Improving Interfaces in All-Solid-State Supercapacitors Using Polymer-Added Activated Carbon Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid Electrolyte Membrane Synthesis
2.2. ASSC Fabrication
3. Results and Discussion
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Wang, Z.Y.; He, Z.J.; Li, Y.J.; Mao, J.; Dai, K.H.; Yan, C.; Zheng, J.C. An advance review of solid-state battery: Challenges, progress and prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar] [CrossRef]
- Olabi, A.; Abbas, Q.; Abdelkareem, M.; Alami, A.; Mirzaeian, M.; Sayed, E. Carbon-Based Materials for Supercapacitors: Recent Progress, Challenges and Barriers. Batteries 2023, 9, 19. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nanosci. Technol. A Collect. Rev. Nat. J. 2009, 7, 320–329. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X. Conducting polymers directly coated on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Böckenfeld, N.; Jeong, S.; Winter, M.; Passerini, S.; Balducci, A. Natural, cheap and environmentally friendly binder for supercapacitors. J. Power Sources 2013, 221, 14–20. [Google Scholar] [CrossRef]
- Bhattarai, R.; Chhetri, K.; Natarajan, S.; Saud, S.; Kim, S.; Mok, Y. Activated carbon derived from cherry flower biowaste with a self-doped heteroatom and large specific surface area for supercapacitor and sodium-ion battery applications. Chemosphere 2022, 303, 135290. [Google Scholar] [CrossRef]
- Tjandra, R.; Liu, W.; Zhang, M.; Yu, A. All-carbon flexible supercapacitors based on electrophoretic deposition of graphene quantum dots on carbon cloth. J. Power Sources 2019, 438, 227009. [Google Scholar] [CrossRef]
- Bai, Y.; Li, N.; Yang, C.; Wu, X.; Yang, H.; Chen, W.; Li, H.; Zhao, B.; Wang, P.; Han, X. Realizing high-voltage and ultralong-life supercapacitors by a universal interfacial engineering strategy. J. Power Sources 2021, 510, 230406. [Google Scholar] [CrossRef]
