting metal-organic frameworks. Coord. Chem. Rev. 2021, 442, 213987. [Google Scholar] [CrossRef]
- Nath, A.; Asha, K.S.; Mandal, S. Conductive Metal-Organic Frameworks: Electronic Structure and Electrochemical Applications. Chem. Eur. J. 2021, 27, 11482–11538. [Google Scholar] [CrossRef]
- Takaishi, S.; Hosoda, M.; Kajiwara, T.; Miyasaka, H.; Yamashita, M.; Nakanishi, Y.; Kitagawa, Y.; Yamaguchi, K.; Kobayashi, A.; Kitagawa, H. Electroconductive Porous Coordination Polymer Cu[Cu(pdt)2] Composed of Donor and Acceptor Building Units. Inorg. Chem. 2009, 49, 9048–9050. [Google Scholar] [CrossRef]
- Park, S.S.; Hontz, E.R.; Sun, L.; Hendon, C.H.; Walsh, A.; Voorhis, T.V.; Dinca, M. Cation-Dependent Intrinsic Electrical Conductivity in Isostructural Tetrathiafulvalene-Based Microporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 1774–1777. [Google Scholar] [CrossRef]
- Hua, C.; Doheny, P.W.; Ding, B.; Chan, B.; Yu, M.; Kepert, C.J.; D’Alessandro, D.M. Through-Space Intervalence Charge Transfer as a Mechanism for Charge Delocalization in Metal-Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 6622–6630. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; Gabaly, F.E.; Yoon, H.P.; et al. Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Pauliukaite, R.; Juodkazytė, J.; Ramanauskas, R. Theodor von Grotthuss’ Contribution to Electrochemistry. Electrochim. Acta 2017, 236, 28–32. [Google Scholar] [CrossRef]
- Xie, X.-X.; Yang, Y.-C.; Dou, B.-H.; Li, Z.-F.; Li, G. Proton conductive carboxylate-based metal-organic frameworks. Coord. Chem. Rev. 2020, 403, 213100. [Google Scholar] [CrossRef]
- Li, A.-L.; Gao, Q.; Xu, J.; Bu, X.-H. Proton-conductive metal-organic frameworks: Recent advances and perspectives. Coord. Chem. Rev. 2017, 344, 54–82. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dinca, M. Electrically Conductive Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef]
- Chen, T.; Dou, J.-H.; Yang, L.; Sun, C.; Libretto, N.J.; Skorupskii, G.; Miller, J.T.; Dinca, M. Continuous Electrical Conductivity Variation in M3(Hexaiminotriphenylene)2 (M = Co, Ni, Cu) MOF Alloys. J. Am. Chem. Soc. 2020, 142, 12367–12373. [Google Scholar] [CrossRef]
- Hmadeh, M.; Lu, Z.; Liu, Z.; Gándara, F.; Furukawa, H.; Wan, S.; Augustyn, V.; Chang, R.; Liao, L.; Zhou, F.; et al. New Porous Crystals of Extended Metal-Catecholates. Chem. Mater. 2012, 24, 3511–3513. [Google Scholar] [CrossRef]
- Day, R.W.; Bediako, D.K.; Rezaee, M.; Parent, L.R.; Skorupskii, G.; Arguilla, M.Q.; Hendon, C.H.; Stassen, I.; Gianneschi, N.C.; Kim, P.; et al. Single Crystals of Electrically Conductive Two-Dimensional Metal-Organic Frameworks: Structural and Electrical Transport Properties. ACS Cent. Sci. 2019, 5, 1959–1964. [Google Scholar] [CrossRef]
- Li, W.-H.; Ding, K.; Tian, H.-R.; Yao, M.-S.; Nath, B.; Deng, W.-H.; Wang, Y.; Xu, G. Conductive Metal-Organic Framework Nanowire Array Electrodes for High-Performance Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Zacher, D.; Baunemann, A.; Hermes, S.; Fischer, R.A. Deposition of microcrystalline [Cu3(btc)2] and [Zn2(bdc)2(dabco)] at alumina and silica surfaces modified with patterned self assembled organic monolayers: Evidence of surface selective and oriented growth. J. Mater. Chem. 2007, 17, 2785–2792. [Google Scholar] [CrossRef]
