Effect of Graphite Morphology on the Electrochemical and Mechanical Properties of SiOx/Graphite Composite Anode
Abstract
:1. Introduction
2. Materials and Methods
3. Results
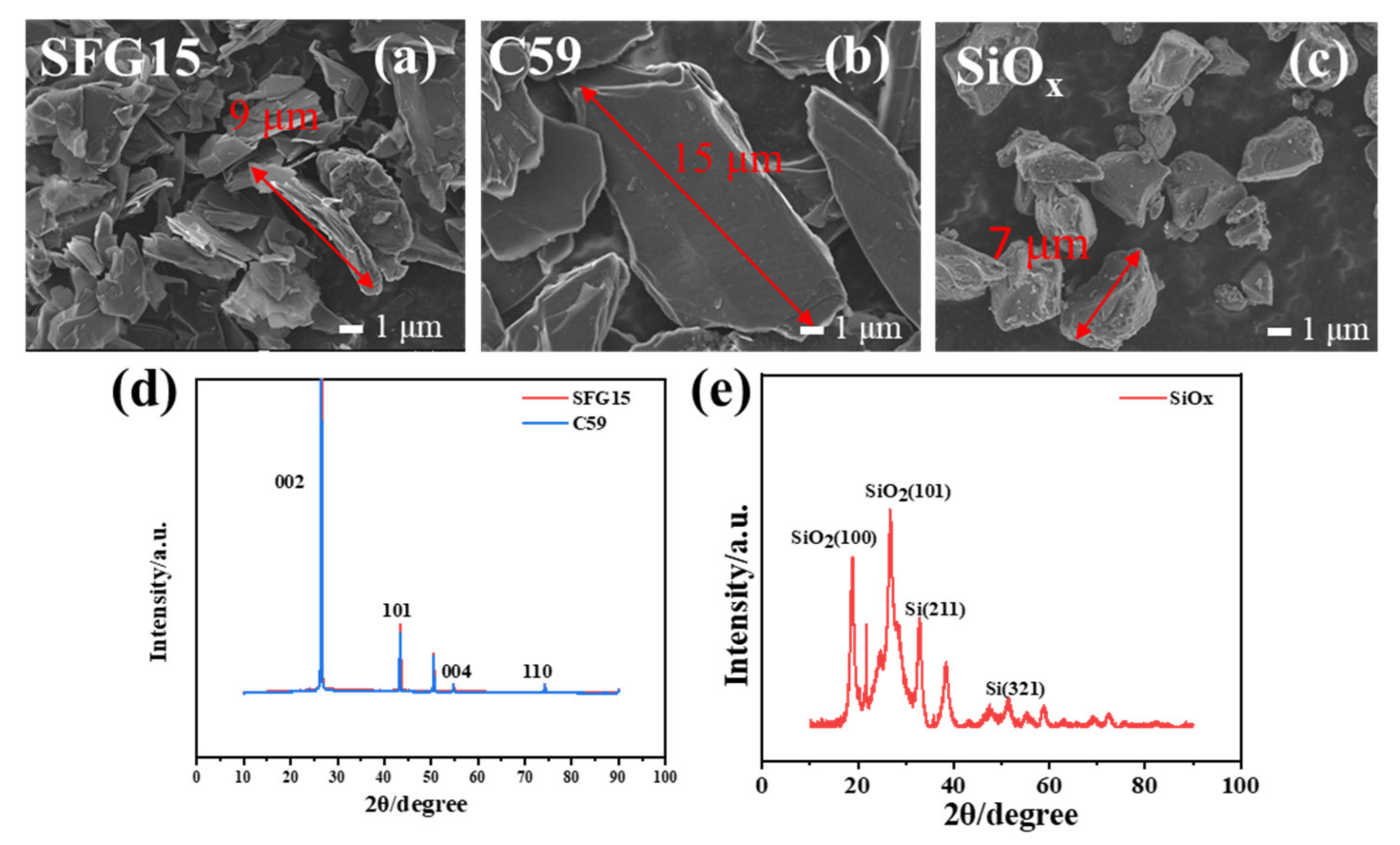
3.1. Physical-Chemical Characteristics of Graphites and SiOx
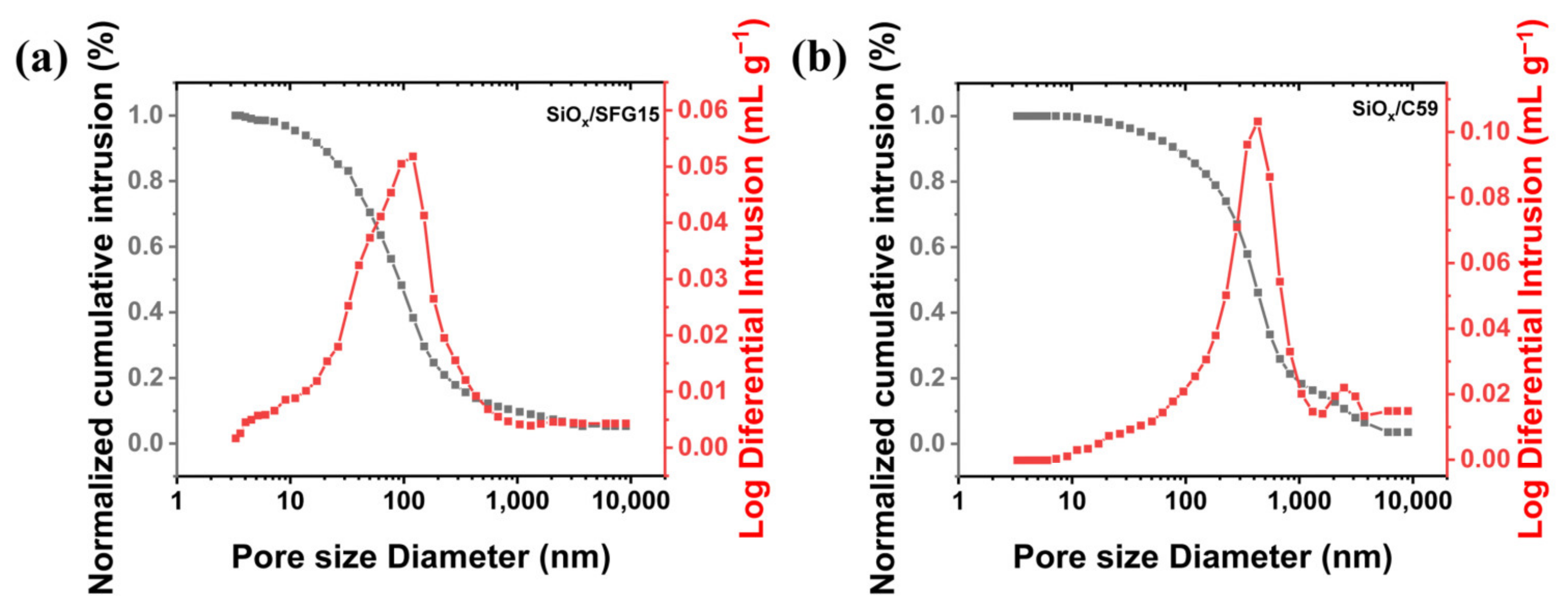
3.2. Pore Structure Characterization of SiOx/C Electrodes
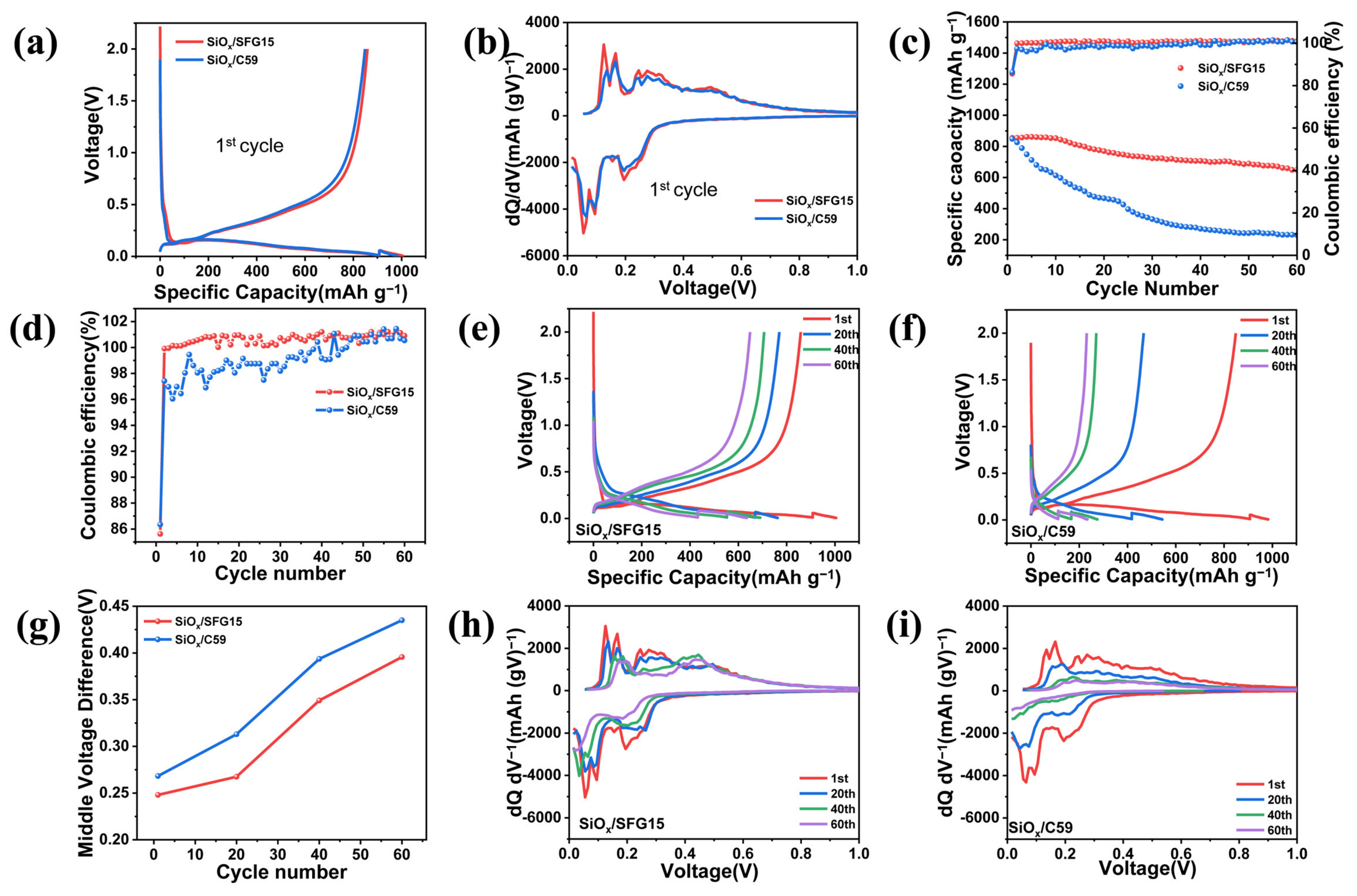