- Ren, J.; Ren, R.; Lv, Y. Stretchable all-solid-state supercapacitors based on highly conductive polypyrrole-coated graphene foam. Chem. Eng. J. 2018, 349, 111–118. [Google Scholar] [CrossRef]
- Xiao, X.; Duan, X.; Song, Z.; Deng, X.; Deng, W.; Hou, H.; Zheng, R.; Zou, G.; Ji, X. High-Throughput Production of Cheap Mineral-Based Heterostructures for High Power. Adv. Funct. Mater. 2022, 32, 202110476. [Google Scholar] [CrossRef]
- Ulihin, A.; Mateyshina, Y.; Uvarov, N. All-solid-state asymmetric supercapacitors with solid composite electrolytes. Solid State Ion. 2013, 251, 62–65. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Wu, J.; Gu, Z.; Xin, X.; Yao, X. In Situ Solidified Gel Polymer Electrolytes for Stable Solid-State Lithium Batteries at High Temperatures. Batteries 2023, 9, 28. [Google Scholar] [CrossRef]
- Asl, M.S.; Hadi, R.; Salehghadimi, L.; Tabrizi, A.G.; Farhoudian, S.; Babapoor, A.; Pahlevani, M. Flexible all-solid-state supercapacitors with high capacitance, long cycle life, and wide operational potential window: Recent progress and future perspectives. J. Energy Storage 2022, 50, 104223. [Google Scholar] [CrossRef]
- Sharma, S.; Dalvi, A. Solid polymer electrolyte membranes using the “polymer-in-ceramic” approach for all-solid-state supercapacitor applications. Solid State Ion. 2022, 387, 116063. [Google Scholar] [CrossRef]
- Singh, M.D.; Dalvi, A.; Phase, D. Novel Na3Zr2Si2PO12–polymer hybrid composites with high ionic conductivity for solid-state ionic devices. Mater. Lett. 2020, 262, 127022. [Google Scholar] [CrossRef]
- Sharma, N.; Dalvi, A. Mechanical milling assisted synthesis of novel LiTi2(PO4)3-glass-ceramic nanocomposites. J. Non-Cryst. Solids 2018, 483, 126–133. [Google Scholar] [CrossRef]