- Pal, T.; Kambe, T.; Kusamoto, T.; Foo, M.L.; Matsuoka, R.; Sakamoto, R.; Nishihara, H. Interfacial Synthesis of Electrically Conducting Palladium Bis(dithiolene) Complex Nanosheet. ChemPlusChem 2015, 80, 1255–1258. [Google Scholar] [CrossRef]
- Sheberla, D.; Sun, L.; Blood-Forsythe, M.A.; Er, S.; Wade, C.R.; Brozek, C.K.; Aspuru-Guzik, A.; Dinca, M. High Electrical Conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a Semiconducting Metal-Organic Graphene Analogue. J. Am. Chem. Soc. 2014, 136, 8859–8862. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Li, Y.; Zheng, C.; Zhang, B.; Di, C.-A.; Li, F.; Jin, C.; Mi, W.; Chen, L.; et al. 2D Semiconducting Metal-Organic Framework Thin Films for Organic Spin Valves. Angew. Chem. Int. Ed. Engl. 2020, 59, 1118–1123. [Google Scholar] [CrossRef]
- Zacher, D.; Shekhah, O.; Woll, C.; Fischer, R.A. Thin films of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1418–1429. [Google Scholar] [CrossRef]
- Summerfield, A.; Cebula, I.; Schroder, M.; Beton, P.H. Nucleation and Early Stages of Layer-by-Layer Growth of Metal Organic Frameworks on Surfaces. J. Phys. Chem. C 2015, 119, 23544–23551. [Google Scholar] [CrossRef]
- Stavila, V.; Volponi, J.; Katzenmeyer, A.M.; Dixon, M.C.; Allendorf, M.D. Kinetics and mechanism of metal–organic framework thin film growth: Systematic investigation of HKUST-1 deposition on QCM electrodes. Chem. Sci. 2012, 3, 1531–1540. [Google Scholar] [CrossRef]
- Ladnorg, T.; Welle, A.; Heissler, S.; Woll, C.; Gliemann, H. Site-selective growth of surface-anchored metal-organic frameworks on self-assembled monolayer patterns prepared by AFM nanografting. Beilstein J. Nanotechnol. 2013, 4, 638–648. [Google Scholar] [CrossRef]
- Arslan, H.K.; Shekhah, O.; Wohlgemuth, J.; Franzreb, M.; Fischer, R.A.; Wöll, C. High-Throughput Fabrication of Uniform and Homogenous MOF Coatings. Adv. Funct. Mater. 2011, 21, 4228–4231. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Strunskus, T.; Cyganik, P.; Zacher, D.; Fischer, R.; Wöll, C. Layer-by-Layer Growth of Oriented Metal Organic Polymers on a Functionalized Organic Surface. Langmuir 2007, 23, 7440–7442. [Google Scholar] [CrossRef]
- Contreras-Pereda, N.; Pané, S.; Puigmartí-Luis, J.; Ruiz-Molina, D. Conductive properties of triphenylene MOFs and COFs. Coord. Chem. Rev. 2022, 460, 214459. [Google Scholar] [CrossRef]
- Li, J.; Cai, Y.; Wu, H.; Yu, Z.; Yan, X.; Zhang, Q.; Gao, T.Z.; Liu, K.; Jia, X.; Bao, Z. Polymers in Lithium-Ion and Lithium Metal Batteries. Adv. Energy Mater. 2021, 11, 2003239. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, C.; Yu, S.; Ge, D.; Zhou, H. Status and challenges facing representative anode materials for rechargeable lithium batteries. J. Energy Chem. 2022, 66, 260–294. [Google Scholar] [CrossRef]