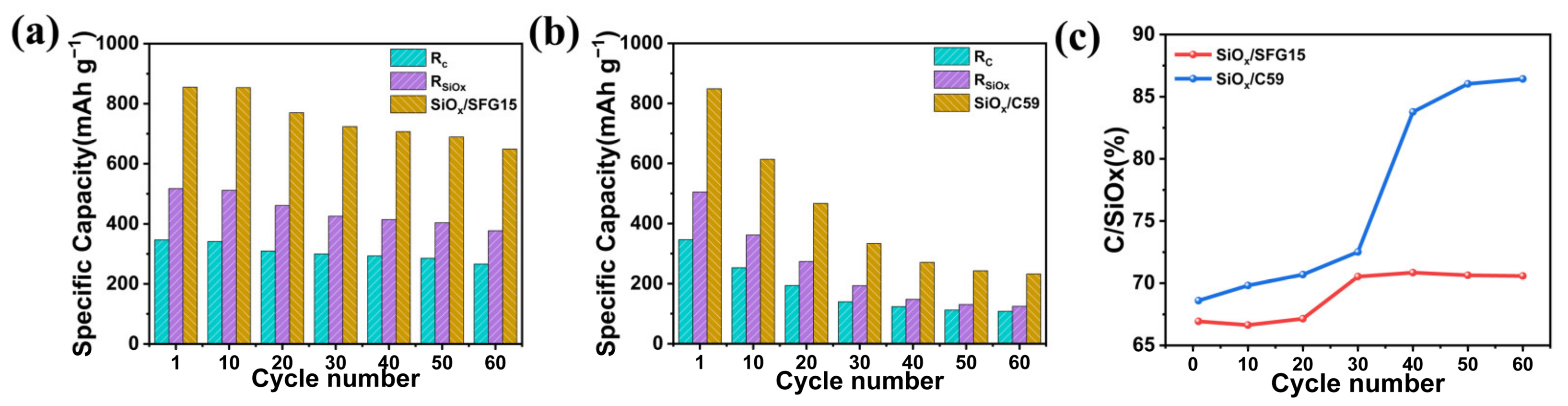
3.3. Electrochemical Performances of SiOx/C Electrodes
3.4. Analysis of Electrode Structure Changes before and after Cycling
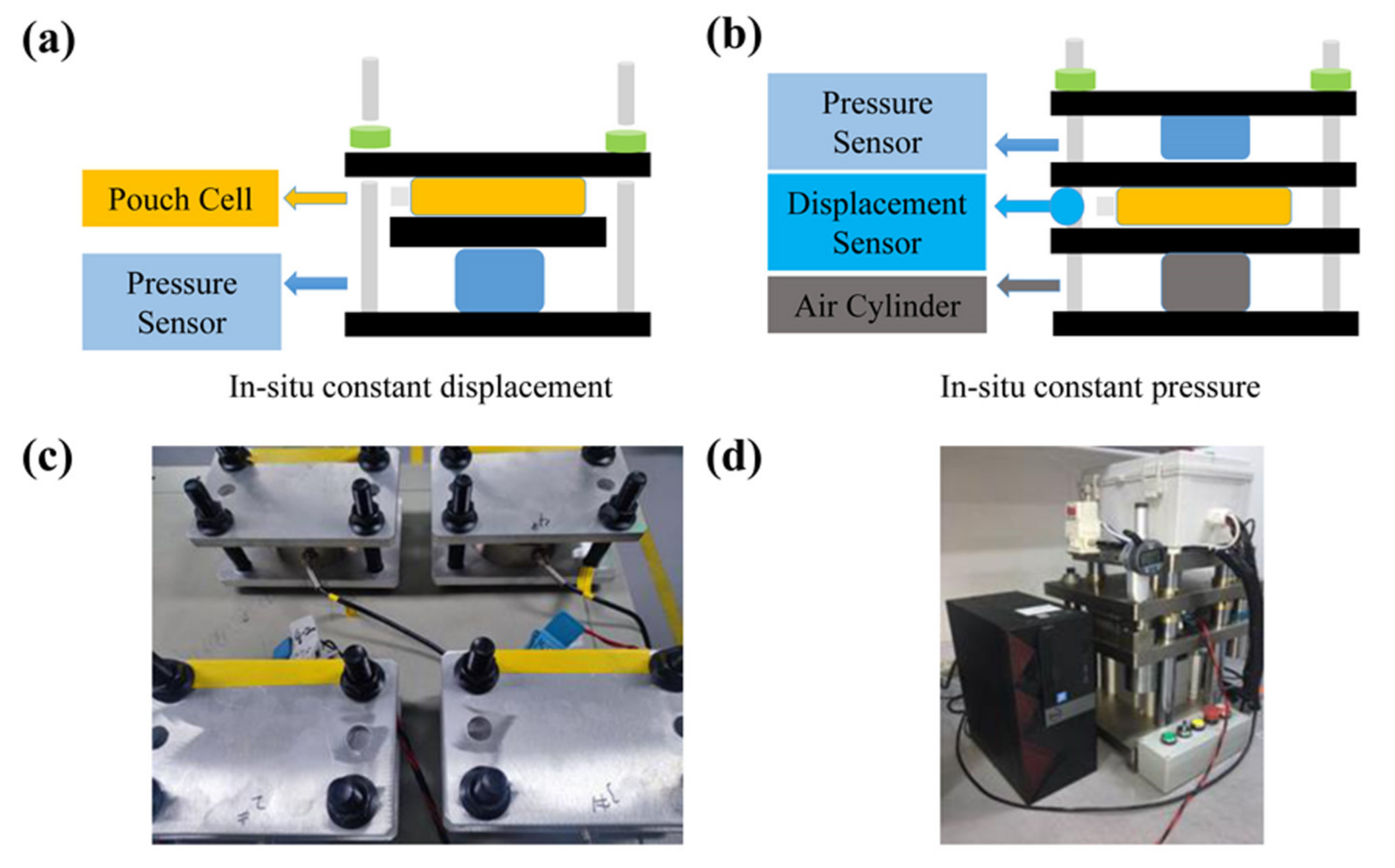
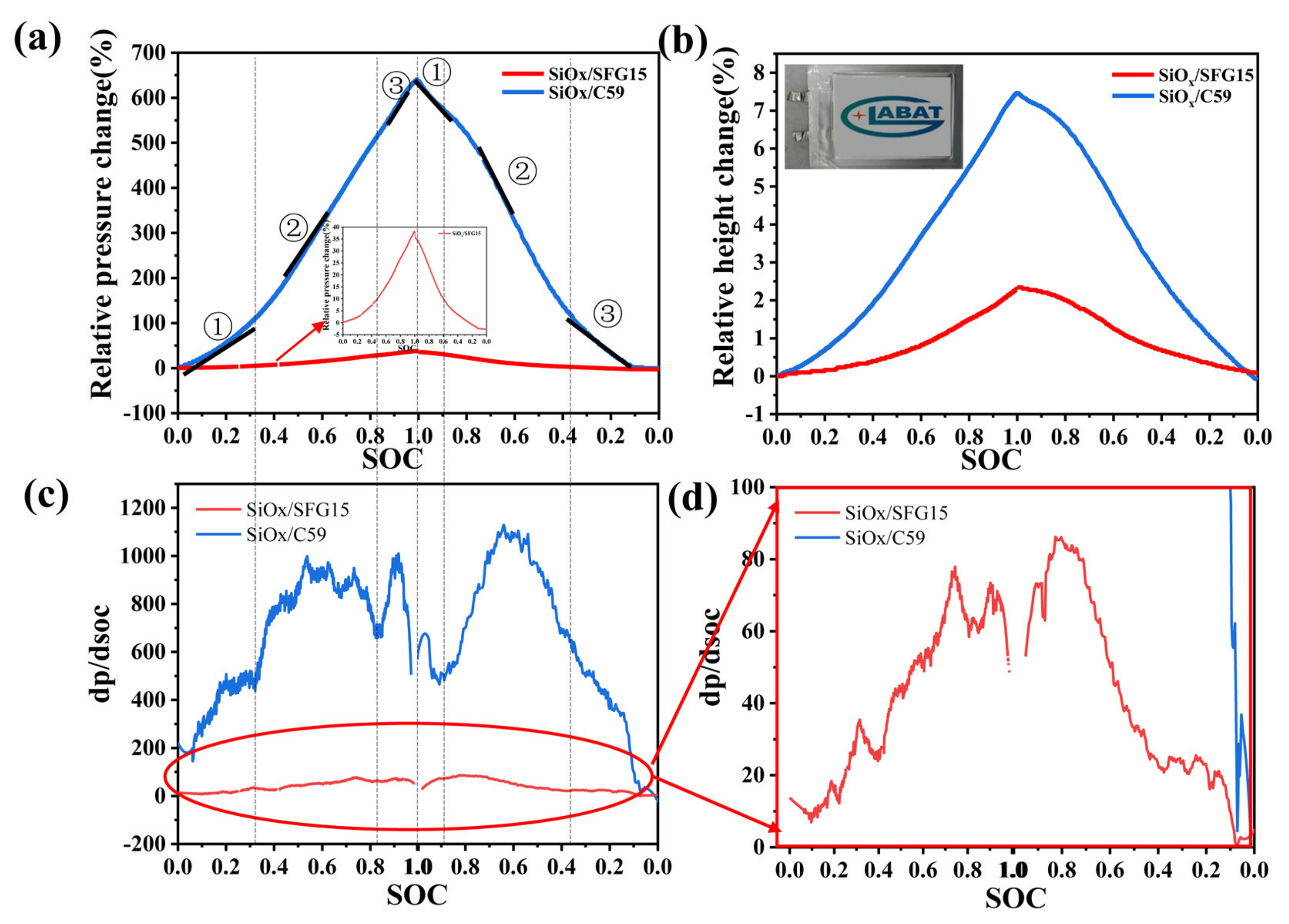
3.5. In-Situ Constant Displacement/Pressure Detection of NCM811|| SiOx/C Pouch Cells
4. Discussion
- (1)
- The results of mercury intrusion porosimetry test and SEM show that the graphite morphology has a large effect on the structure of the composite electrode. The large-size C59 graphite particles are mixed with SiOx particles to make SiOx/C59 composite electrode, in which the SiOx particles agglomerate between the large-size graphite sheets. The conductive network in the electrode is more easily damaged due to the expanding of the SiOx particles with local agglomeration. While the small-size SFG15 graphite particles contribute to build a composite electrode with random and uniform particle distribution. This facilitates the construction of the stable conductive network for the composite electrode.
- (2)
- Furthermore, the difference in electrode structure affects the cycle performance of the electrode. The SiOx/C59 electrode shows poor cycling performance remaining charging specific capacity of only 231.3 mAh g−1 after 60 cycles at 0.1 C. SEM images show that the failure of the SiOx/C59 electrode structure is the main cause of the deactivation of the active material and rapid capacity decay, while, the SiOx/SFG15 electrode shows better cycling performance with charging specific capacity of 648.9 mAh g−1 after 60 cycles at 0.1 C due to its stable electrode structure.