- Kwatek, K.; Nowiński, J. Electrical properties of LiTi2(PO4)3 and Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes containing ionic liquid. Solid State Ion. 2017, 302, 54–60. [Google Scholar] [CrossRef]
- Cheng, S.H.-S.; He, K.-Q.; Liu, Y.; Zha, J.-W.; Kamruzzaman, M.; Ma, R.L.-W.; Dang, Z.-M.; Li, R.K.; Chung, C. Electrochemical performance of all-solid-state lithium batteries using inorganic lithium garnets particulate reinforced PEO/LiClO4 electrolyte. Electrochim. Acta 2017, 253, 430–438. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Dalvi, A.; Singh, M.D.; Nayak, B.; Choudhury, B.; Sarit, A. Li+-NASICON crystallites in PEO-LiCF3SO3 matrix Characterization of a novel hybrid electrolyte. Solid State Ionics 2017, 311, 20–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Kaur, G.; Sivasubramanian, S.C.; Dalvi, A. Solid-state supercapacitors using ionic liquid dispersed Li+-NASICONs as electrolytes. Electrochim. Acta 2022, 434, 141311. [Google Scholar] [CrossRef]
- Parejiya, A.; Amin, R.; Dixit, M.; Essehli, R.; Jafta, C.; Wood, D.; Belharouak, I. Improving Contact Impedance via Electrochemical Pulses Applied to Lithium-Solid Electrolyte Interface in Solid-State Batteries. ACS Energy Lett. 2021, 6, 3669–3675. [Google Scholar] [CrossRef]
- Richards, W.D.; Miara, L.J.; Wang, Y.; Kim, J.C.; Ceder, G. Interface Stability in Solid-State Batteries. Chem. Mater. 2016, 28, 266–273. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, M.; Dalvi, A. All-solid-state electric double layer supercapacitors using Li1.3Al0.3Ti1.7(PO4)3 reinforced solid polymer electrolyte. J. Energy Storage 2022, 49, 104178. [Google Scholar] [CrossRef]
- Singh, M.; Dalvi, A.; Phase, D.; Kumar, Y. Materials composites: Assessment of enhanced Li + ion transport and potential for solid-state supercapacitor applications. J. Mater. Sci. 2020, 55, 3951–3963. [Google Scholar] [CrossRef]