- Su, Y.-S.; Hsiao, K.-C.; Sireesha, P.; Huang, J.-Y. Lithium Silicates in Anode Materials for Li-Ion and Li Metal Batteries. Batteries 2022, 8, 2. [Google Scholar] [CrossRef]
- Liu, W.; Lu, B.; Liu, X.; Gan, Y.; Zhang, S.; Shi, S. In Situ Synthesis of the Peapod-Like Cu–SnO2 @Copper Foam as Anode with Excellent Cycle Stability and High Area Specific Capacity. Adv. Funct. Mater. 2021, 31, 2101999. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Zhang, J.; Zhang, S.; Shi, S. In-Situ synthesis of freestanding porous SnOx-decorated Ni3Sn2 composites with enhanced Li storage properties. Chem. Eng. J. 2021, 412, 128591. [Google Scholar] [CrossRef]
- Liu, W.; Xiang, P.; Dong, X.; Yin, H.; Yu, H.; Cheng, P.; Zhang, S.; Shi, S. Two advantages by a single move: Core-bishell electrode design for ultrahigh-rate capacity and ultralong-life cyclability of lithium ion batteries. Compos. B Eng. 2021, 216, 108883. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Sun, X.; Zhang, J.; Hou, L.; Yuan, C. Construction of 1D conductive Ni-MOF nanorods with fast Li+ kinetic diffusion and stable high-rate capacities as an anode for lithium ion batteries. Nanoscale Adv. 2019, 1, 4688–4691. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Zhang, W.; Hou, L.; Liang, L.; Liu, Y.; Yuan, C. Bottom-Up Fabrication of 1D Cu-based Conductive Metal-Organic Framework Nanowires as a High-Rate Anode Towards Efficient Lithium Storage. ChemSusChem 2019, 12, 5051–5058. [Google Scholar] [CrossRef]
- Mao, P.; Fan, H.; Liu, C.; Lan, G.; Huang, W.; Li, Z.; Mahmoud, H.; Zheng, R.; Wang, Z.; Sun, H.; et al. Conductive Co-based metal organic framework nanostructures for excellent potassium- and lithium-ion storage: Kinetics and mechanism studies. Sustain. Energy Fuels 2022, 6, 4075–4084. [Google Scholar] [CrossRef]
- Nazir, A.; Le, H.T.T.; Min, C.-W.; Kasbe, A.; Kim, J.; Jin, C.S.; Park, C.J. Coupling of a conductive Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2 metal-organic framework with silicon nanoparticles for use in high-capacity lithium-ion batteries. Nanoscale 2020, 12, 1629–1642. [Google Scholar] [CrossRef]
- Meng, C.; Hu, P.; Chen, H.; Cai, Y.; Zhou, H.; Jiang, Z.; Zhu, X.; Liu, Z.; Wang, C.; Yuan, A. 2D conductive MOFs with sufficient redox sites: Reduced graphene oxide/Cu-benzenehexathiolate composites as high capacity anode materials for lithium-ion batteries. Nanoscale 2021, 13, 7751–7760. [Google Scholar] [CrossRef]
- Yan, J.; Cui, Y.; Xie, M.; Yang, G.-Z.; Bin, D.S.; Li, D. Immobilizing Redox-Active Tricycloquinazoline into a 2D Conductive Metal-Organic Framework for Lithium Storage. Angew. Chem. Int. Ed. 2021, 60, 24467–24472. [Google Scholar] [CrossRef]
- Gu, S.; Bai, Z.; Majumder, S.; Huang, B.; Chen, G. Conductive metal–organic framework with redox metal center as cathode for high rate performance lithium ion battery. J. Power Sources 2019, 429, 22–29. [Google Scholar] [CrossRef]