- (3)
- Besides, the in-situ constant displacement/pressure method was used to study the electrode stress and strain characteristics. The results show that the SiOx/C electrode constructed by small particle graphite with small anisotropy could effectively reduce the stress and strain of the electrode during the charging/discharging. Thus, small particle graphite can improve the stability of the electrode structure, and then restrain the capacity decay of the SiOx/C composite electrode upon cycling.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Limthongkul, P.; Jang, Y.-I.; Dudney, N.J.; Chiang, Y.-M. Electrochemically-driven solid-state amorphization in lithium-silicon alloys and implications for lithium storage. Acta Mater. 2003, 51, 1103–1113. [Google Scholar] [CrossRef]
- Beattie, S.D.; Loveridge, M.J.; Lain, M.J.; Ferrari, S.; Polzin, B.J.; Bhagat, R.; Dashwood, R. Understanding capacity fade in silicon based electrodes for lithium-ion batteries using three electrode cells and upper cut-off voltage studies. J. Power Sources 2016, 302, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Zhou, Q.; Peng, Y.; Yang, H. Enhancing electrochemical properties of silicon-graphite anodes by the introduction of cobalt for lithium-ion batteries. J. Power Sources 2015, 290, 71–79. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.-Y.; Sun, J.-K.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Watermelon-Inspired Si/C Microspheres with Hierarchical Buffer Structures for Densely Compacted Lithium-Ion Battery Anodes. Adv. Energy Mater. 2017, 7, 1601481. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.; Kim, B.H.; Kim, Y.J.; Yang, K.S.; Park, M.S. Facile Synthesis of Carbon-Coated Silicon/Graphite Spherical Composites for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 12109–12117. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Ma, J.; Kim, N.; Lee, H.-W.; Cui, Y.; Cho, J. Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries. Nat. Energy 2016, 1, 16113. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Q.; Yuca, N.; Ling, M.; Higa, K.; Battaglia, V.S.; Parkinson, D.Y.; Srinivasan, V.; Liu, G. A Convenient and Versatile Method To Control the Electrode Microstructure toward High-Energy Lithium-Ion Batteries. Nano Lett. 2016, 16, 4686–4690. [Google Scholar] [CrossRef] [Green Version]
- Jeong, G.; Lee, S.M.; Choi, N.S.; Kim, Y.-U.; Lee, C.K. Stabilizing dimensional changes in Si-based composite electrodes by controlling the electrode porosity: An in situ electrochemical dilatometric study. Electrochim. Acta 2011, 56, 5095–5101. [Google Scholar] [CrossRef]
- Bitsch, B.; Gallasch, T.; Schroeder, M.; Börner, M.; Winter, M.; Willenbacher, N. Capillary suspensions as beneficial formulation concept for high energy density Li-ion battery electrodes. J. Power Sources 2016, 328, 114–123. [Google Scholar] [CrossRef]
- Diehm, R.; Kumberg, J.; Dörrer, C.; Müller, M.; Bauer, W.; Scharfer, P.; Schabel, W. In Situ Investigations of Simultaneous Two-Layer Slot Die Coating of Component-Graded Anodes for Improved High-Energy Li-Ion Batteries. Energy Technol. 2020, 8, 1901251. [Google Scholar] [CrossRef]
- Liu, D.; Chen, L.-C.; Liu, T.-J.; Chu, W.-B.; Tiu, C. Improvement of Lithium-Ion Battery Performance by Two-Layered Slot-Die Coating Operation. Energy Technol. 2017, 5, 1235–1241. [Google Scholar] [CrossRef]