- Neha; Dalvi, A. Fast ionic PEO-NaCF3SO3-Na3Zr2Si2P3O12 membranes for all-solid-state energy storage devices. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2023, 289, 116252. [Google Scholar] [CrossRef]
- Ma, W.; Chen, S.; Yang, S.; Chen, W.; Weng, W.; Zhu, M. Bottom-Up Fabrication of Activated Carbon Fiber for All-Solid-State Supercapacitor with Excellent Electrochemical Performance. ACS Appl. Mater. Interfaces 2016, 8, 14622–14627. [Google Scholar] [CrossRef]
- Singh, M.D.; Dalvi, A. Ionic transport in NASICON-polymer hybrids: An assessment using X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2021, 536, 147792. [Google Scholar] [CrossRef]
- Aono, H.; Sato, M.; Traversa, E.; Sakamoto, M.; Sadaoka, Y. Design of ceramic materials for chemical sensors: Effect of SmFeO3 processing on surface and electrical properties. J. Am. Ceram. Soc. 2001, 84, 341–347. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. All-solid state supercapacitors operating at 3.5 V by using ionic liquid based polymer electrolytes. J. Power Sources 2015, 279, 472–480. [Google Scholar] [CrossRef]
- Vicentini, R.; Da Silva, L.; Cecilio, E.; Alves, T.; Nunes, W.; Zanin, H. How to measure and calculate equivalent series resistance of electric double-layer capacitors. Molecules 2019, 24, 1452. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, M.; Yadav, N.; Hashmi, S. High performance quasi-solid-state supercapacitors with peanut-shell- derived porous carbon. J. Power Sources 2018, 402, 133–146. [Google Scholar] [CrossRef]
- Bhat, Y.; Yadav, N.; Hashmi, S. Pinecone-derived porous activated carbon for high performance all-solid-state electrical double layer capacitors fabricated with flexible gel polymer electrolytes. Electrochim. Acta 2019, 304, 94–108. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, A.; Hashmi, S. Electrochemical redox supercapacitors using PVdF-HFP based gel electrolytes and polypyrrole as conducting polymer electrode. Solid State Ion. 2006, 177, 2979–2985. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, J.; Cao, L.; Kong, W.; Sun, Z.; Cheng, H. Cross-linking of polymer and ionic liquid as high-performance gel electrolyte for fl exible solid-state supercapacitors. Electrochim. Acta 2017, 244, 112–118. [Google Scholar] [CrossRef]
- Pandey, G.P.; Kumar, Y.; Hashmi, S. Ionic liquid incorporated PEO based polymer electrolyte for electrical double layer capacitors: A comparative study with lithium and magnesium systems. Solid State Ion. 2011, 190, 93–98. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Latham, R.J.; Linford, R.G.; Schlindwein, W.S. Polymer Electrolyte Based Solid State Redox Supercapacitors with Poly(3-Methyl Thiophene)and Polypyrrole Conducting Polymer Electrodes. Ionics 1997, 3, 177–183. [Google Scholar] [CrossRef]
- Lim, C.-S.; Teoh, K.H.; Liew, C.-W.; Ramesh, S. Electric double layer capacitor based on activated carbon electrode and biodegradable composite polymer electrolyte. Ionics 2014, 20, 251–258. [Google Scholar] [CrossRef]
- Jang, H.S.; Raj, C.J.; Lee, W.-G.; Kim, B.C.; Yu, K.H. Enhanced supercapacitive performances of functionalized activated carbon in novel gel polymer electrolytes with ionic liquid redox-mediated poly(vinyl alcohol)/phosphoric acid. RSC Adv. 2016, 6, 75376–75383. [Google Scholar] [CrossRef]
- Lota, G.; Tyczkowski, J.; Kapica, R.; Lota, K.; Frackowiak, E. Carbon materials modified by plasma treatment as electrodes for supercapacitors. J. Power Sources 2010, 195, 7535–7539. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Shinya, N.; Qin, L. Polyaniline modified graphene and carbon nanotube composite electrode for asymmetric supercapacitors of high energy density. J. Power Sources 2013, 241, 423–428. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Lee, S.M.; Kim, Y.J.; Kahng, Y.H.; Lee, K. A new approach of structural and chemical modification on graphene electrodes for high-performance supercapacitors. Carbon 2016, 100, 7–15. [Google Scholar] [CrossRef]