- Wu, Z.; Adekoya, D.; Huang, X.; Kiefel, M.J.; Xie, J.; Xu, W.; Zhang, Q.; Zhu, D.; Zhang, S. Highly Conductive Two-Dimensional Metal-Organic Frameworks for Resilient Lithium Storage with Superb Rate Capability. ACS Nano 2020, 14, 12016–12026. [Google Scholar] [CrossRef]
- Dong, H.; Gao, H.; Geng, J.; Hou, X.; Gao, S.; Wang, S.; Chou, S. Quinone-Based Conducting Three-Dimensional Metal–Organic Framework as a Cathode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2021, 125, 20814–20820. [Google Scholar] [CrossRef]
- Gu, S.; Xu, S.; Song, X.; Li, H.; Wang, Y.; Zhou, G.; Wang, N.; Chang, H. Electrostatic Potential-Induced Co-N4 Active Centers in a 2D Conductive Metal-Organic Framework for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2022, 14, 50815–50826. [Google Scholar] [CrossRef]
- Zang, Y.; Pei, F.; Huang, J.; Fu, Z.; Xu, G.; Fang, X. Large-Area Preparation of Crack-Free Crystalline Microporous Conductive Membrane to Upgrade High Energy Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1802052. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, Y.; Chen, C.; Yang, J.; Gao, C.; Chen, Y.; Wu, J.; Shen, Y.; Zhang, W.; Li, S.; et al. Conductive MOF-Modified Separator for Mitigating the Shuttle Effect of Lithium-Sulfur Battery through a Filtration Method. ACS Appl. Mater. Interfaces 2019, 11, 11459–11465. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, Y.; Guo, S.; Wang, J.; Ouyang, Y.; Li, D.; Zeng, Q.; Gong, W.; Gan, L.; Zhang, Q.; et al. An ultralight electroconductive metal-organic framework membrane for multistep catalytic conversion and molecular sieving in lithium-sulfur batteries. Energy Storage Mater. 2022, 51, 882–889. [Google Scholar] [CrossRef]
- Cai, D.; Lu, M.; Li, L.; Cao, J.; Chen, D.; Tu, H.; Li, J.; Han, W. A Highly Conductive MOF of Graphene Analogue Ni3 (HITP)2 as a Sulfur Host for High-Performance Lithium-Sulfur Batteries. Small 2019, 15, 1902605. [Google Scholar] [CrossRef]
- Wang, S.; Huang, F.; Zhang, Z.; Cai, W.; Jie, Y.; Wang, S.; Yan, P.; Jiao, S.; Cao, R. R. Conductive metal-organic frameworks promoting polysulfides transformation in lithium-sulfur batteries. J. Energy Chem. 2021, 63, 336–343. [Google Scholar] [CrossRef]
- Lv, Q.; Zhu, Z.; Ni, Y.; Wen, B.; Jiang, Z.; Fang, H.; Li, F. Atomic Ruthenium-Riveted Metal–Organic Framework with Tunable d-Band Modulates Oxygen Redox for Lithium–Oxygen Batteries. J. Am. Chem. Soc. 2022, 144, 23239–23246. [Google Scholar] [CrossRef] [PubMed]
- Majidi, L.; Ahmadiparidari, A.; Shan, N.; Singh, S.K.; Zhang, C.; Huang, Z.; Rastegar, S.; Kumar, K.; Hemmat, Z.; Ngo, A.T.; et al. Nanostructured Conductive Metal Organic Frameworks for Sustainable Low Charge Overpotentials in Li–air Batteries. Small 2022, 18, 2102902. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, H.; Liang, F.; Xue, D. Microwave-irradiation-assisted combustion toward modified graphite as lithium ion battery anode. ACS Appl. Mater. Interfaces 2018, 10, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, Z.; Qi, S.; Wang, P.; Jensen, L.; Johansen, M.; Christensen, C.; Zhang, Y.; Ravnsbæk, D.; Yue, Y. Metal-organic framework glass anode with an exceptional cycling-induced capacity enhancement for lithium-ion batteries. Adv. Mater. 2022, 34, 2110048. [Google Scholar] [CrossRef]