- Chen, L.-C.; Liu, D.; Liu, T.-J.; Tiu, C.; Yang, C.-R.; Chu, W.-B.; Wan, C.-C. Improvement of lithium-ion battery performance using a two-layered cathode by simultaneous slot-die coating. J. Energy Storage 2016, 5, 156–162. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Sun, S.; Sun, X.; Cao, F.; Ohsaka, T.; Wu, J. Analysis of the relationship between vertical imparity distribution of conductive additive and electrochemical behaviors in lithium ion batteries. Electrochim. Acta 2018, 269, 422–428. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, L.; Yao, H. Improving the electrochemical performance of Si-based anode via gradient Si concentration. Mater. Des. 2019, 177, 107851. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, G.; Cheng, X.; Zuo, P. Enhanced lithium storage performance of silicon anode via fabricating into sandwich electrode. Electrochim. Acta 2011, 56, 4403–4407. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Ji, J.; Qiu, B.; Zhang, K.; Liu, Z. Superior cycling performance of a sandwich structure Si/C anode for lithium ion batteries. RSC Adv. 2016, 6, 12107–12113. [Google Scholar] [CrossRef]
- Huang, C.; Kim, A.; Chung, D.J.; Park, E.; Young, N.P.; Jurkschat, K.; Kim, H.; Grant, P.S. Multiscale Engineered Si/SiO x Nanocomposite Electrodes for Lithium-Ion Batteries Using Layer-by-Layer Spray Deposition. ACS Appl. Mater. Interfaces 2018, 10, 15624–15633. [Google Scholar] [CrossRef]
- Wu, J.; Qin, X.; Zhang, H.; He, Y.-B.; Li, B.; Ke, L.; Lv, W.; Du, H.; Yang, Q.-H.; Kang, F. Multilayered silicon embedded porous carbon/graphene hybrid film as a high performance anode. Carbon 2015, 84, 434–443. [Google Scholar] [CrossRef]
- Jeschull, F.; Surace, Y.; Zürcher, S.; Lari, G.; Spahr, M.E.; Novák, P.; Trabesinger, S. Graphite Particle-Size Induced Morphological and Performance Changes of Graphite–Silicon Electrodes. J. Electrochem. Soc. 2020, 167, 100535. [Google Scholar] [CrossRef]
- Gómez-Cámer, J.L.; Bünzli, C.; Hantel, M.M.; Poux, T.; Novák, P. On the correlation between electrode expansion and cycling stability of graphite/Si electrodes for Li-ion batteries. Carbon 2016, 105, 42–51. [Google Scholar] [CrossRef]
- Rieger, B.; Schlueter, S.; Erhard, S.V.; Schmalz, J.; Reinhart, G.; Jossen, A. Multi-scale investigation of thickness changes in a commercial pouch type lithium-ion battery. J. Energy Storage 2016, 6, 213–221. [Google Scholar] [CrossRef]
- Oh, K.-Y.; Siegel, J.B.; Secondo, L.; Kim, S.U.; Samad, N.A.; Qin, J.; Anderson, D.; Garikipati, K.; Knobloch, A.; Epureanu, B.I.; et al. Rate dependence of swelling in lithium-ion cells. J. Power Sources 2014, 267, 197–202. [Google Scholar] [CrossRef]
- Wagner, M.R.; Raimann, P.R.; Trifonova, A.; Moller, K.C.; Besenhard, J.O.; Winter, M. Dilatometric and mass spectrometric investigations on lithium ion battery anode materials. Anal. Bioanal. Chem. 2004, 379, 272–276. [Google Scholar] [CrossRef]
- Hahn, M.; Buqa, H.; Ruch, P.; Goers, D.; Spahr, M.; Ufheil, J.; Nova, P.; Kotz, R. A Dilatometric Study of Lithium Intercalation into Powder-Type Graphite Electrodes. Electrochem. Solid State 2008, 11, A151. [Google Scholar] [CrossRef]
- Gómez Cámer, J.L.; Morales, J.; Sánchez, L.; Ruch, P.; Ng, S.H.; Kötz, R.; Novák, P. Nanosized Si/cellulose fiber/carbon composites as high capacity anodes for lithium-ion batteries: A galvanostatic and dilatometric study. Electrochim. Acta 2009, 54, 6713–6717. [Google Scholar] [CrossRef]
- Louli, A.J.; Li, J.; Trussler, S.; Fell, C.R.; Dahn, J.R. Volume, Pressure and Thickness Evolution of Li-Ion Pouch Cells with Silicon-Composite Negative Electrodes. J. Electrochem. Soc. 2017, 164, A2689–A2696. [Google Scholar] [CrossRef]
- Park, S.; Kim, T.; Oh, S.M. Electrochemical Dilatometry Study on Si-Embedded Carbon Nanotube Powder Electrodes. Electrochem. Solid-State Lett. 2007, 10, A142. [Google Scholar] [CrossRef]
- Hwang, S.S.; Cho, C.G.; Kim, H. Polymer microsphere embedded Si/graphite composite anode material for lithium rechargeable battery. Electrochim. Acta 2010, 55, 3236–3239. [Google Scholar] [CrossRef]
- Berckmans, G.; De Sutter, L.; Marinaro, M.; Smekens, J.; Jaguemont, J.; Wohlfahrt-Mehrens, M.; van Mierlo, J.; Omar, N. Analysis of the effect of applying external mechanical pressure on next generation silicon alloy lithium-ion cells. Electrochim. Acta 2019, 306, 387–395. [Google Scholar] [CrossRef]