- Masarapu, C.; Wang, L.-P.; Li, X.; Wei, B. Tailoring Electrode/Electrolyte Interfacial Properties in Flexible Supercapacitors by Applying Pressure. Adv. Energy Mater. 2012, 2, 546–552. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, H.W.; Stoller, M.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S.; Suh, K.S. High-Performance Supercapacitors Based on Poly(ionic liquid)-Modified Graphene Electrodes. ACS Nano 2011, 5, 436–442. [Google Scholar] [CrossRef]
- Pandey, G.; Rastogi, A.; Westgate, C. All-solid-state supercapacitors with poly(3,4-ethylenedioxythiophene)- coated carbon fiber paper electrodes and ionic liquid gel polymer electrolyte. J. Power Sources 2014, 245, 857–865. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Li, T.; Ding, B.; Liu, S.; Henzie, J.; Amin, M.; Yuliarto, B.; Sugahara, Y.; Yamauchi, Y. N-doped hollow carbon nanoplates with mesoporous thin shells towards high-performance supercapacitors. J. Power Sources 2022, 542, 231776. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, G.; Deng, X.; Tian, Y.; Xiao, X.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Strongly Coupled Interfacial Engineering Inspired by Robotic Arms Enable High-Performance Sodium-Ion Capacitors. Adv. Funct. Mater. 2022, 32, 2205453. [Google Scholar] [CrossRef]
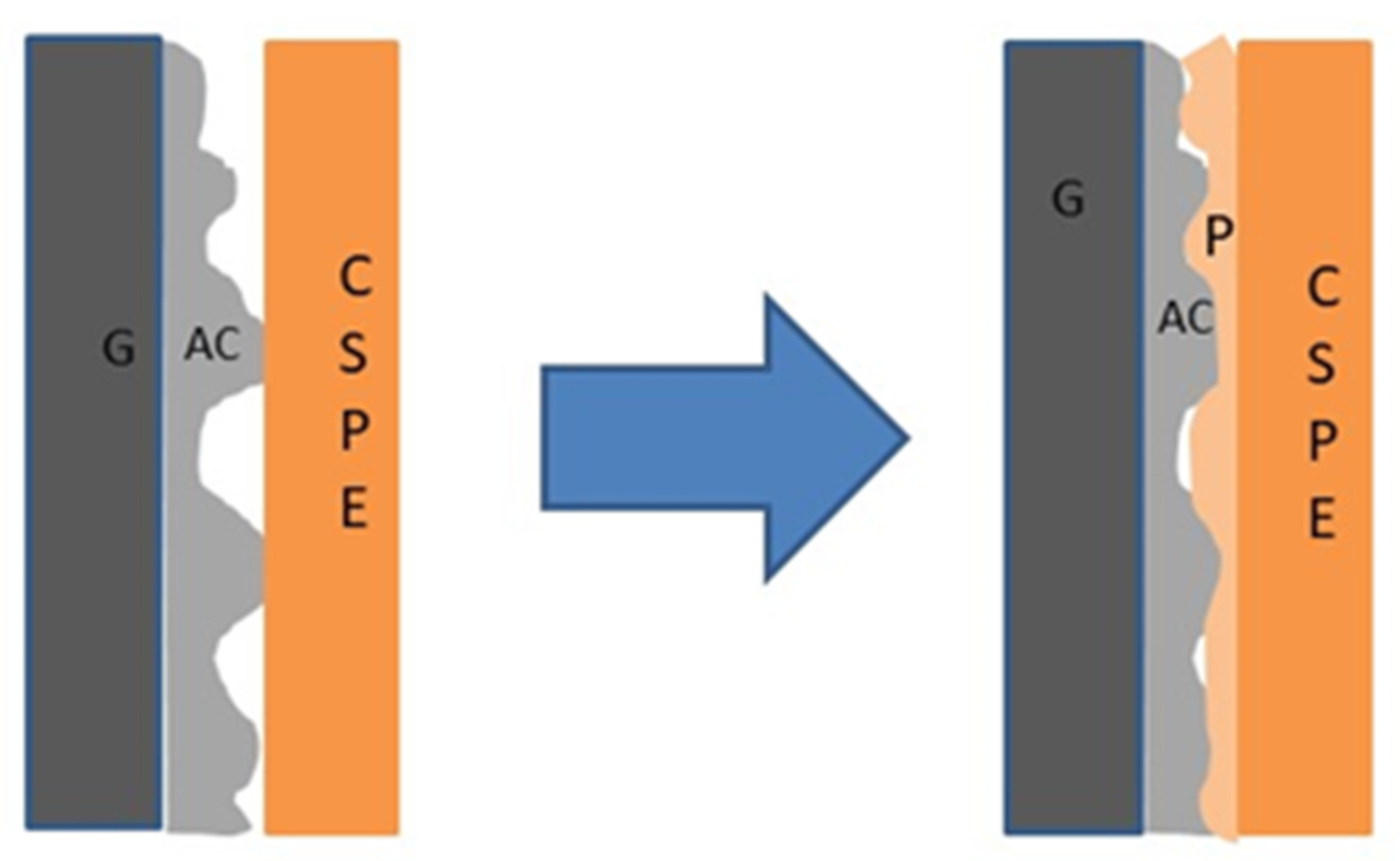
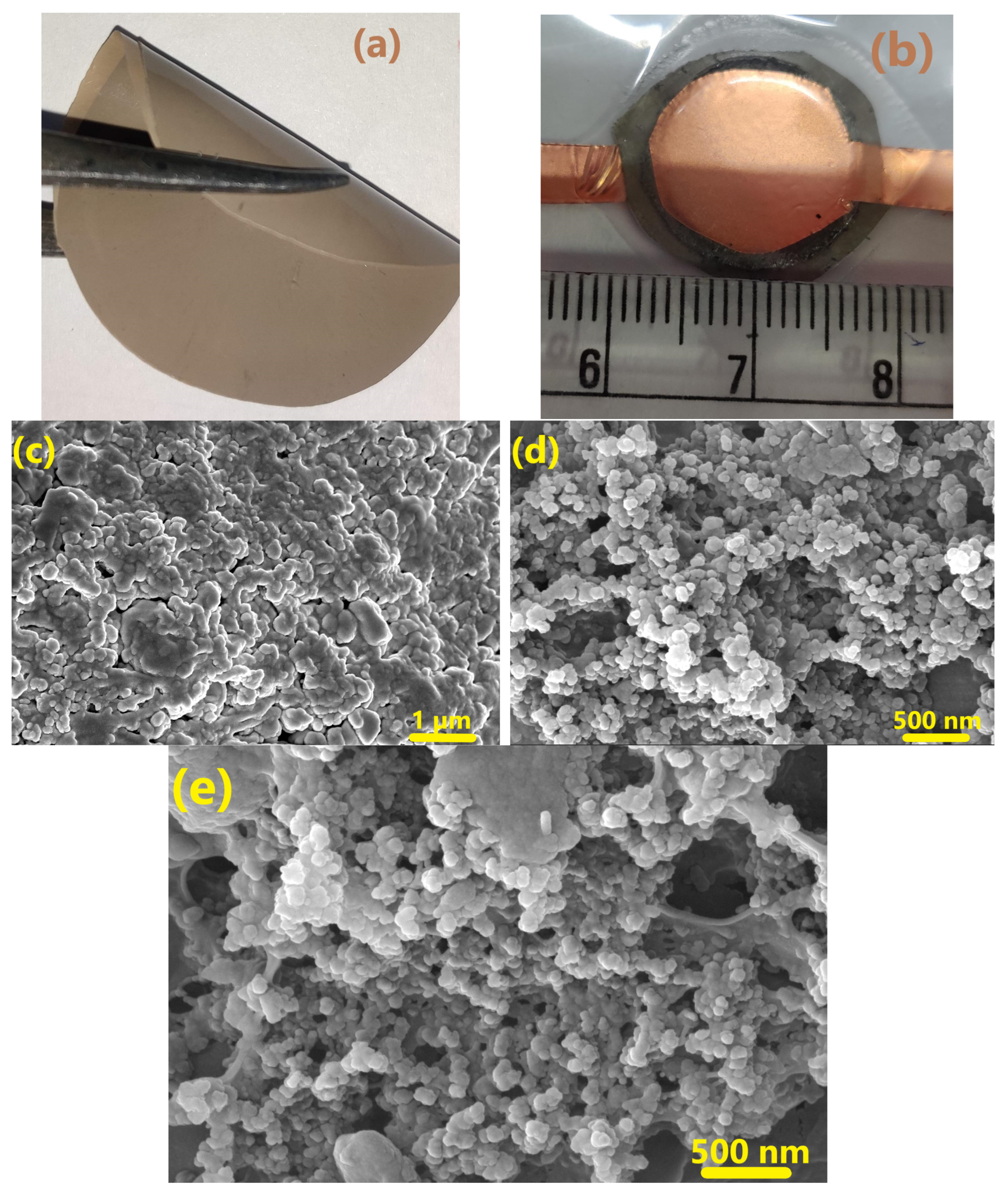

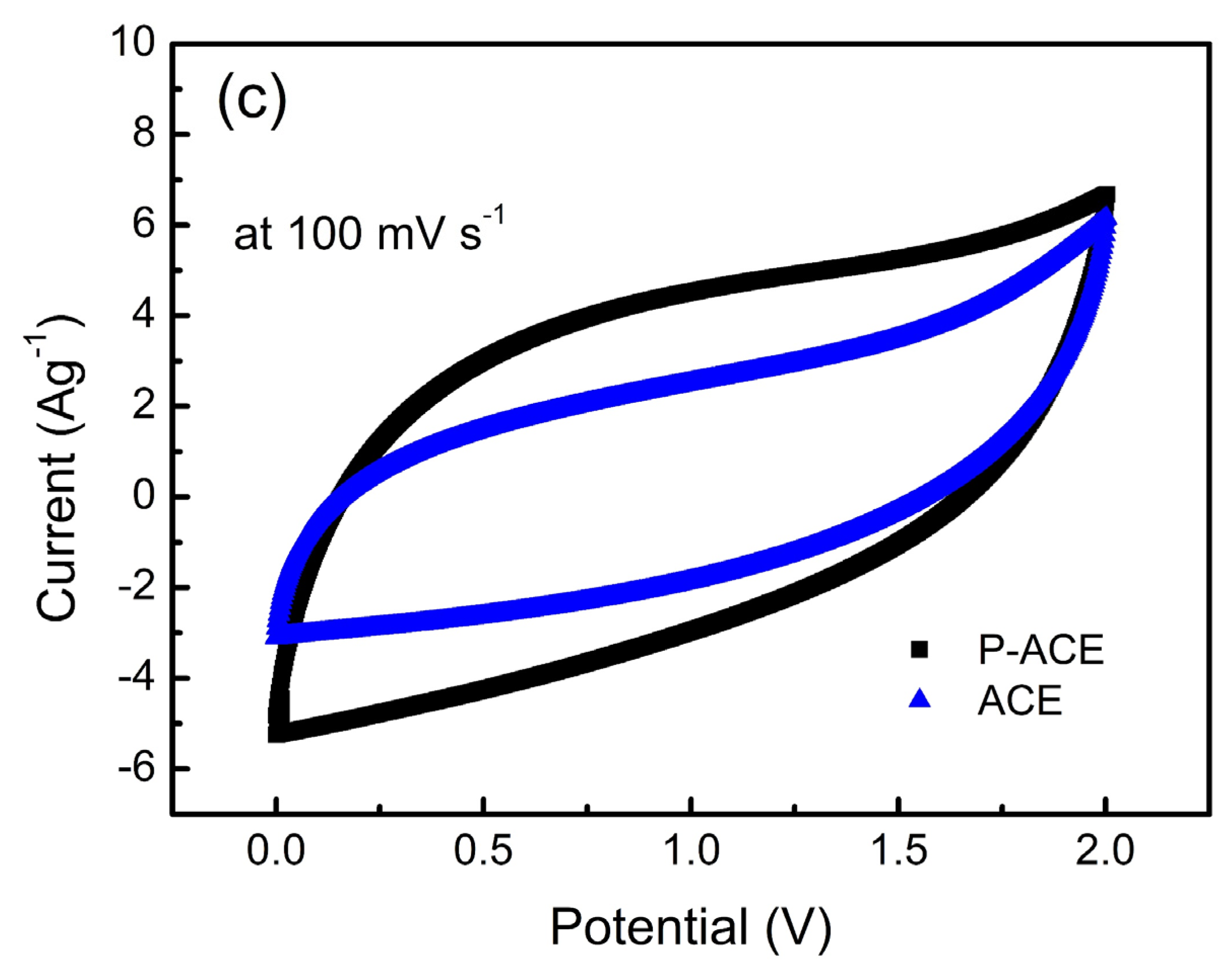

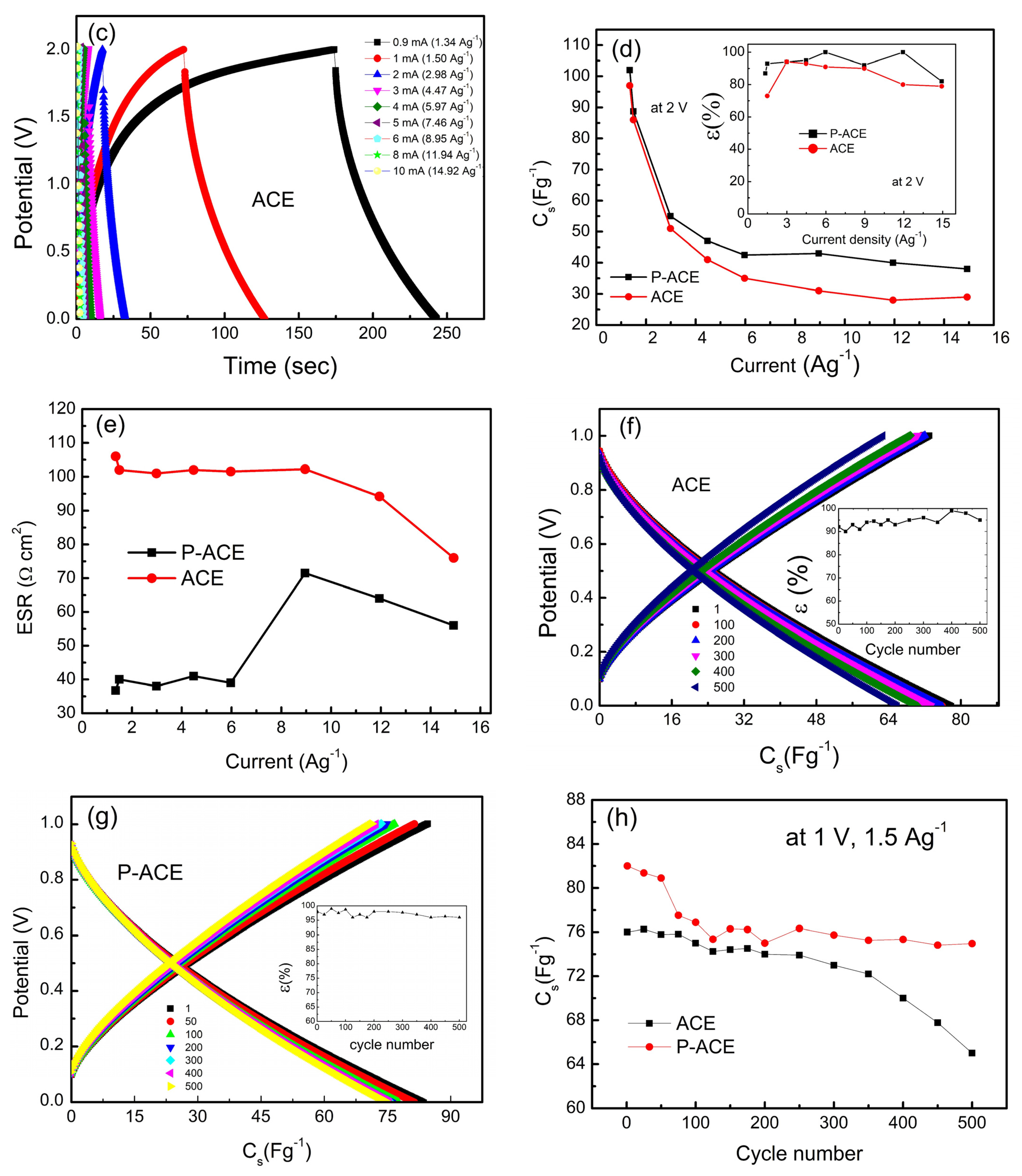
| CSPE MEMBRANE COMPOSITON | Cs (Fg−1) | E (Wh kg−1) | P (W kg−1) | ESR (Ω cm2) | ɛ (%) |
|---|---|---|---|---|---|
| 65AC:8SA:7AB:20P (65 P-ACE) | 96.30 | 12.36 | 890 | 59.23 | 96 |
| 65AC:17.5SA:17.5AB (65ACE) | 71.93 | 9.06 | 881 | 114.01 | 93 |