- Zhong, M.; Yan, J.; Wang, L.; Huang, Y.; Li, L.; Gao, S.; Tian, Y.; Shen, W.; Zhang, J.; Guo, S. Hierarchic porous graphite/reduced graphene oxide composites generated from semi-coke as high-performance anodes for lithium-ion batteries. Sustain. Mater. Techno. 2022, 33, e00476. [Google Scholar] [CrossRef]
- Qiu, T.; Yu, Z.; Xie, W.; He, Y.; Wang, H.; Zhang, T. Preparation of Onion-like Synthetic Graphite with a Hierarchical Pore Structure from Anthracite and Its Electrochemical Properties as the Anode Material of Lithium-Ion Batteries. Energy Fuels 2022, 36, 8256–8266. [Google Scholar] [CrossRef]
- Kwon, G.D.; Moyen, E.; Lee, Y.J.; Joe, J.; Pribat, D. Graphene-coated aluminum thin film anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 29486–29495. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, G.; Guo, Z.; Li, X.; Sun, D.; Yu, X. Boron and nitrogen co-doped porous carbon nanotubes webs as a high-performance anode material for lithium ion batteries. Int. J. Hydrogen Energy 2016, 41, 14252–14260. [Google Scholar] [CrossRef]
- Zhang, D.; Su, W.; Li, Z.; Wang, Q.; Yuan, F.; Sun, H.; Li, Y.; Zhang, Y.; Wang, B. Three-dimensional interconnected porous carbon nanoflakes with improved electron transfer and ion storage for lithium-ion batteries. J. Alloys Compd. 2022, 904, 164122. [Google Scholar] [CrossRef]
- Kon, K.; Uchida, K.; Fuku, K.; Yamanaka, S.; Wu, B.; Yamazui, D.; Iguchi, H.; Kobayashi, H.; Gambe, Y.; Honma, I.; et al. Electron-Conductive Metal-Organic Framework, Fe(dhbq)(dhbq = 2,5-Dihydroxy-1,4-benzoquinone): Coexistence of Microporosity and Solid-State Redox Activity. ACS Appl. Mater. Interfaces 2021, 13, 38188–38193. [Google Scholar] [CrossRef]
- Cheng, Q.; Yin, Z.; Pan, Z.; Zhong, X.; Rao, H. Lightweight Free-Standing 3D Nitrogen-Doped Graphene/TiN Aerogels with Ultrahigh Sulfur Loading for High Energy Density Li–S Batteries. ACS Appl. Energy Mater. 2021, 4, 7599–7610. [Google Scholar] [CrossRef]
- McCreary, C.; An, Y.; Kim, S.U.; Hwa, Y. A Perspective on Li/S Battery Design: Modeling and Development Approaches. Batteries 2021, 7, 82. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.T.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li–air Versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhang, X.; Hu, S.; Yu, Y. Investigation of the discharging behaviors of different doped silicon nanowires in alkaline Si-air batteries. J. Ind. Eng. Chem. 2022, 112, 271–278. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-Energy Batteries: Beyond Lithium-Ion and Their Long Road to Commercialisation. Nano-Micro Lett. 2022, 14, 94. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, S.; Hu, S. Si modified by Zn and Fe as anodes in Si-air batteries with ameliorative properties. J. Alloys Compd. 2021, 883, 160902. [Google Scholar] [CrossRef]
