An Insoluble Amino-Functionalized Hexaazatriphenylene as Stable Organic Cathode in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of HATN-[NH2]3 Molecules
2.3. Material Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
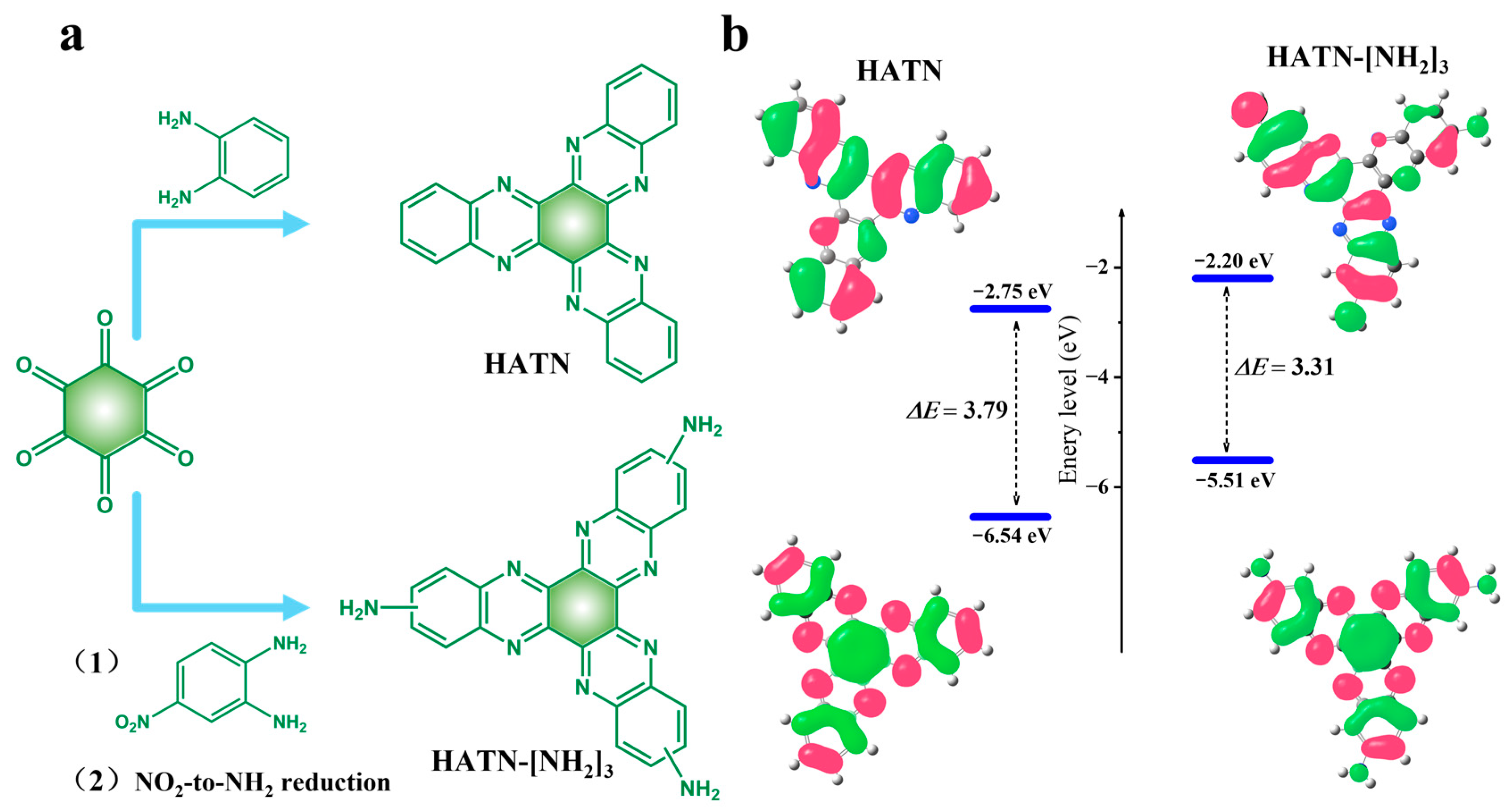
3.1. Preparation and Characterization of HATN-[NH2]3
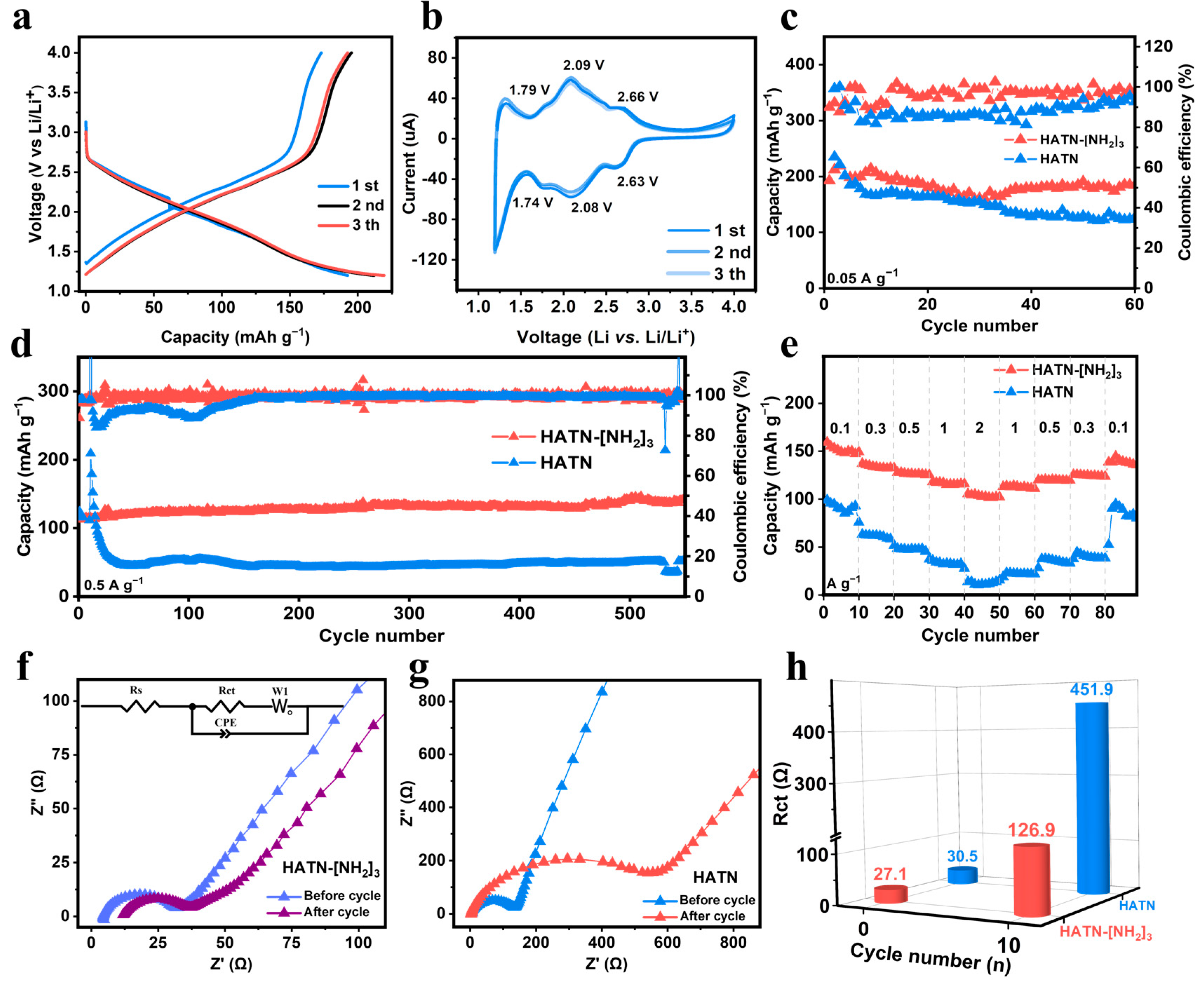
3.2. Electrochemical Performance
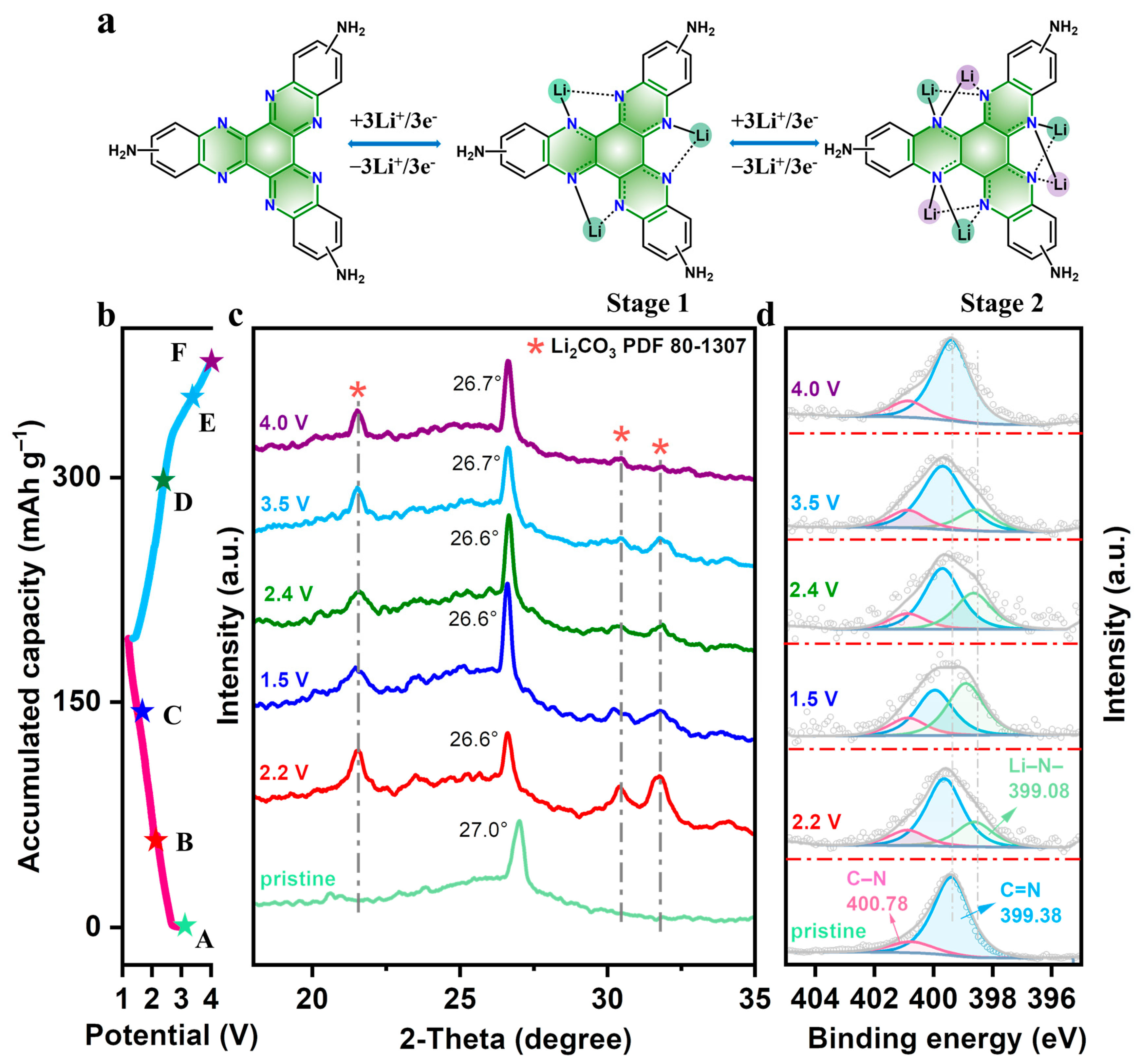
3.3. Lithium Storage Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, H.-K.; Lee, K.T.; Kim, M.G.; Nazar, L.F.; Cho, J. Recent Progress in Nanostructured Cathode Materials for Lithium Secondary Batteries. Adv. Funct. Mater. 2010, 20, 3818–3834. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Häupler, B.; Wild, A.; Schubert, U.S. Carbonyls: Powerful Organic Materials for Secondary Batteries. Adv. Energy Mater. 2015, 5, 1402034. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Wang, R.; Shi, M.; Li, L.; Zhao, Y.; Zhao, L.; Yan, C. In-situ investigation and application of cyano-substituted organic electrode for rechargeable aqueous Na-ion batteries. Chem. Eng. J. 2023, 451, 138652. [Google Scholar] [CrossRef]