| 75AC:8SA:7AB:10P (75 P-ACE) | 99.33 | 12.14 | 754 | 80.89 | 86 |
| 75AC:12.5SA:12.5AB (75ACE) | 97.48 | 11.92 | 740 | 108.13 | 80 |
| 80AC:8SA:7AB:5P (80 P-ACE) | 100.23 | 12.38 | 718 | 40.12 | 95 |
| 80AC:10SA:10AB (80 ACE) | 68.48 | 10.35 | 697.60 | 102.85 | 94 |
| Electrode Material | Strategy Adopted | Electrolyte Composition | Important Outcomes |
|---|---|---|---|
| Carbon [44] | Plasma treatment | 1 M H2SO4 | Surface functionality, improved cycling |
| Graphene CNT composite [45] | Modification using PANI nano-cones | 1 M KCl, EMI-TFSI | Improved specific capacitance, energy and power density |
| Reduced Graphene oxide [46] | ZnO-template assisted alkaline treatment | 1 M H2SO4 | Interconnected porous structure, high energy density and power density |
| Activated carbon [47] | Tailoring the interface by applying pressure | KOH, NaOH, LiCl etc. | Two orders of magnitude rise in capacitance |
| PIL-RGO [48] | Incorporation of polymer ionic liquid | EMIM NTF2 | Enhancement in power density, energy density, and specific capacitance |
| Activated carbon [8] | Natural cellulose as a binder | 1 M Et4NBF4, PYR14TFSI, Na2SO4 | Improved thermal and cycling stability |
| Carbon Cloth [10] | Incorporation of graphene quantum dots | 1 M H2SO4, PVA/H2SO4 | Enhanced flexibility and electrochemical stability window |
| Carbon Fiber Paper [49] | Deposition of polymerized poly(3,4-ethylenedioxythiophene) (PEDOT) over carbon fiber paper | 0.01 M of EDOT and 0.1 M LiClO4 in acetonitrile, gel polymer electrolyte | Good capacitive performance and specific energy. Improved electrochemical performance |
| Mesoporous carbon [50] | Hollow ordered mesoporous carbon materials by self-assembling Resol F12 micelles on MOF nanoplate | 6 M KOH | Improved capacitive properties and shortened diffusion pathways provide smooth channels to facilitate ion transport |