- Li, F.; Zhao, J. Atomic Sulfur Anchored on Silicene, Phosphorene, and Borophene for Excellent Cycle Performance of Li–S Batteries. ACS Appl. Mater. Interfaces 2017, 9, 42836–42844. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Hu, J.; Feng, Y.; He, P.; Ma, J. Recent advances in cathode materials for rechargeable lithium-sulfur batteries. Nanoscale 2019, 11, 15418–15439. [Google Scholar] [CrossRef]
- Song, Y.; Cai, W.; Kong, L.; Cai, J.; Zhang, Q.; Sun, J. Rationalizing Electrocatalysis of Li–S Chemistry by Mediator Design: Progress and Prospects. Adv. Energy Mater. 2019, 10, 1901075. [Google Scholar] [CrossRef]
- Ye, H.; Li, M.; Liu, T.; Li, Y.; Lu, J. Activating Li2S as the Lithium-Containing Cathode in Lithium–Sulfur Batteries. ACS Energy Lett. 2020, 5, 2234–2245. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Bernussi, A.A.; Fan, Z. 3-D Edge-Oriented Electrocatalytic NiCo2S4 Nanoflakes on Vertical Graphene for Li–S Batteries. Energy Mater. Adv. 2021, 2021, 2712391. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, W.; Kong, L.; Zhang, L.; Zhu, X.; Shao, Y.; Ding, F.; Zhang, Q.; Sun, J.; Liu, Z. Synchronous immobilization and conversion of polysulfides on a VO2–VN binary host targeting high sulfur load Li–S batteries. Energy Environ. Sci. 2018, 11, 2620–2630. [Google Scholar] [CrossRef]
- Li, S.; Leng, D.; Li, W.; Qie, L.; Dong, Z.; Cheng, Z.; Fan, Z. Recent progress in developing Li2S cathodes for Li–S batteries. Energy Storage Mater. 2020, 27, 279–296. [Google Scholar] [CrossRef]
- Wu, T.; Yang, T.; Zhang, J.; Zheng, X.; Liu, K.; Wang, C.; Chen, M. CoB and BN composites enabling integrated adsorption/catalysis to polysulfides for inhibiting shuttle-effect in Li–S batteries. J. Energy Chem. 2021, 59, 220–228. [Google Scholar] [CrossRef]
- Kim, Y.; Noh, Y.; Bae, J.; Ahn, H.; Kim, M.; Kim, W.B. N-doped carbon-embedded TiN nanowires as a multifunctional separator for Li–S batteries with enhanced rate capability and cycle stability. J. Energy Chem. 2021, 57, 10–18. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.; Zhang, Y.; Jia, J.; He, X.; Yu, L.; Pan, Y.; Liao, J.; Sun, M.; He, J. Interconnected NiCo2O4 nanosheet arrays grown on carbon cloth as a host, adsorber and catalyst for sulfur species enabling high-performance Li–S batteries. Nanoscale Adv. 2021, 3, 1690–1698. [Google Scholar] [CrossRef]
- Pan, Z.; Brett, D.J.L.; He, G.; Parkin, I.P. Progress and Perspectives of Organosulfur for Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103483. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, W.; Wei, N.; Zhang, L.; Ding, F.; Liu, Z.; Sun, J. In-situ PECVD-enabled graphene-V2O3 hybrid host for lithium–sulfur batteries. Nano Energy 2018, 53, 432–439. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, S.; Chen, Y.; Cai, J.; Li, J.; Yang, Q.; Sun, J.; Liu, Z. Enhanced Sulfur Redox and Polysulfide Regulation via Porous VN-Modified Separator for Li–S Batteries. ACS Appl. Mater. Interfaces 2019, 11, 5687–5694. [Google Scholar] [CrossRef]