- De Sutter, L.; Berckmans, G.; Marinaro, M.; Wohlfahrt-Mehrens, M.; Berecibar, M.; Van Mierlo, J. Mechanical behavior of Silicon-Graphite pouch cells under external compressive load: Implications and opportunities for battery pack design. J. Power Sources 2020, 451, 227774. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lim, H.K.; Seo, J.-H.; Shin, W.J.; Choi, J.H.; Park, J.H. Development of Standardized Battery Pack for Next-Generation PHEVs in Considering the Effect of External Pressure on Lithium-Ion Pouch Cells. SAE Int. J. Altern. Powertrains 2018, 7, 195–205. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Y.; Yang, Y.; Fu, H.; Sun, X.; Ding, X. Insight into the electrochemical behavior of lithium-sulfur cells assisted by potassium hydroxide activated carbon black and polyaniline nanorods. Electrochim. Acta 2016, 209, 643–653. [Google Scholar] [CrossRef]
- Buqa, H.; Würsig, A.; Vetter, J.; Spahr, M.E.; Krumeich, F.; Novák, P. SEI film formation on highly crystalline graphitic materials in lithium-ion batteries. J. Power Sources 2006, 153, 385–390. [Google Scholar] [CrossRef]
- Dudley, J.T.; Wilkinson, D.P.; Thomas, G.; LeVae, R.; Woo, S.; Blom, H.; Dahn, J.R. Conductivity of electrolytes for rechargeable lithium batteries. J. Power Sources 1991, 35, 59–82. [Google Scholar] [CrossRef]
- Oumellal, Y.; Delpuech, N.; Mazouzi, D.; Dupré, N.; Gaubicher, J.; Moreau, P.; Soudan, P.; Lestriez, B.; Guyomard, D. The failure mechanism of nano-sized Si-based negative electrodes for lithium ion batteries. J. Mater. Chem. 2011, 21, 6201–6208. [Google Scholar] [CrossRef]
- Zhao, K.-J.; Li, Y.-G.; Brassart, L. Pressure-sensitive plasticity of lithiated silicon in Li-ion batteries. Acta Mech. Sin. 2013, 29, 379–387. [Google Scholar] [CrossRef]
- Pan, K.; Zou, F.; Canova, M.; Zhu, Y.; Kim, J.-H. Systematic electrochemical characterizations of Si and SiO anodes for high-capacity Li-Ion batteries. J. Power Sources 2019, 413, 20–28. [Google Scholar] [CrossRef]
- Zhao, K.; Tritsaris, G.A.; Pharr, M.; Wang, W.L.; Okeke, O.; Suo, Z.; Vlassak, J.J.; Kaxiras, E. Reactive flow in silicon electrodes assisted by the insertion of lithium. Nano Lett 2012, 12, 4397–4403. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhu, T. Atomistic mechanisms of lithium insertion in amorphous silicon. J. Power Sources 2011, 196, 3664–3668. [Google Scholar] [CrossRef]


| D10 (μm) | D50 (μm) | D90 (μm) | Specific Surface Area (m2 g−1) | Tap Density g cm−3 | Resistivity Ω·cm | |
|---|---|---|---|---|---|---|
| SFG15 | 4.76 | 9.34 | 15.7 | 8.757 | 0.212 | 0.14 × 103 |
| C59 | 7.79 | 15.7 | 26.3 | 1.525 | 0.940 | 2.41 × 103 |
| SiOx | 4.13 | 7.73 | 10.5 | 1.791 | 0.202 | — |
| Porosity (%) | Total Intrusion Volume (mL/g) | Median Proe Diameter (μm) | |
|---|---|---|---|
| SiOx/SFG15 | 33.83 | 0.059 | 0.10 |
| SiOx/C59 | 39.05 | 0.082 | 0.39 |
| First Lithiation Specific Capacity (mAh g−1) | First Delithiation Specific Capacity (mAh g−1) | First Coulombic Efficiency (%) | 60 Cycles Capacity Retention (%) | Average Coulombic Efficiency (%) | |
|---|---|---|---|---|---|
| SiOx/SFG15 | 1002.8 | 858.6 | 85.62 | 75.15 | 100.43 |
| SiOx/C59 | 982.8 | 848.7 | 86.35 | 27.25 | 98.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Ma, T.; Liu, X.; Liu, Z.; Chang, Z.; Pang, J. Effect of Graphite Morphology on the Electrochemical and Mechanical Properties of SiOx/Graphite Composite Anode. Batteries 2023, 9, 78. https://doi.org/10.3390/batteries9020078
Wang C, Ma T, Liu X, Liu Z, Chang Z, Pang J. Effect of Graphite Morphology on the Electrochemical and Mechanical Properties of SiOx/Graphite Composite Anode. Batteries. 2023; 9(2):78. https://doi.org/10.3390/batteries9020078
Chicago/Turabian StyleWang, Chenyang, Tianyi Ma, Xingge Liu, Zhi Liu, Zenghua Chang, and Jing Pang. 2023. "Effect of Graphite Morphology on the Electrochemical and Mechanical Properties of SiOx/Graphite Composite Anode" Batteries 9, no. 2: 78. https://doi.org/10.3390/batteries9020078