| Carbon [11] | Coating dielectric film using atomic layer deposition technique | 1 M MeEt3NBF4/AN organic electrolyte | 83% capacity retention at 0.5 Ag−1 at 3.5 V after 20,000 cycles, high energy density |
| Activated carbon [13] | Heterostructure FeS2/TiO2 | NaClO4 ester-based solution | Improved cycling stability, rate capability, and reduction in diffusion energy barrier, leading to rapid Na+ transport |
| Activated carbon [51] | VS4-CNT | Ether based electrolyte | Strong interfacial coupling leading to conducting solid electrolyte film at the interface, leading to elevated electrochemical performance |
| Graphene [12] | Addition of polypyrrole (PPy) | PVA/H3PO4 gel | High flexibility, stretchability, and excellent electrochemical performance. |
| Activated Carbon (present work) | Addition of polymer in the electrode | 40 LATP (CSPE) | Improved specific capacitance and cycling, specific energy, and power density reduction in ESR. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Kaur, G.; Dalvi, A. Improving Interfaces in All-Solid-State Supercapacitors Using Polymer-Added Activated Carbon Electrodes. Batteries 2023, 9, 81. https://doi.org/10.3390/batteries9020081
Sharma S, Kaur G, Dalvi A. Improving Interfaces in All-Solid-State Supercapacitors Using Polymer-Added Activated Carbon Electrodes. Batteries. 2023; 9(2):81. https://doi.org/10.3390/batteries9020081
Chicago/Turabian StyleSharma, Shrishti, Gurpreet Kaur, and Anshuman Dalvi. 2023. "Improving Interfaces in All-Solid-State Supercapacitors Using Polymer-Added Activated Carbon Electrodes" Batteries 9, no. 2: 81. https://doi.org/10.3390/batteries9020081
APA StyleSharma, S., Kaur, G., & Dalvi, A. (2023). Improving Interfaces in All-Solid-State Supercapacitors Using Polymer-Added Activated Carbon Electrodes. Batteries, 9(2), 81. https://doi.org/10.3390/batteries9020081