- Bonnett, B.L.; Smith, E.D.; Garza, M.D.L.; Cai, M.; Haag, J.V.; Serrano, J.M.; Cornell, H.D.; Gibbons, B.; Martin, S.M.; Morris, A.J. PCN-222 Metal-Organic Framework Nanoparticles with Tunable Pore Size for Nanocomposite Reverse Osmosis Membranes. ACS Appl. Mater. Interfaces 2020, 12, 15765–15773. [Google Scholar] [CrossRef]
- Gong, X.; Gnanasekaran, K.; Chen, Z.; Robison, L.; Wasson, M.C.; Bentz, K.C.; Cohen, S.M.; Farha, O.K.; Gianneschi, N.C. Insights into the Structure and Dynamics of Metal-Organic Frameworks Via Transmission Electron Microscopy. J. Am. Chem. Soc. 2020, 142, 17224–17235. [Google Scholar] [CrossRef]
- Saroha, R.; Oh, J.H.; Seon, Y.H.; Kang, Y.C.; Lee, J.S.; Jeong, D.W.; Cho, J.S. Freestanding interlayers for Li–S batteries: Design and synthesis of hierarchically porous N-doped C nanofibers comprising vanadium nitride quantum dots and MOF-derived hollow N-doped C nanocages. J. Mater. Chem. A 2021, 9, 11651–11664. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, G.; Kang, W.; Deng, N.; Cheng, B. Taming polysulfides and facilitating lithium-ion migration: Novel electrospinning MOFs@PVDF-based composite separator with spiderweb-like structure for Li–S batteries. Electrochim. Acta 2021, 365, 137344. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Liu, X.; Zhao, M. Novel Conductive Metal-Organic Framework for a High-Performance Lithium-Sulfur Battery Host: 2D Cu-Benzenehexathial (BHT). ACS Appl. Mater. Interfaces 2018, 10, 15012–15020. [Google Scholar] [CrossRef]
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A lithium-oxygen battery with a long cycle life in an air-like atmosphere. Nature 2018, 555, 502–506. [Google Scholar] [CrossRef]
- Balaish, M.; Jung, J.-W.; Kim, I.-D.; Ein-Eli, Y. A Critical Review on Functionalization of Air-Cathodes for Nonaqueous Li–O2 Batteries. Adv. Funct. Mater. 2019, 30, 1808303. [Google Scholar] [CrossRef]
- Majidi, L.; Yasaei, P.; Warburton, R.E.; Fuladi, S.; Cavin, J.; Hu, X.; Hemmat, Z.; Cho, S.B.; Abbasi, P.; Voros, M.; et al. New Class of Electrocatalysts Based on 2D Transition Metal Dichalcogenides in Ionic Liquid. Adv. Mater. 2019, 31, 1804453. [Google Scholar] [CrossRef]
- Majidi, L.; Hemmat, Z.; Warburton, R.E.; Kumar, K.; Ahmadiparidari, A.; Hong, L.; Guo, J.; Zapol, P.; Klie, R.F.; Cabana, J.; et al. Highly Active Rhenium-, Ruthenium-, and Iridium-Based Dichalcogenide Electrocatalysts for Oxygen Reduction and Oxygen Evolution Reactions in Aprotic Media. Chem. Mater. 2020, 32, 2764–2773. [Google Scholar] [CrossRef]
- Wu, F.; Yu, Y. Toward True Lithium-Air Batteries. Joule 2018, 2, 815–817. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Sun, X.; Yao, Y.; Hong, G. Recent advances of electrically conductive metal-organic frameworks in electrochemical applications. Mater. Today Nano 2021, 13, 100105. [Google Scholar] [CrossRef]