- Lee, M.; Hong, J.; Lee, B.; Ku, K.; Lee, S.; Park, C.B.; Kang, K. Multi-Electron Redox Phenazine for Ready-to-Charge Organic Batteries. Green Chem. 2017, 19, 2980–2985. [Google Scholar] [CrossRef]
- Liang, Y.; Tao, Z.; Chen, J. Organic Electrode Materials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Schon, T.B.; McAllister, B.T.; Li, P.-F.; Seferos, D.S. The Rise of Organic Electrode Materials for Energy Storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Zhou, H. Towards Sustainable and Versatile Energy Storage Devices: An Overview of Organic Electrode Materials. Energy Environ. Sci. 2013, 6, 2280–2301. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Riduan, S.N. Strategies toward Improving the Performance of Organic Electrodes in Rechargeable Lithium (Sodium) Batteries. J. Mater. Chem. A 2016, 4, 14902–14914. [Google Scholar] [CrossRef]
- Chen, M.; Yang, C.; Xu, Z.; Tang, Y.; Jiang, J.; Liu, P.; Su, Y.; Wu, D. A Facile Self-Assembly Strategy towards Naphthalene Diimide/Graphene Hybrids as high performance Organic Cathodes for Lithium-Ion Batteries. RSC Adv. 2016, 6, 13666–13669. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Qi, H.; Hambsch, M.; Rawle, J.; Vázquez, A.R.; Nia, A.S.; Pashkin, A.; Schneider, H.; Polozij, M.; et al. A Two-Dimensional Polyimide-Graphene Heterostructure with Ultra-Fast Interlayer Charge Transfer. Angew. Chem. Int. Ed. 2021, 133, 13978–13983. [Google Scholar] [CrossRef]
- Hanyu, Y.; Ganbe, Y.; Honma, I. Application of Quinonic Cathode Compounds for Quasi-Solid Lithium Batteries. J. Power Sources 2013, 221, 186–190. [Google Scholar] [CrossRef]
- Häupler, B.; Hagemann, T.; Friebe, C.; Wild, A.; Schubert, U.S. Dithiophenedione-Containing Polymers for Battery Application. ACS Appl. Mater. Interfaces 2015, 7, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, T.; Sato, K.; Oyaizu, K.; Nishide, H. Efficient Charge Transport of a Radical Polyether/SWCNT Composite Electrode for an Organic Radical Battery with High Charge-Storage Density. RSC Adv. 2015, 5, 15448–15452. [Google Scholar] [CrossRef]
- Shimizu, A.; Kuramoto, H.; Tsujii, Y.; Nokami, T.; Inatomi, Y.; Hojo, N.; Suzuki, H.; Yoshida, J. Introduction of Two Lithiooxycarbonyl Groups Enhances Cyclability of Lithium Batteries with Organic Cathode Materials. J. Power Sources 2014, 260, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yang, J.; Sun, P.; Xu, Y. Sodium Sulfonate Groups Substituted Anthraquinone as an Organic Cathode for Potassium Batteries. Electrochem. Commun. 2018, 86, 34–37. [Google Scholar] [CrossRef]