- Dou, J.-H.; Arguilla, M.Q.; Luo, Y.; Li, J.; Zhang, W.; Sun, L.; Mancuso, J.L.; Yang, L.; Chen, T.; Parent, L.R.; et al. Atomically precise single-crystal structures of electrically conducting 2D metal-organic frameworks. Nat. Mater. 2021, 20, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lu, R.; Xia, L.; Liu, Q.; Wang, H.; Zhao, K.; Wang, Z.; Zhao, Y. Density Functional Theory for Electrocatalysis. Energy Environ. Mater. 2021, 5, 157–185. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Y.; Wang, D.; Chen, D.; Zhang, X.; Yu, Y. Graphene-coated Ge as anodes in Ge-air batteries with enhanced performance. Carbon 2023, 205, 86–96. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Hou, J. A novel two-dimensional main group metal organic framework Ga3C6N6 as a promising anode material for Li/Na-Ion batteries. Appl. Surf. Sci. 2022, 599, 153958. [Google Scholar] [CrossRef]



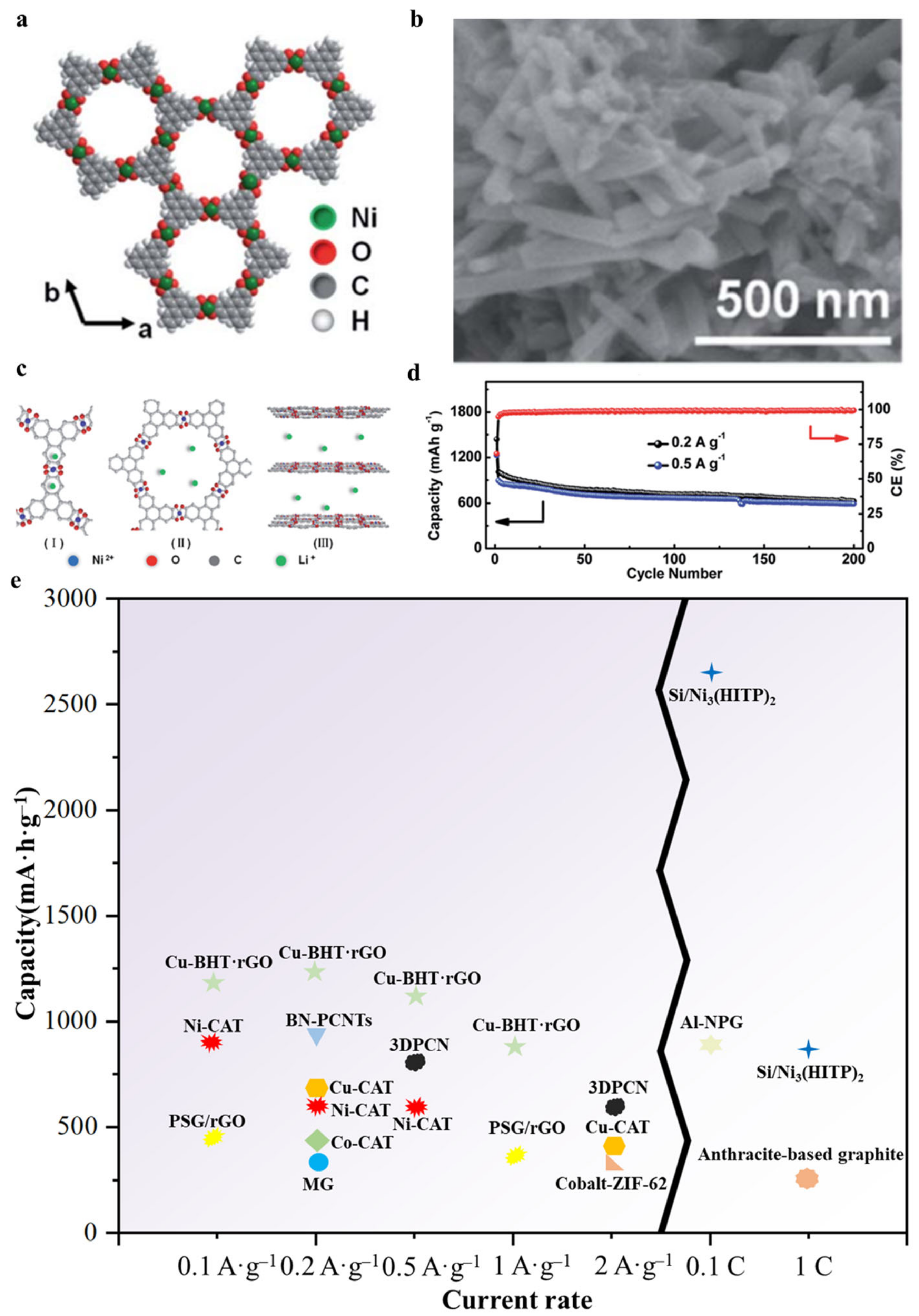
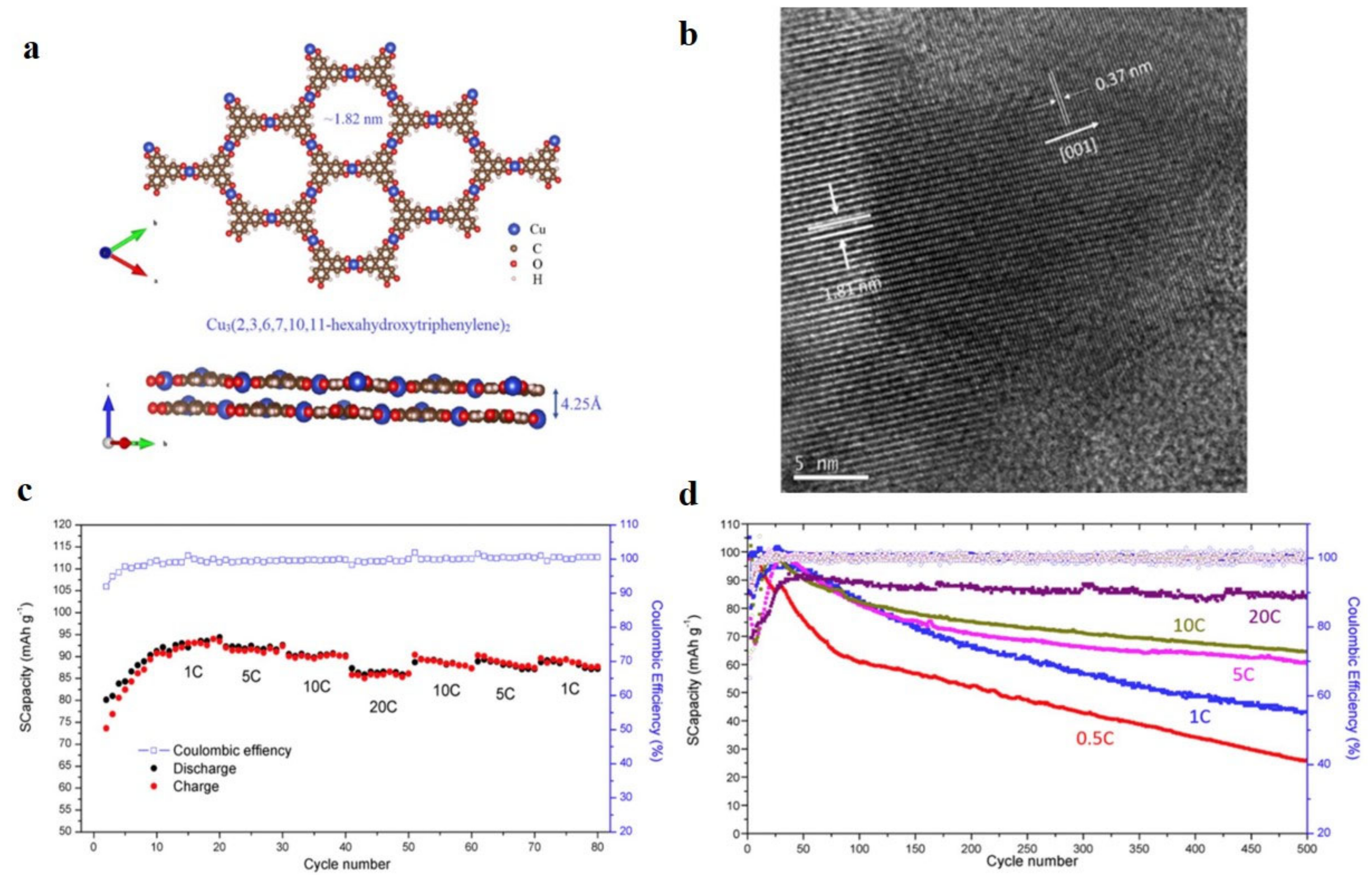
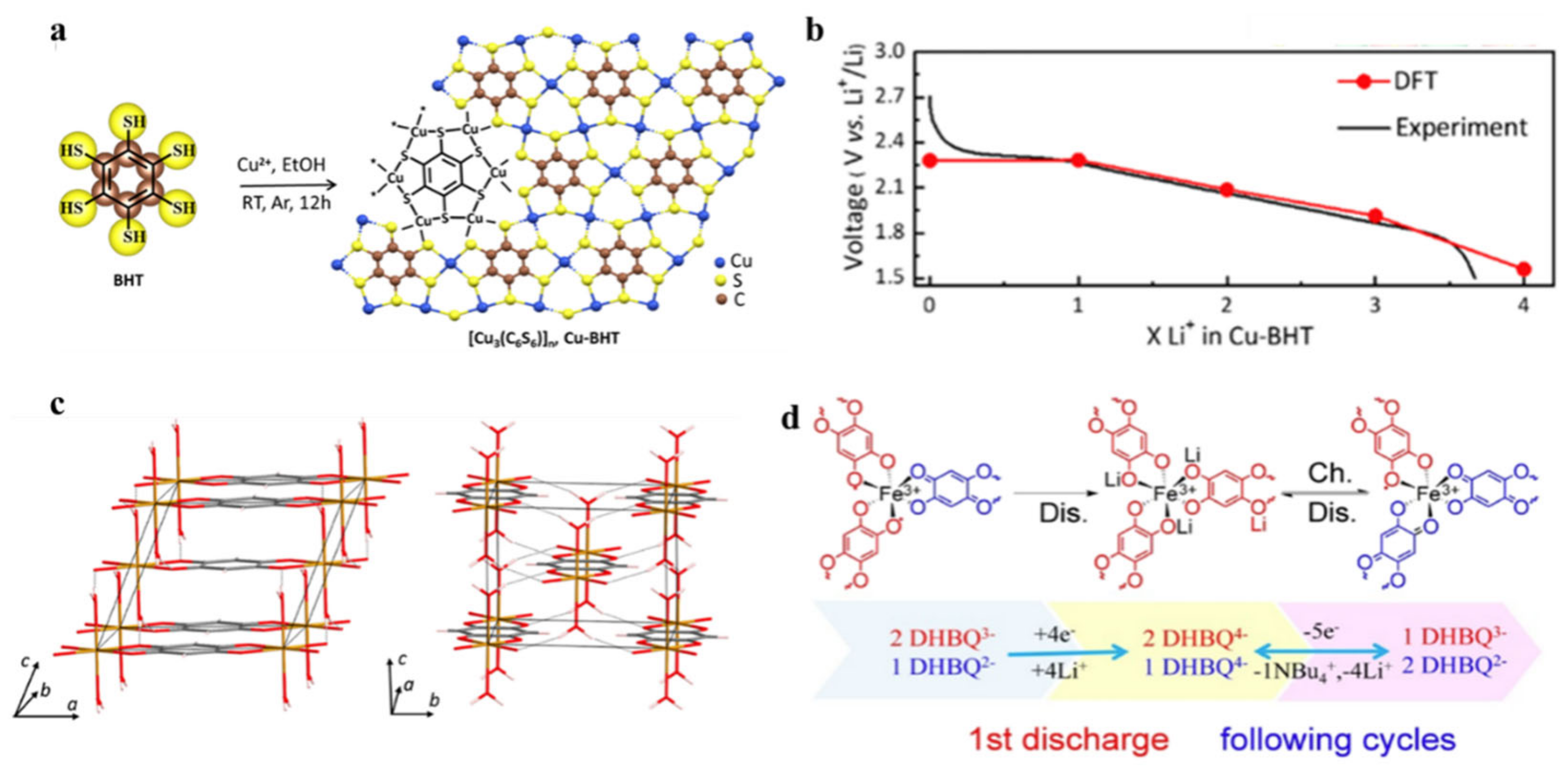
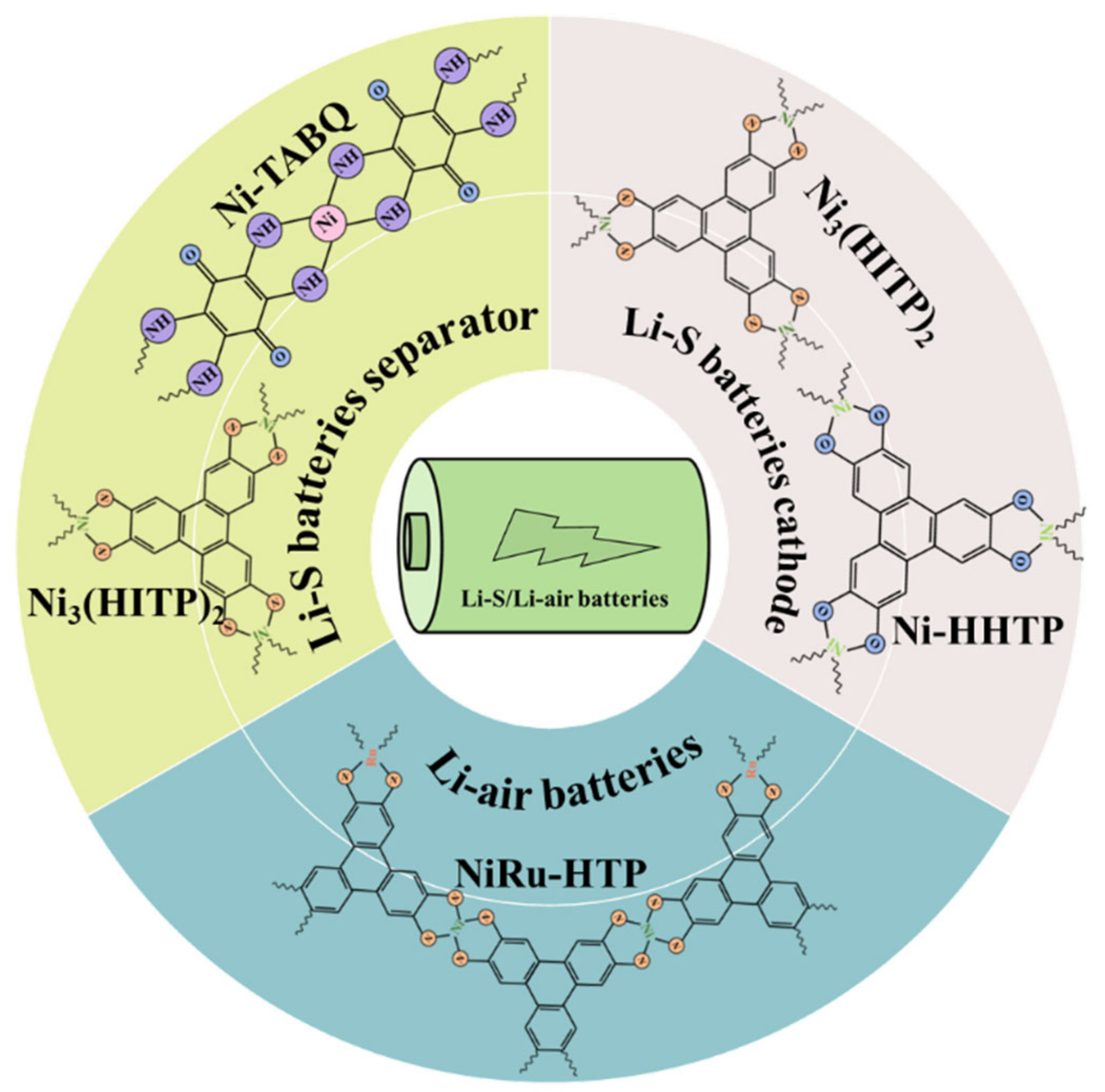
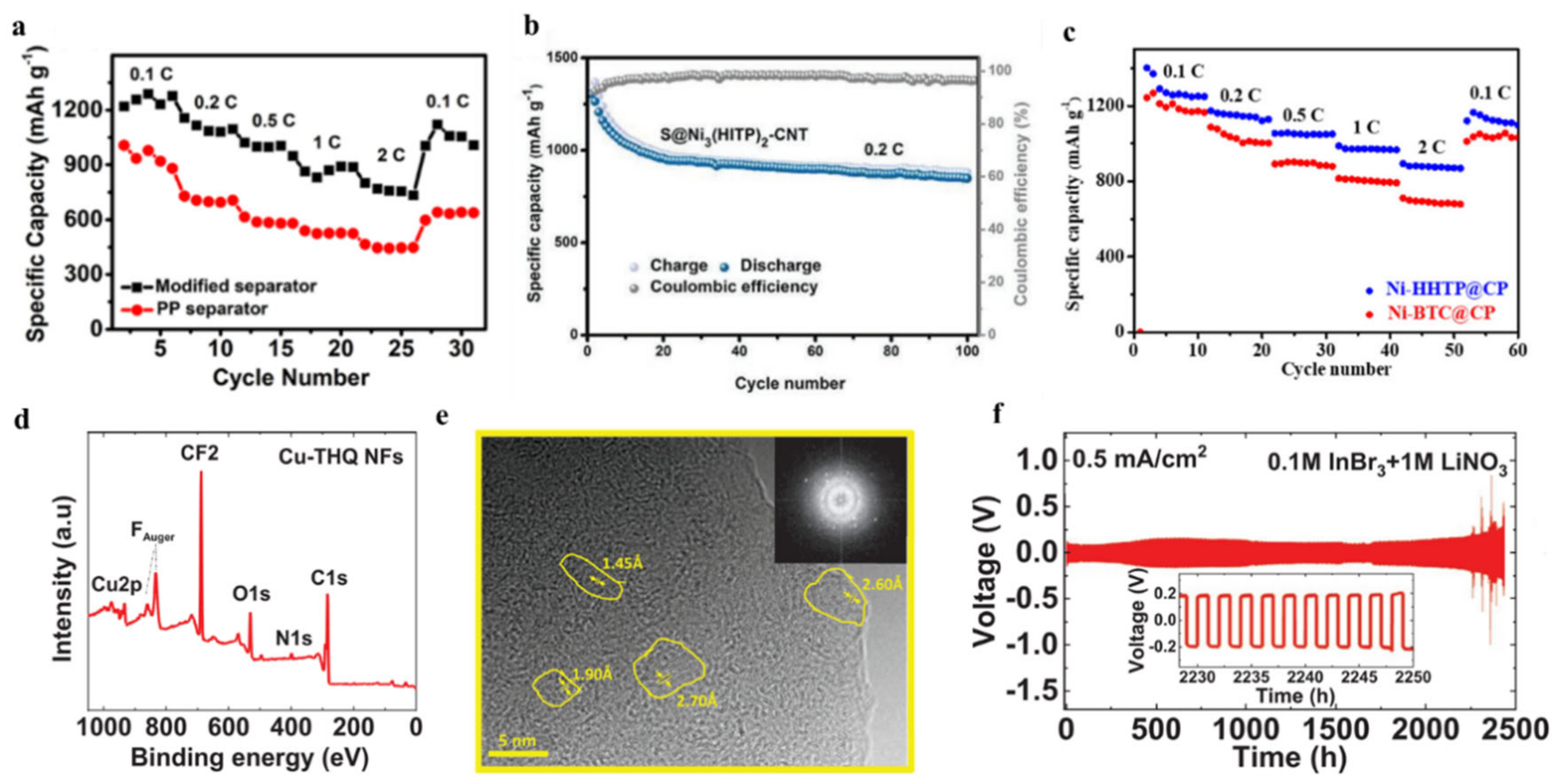

| c-MOFs | Dimension | Application | Current Rate | Cycles | Capacity (mA·h·g−1) | Reference |
|---|---|---|---|---|---|---|
| Ni–CAT | 1 | LIBs anode | 0.1 A·g−1 0.2 A·g−1 0.5 A·g−1 | / 200 200 | 889 626 592 | [77] |
| Cu–CAT | 1 | LIBs anode | 0.2 A·g−1 2.0 A·g−1 | 320 / | 646 381 | [78] |
| Co–CAT | / | LIBs anode | 200 mA·g−1 | 100 | 404 | [79] |
| Si/Ni3(HITP)2 | 2 | LIBs anode | 0.1 C 1 C | 100 1000 | 2657 876 | [80] |
| Cu–BHT·rGO | 2 | LIBs anode | 100 mA·g−1 200 mA·g−1 500 mA·g−1 1000 mA·g−1 | / / / / | 1190.4 1230.8 1131.4 898.7 | [81] |
| Cu–HHTQ | 2 | LIBs anode | 600 mA·g−1 | 200 | 657.6 | [82] |
| Cu3(HHTP)2 | / | LIBs cathode | 1 C | 20 | 94.9 | [83] |
| Cu–BHT | 2 | LIBs cathode | 300 mA·g−1 | 500 | 175 | [84] |
| (NBu4)2Fe2(DHBQ)3 | 3 | LIBs cathode | 500 mA·g−1 | 350 | 103.1 | [85] |
| Co3(HITP)2 | 2 | Li–S batteries separator | / | / | 762 | [86] |
| Ni3(HITP)2 | 2 | Li–S batteries separator | 1 C | 500 | 716 | [87] |
| Ni3(HITP)2 | 2 | Li–S batteries separator | 0.5 C | 300 | 585.4 | [88] |
| Ni–TABQ | 2 | Li–S batteries separator | 1 C | 1000 | 820 | [89] |
| Ni3(HITP)2 | 2 | Li–S batteries cathode | 0.2 C 0.5 C 1 C | 100 150 300 | 1302.9 807.4 629.6 | [90] |
| Ni–HHTP@CP | / | Li–S batteries cathode | 0.2 C | 200 | 910 | [91] |
| NiRu–HTP | / | Li–air batteries cathode | 500 mA·g−1 | 200 | / | [92] |
| Cu–THQ | / | Li–air batteries cathode | 1–2 A·g−1 | 100–300 | 1000–2000 | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, F.; Zhang, Y.; Yu, Y. Conductive Metal–Organic Frameworks for Rechargeable Lithium Batteries. Batteries 2023, 9, 109. https://doi.org/10.3390/batteries9020109
Deng F, Zhang Y, Yu Y. Conductive Metal–Organic Frameworks for Rechargeable Lithium Batteries. Batteries. 2023; 9(2):109. https://doi.org/10.3390/batteries9020109
Chicago/Turabian StyleDeng, Fengjun, Yuhang Zhang, and Yingjian Yu. 2023. "Conductive Metal–Organic Frameworks for Rechargeable Lithium Batteries" Batteries 9, no. 2: 109. https://doi.org/10.3390/batteries9020109
APA StyleDeng, F., Zhang, Y., & Yu, Y. (2023). Conductive Metal–Organic Frameworks for Rechargeable Lithium Batteries. Batteries, 9(2), 109. https://doi.org/10.3390/batteries9020109