- Wan, W.; Lee, H.; Yu, X.; Wang, C.; Nam, K.-W.; Yang, X.-Q.; Zhou, H. Tuning the Electrochemical Performances of Anthraquinone Organic Cathode Materials for Li-Ion Batteries through the Sulfonic Sodium Functional Group. RSC Adv. 2014, 4, 19878–19882. [Google Scholar] [CrossRef]
- Gottis, S.; Barrès, A.-L.; Dolhem, F.; Poizot, P. Voltage Gain in Lithiated Enolate-Based Organic Cathode Materials by Isomeric Effect. ACS Appl. Mater. Interfaces 2014, 6, 10870–10876. [Google Scholar] [CrossRef]
- Yokoji, T.; Kameyama, Y.; Sakaida, S.; Maruyama, N.; Satoh, M.; Matsubara, H. Steric Effects on the Cyclability of Benzoquinone-Type Organic Cathode Active Materials for Rechargeable Batteries. Chem. Lett. 2015, 44, 1726–1728. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Q.; Chen, Y.; Fan, K.; Zhang, C.; Zhang, G.; Xu, M.; Mao, M.; Ma, J.; Hu, W.; et al. A Small Molecular Symmetric All-Organic Lithium-Ion Battery. Angew. Chem. Int. Ed. 2022, 61, e202207221. [Google Scholar] [CrossRef]
- Wang, C.; Tang, W.; Jia, S.; Yan, Y.; Li, D.; Hu, Y.; Gao, J.; Wu, H.; Wang, M.; Liu, S.; et al. Benzene-bridged anthraquinones as a high-rate and long-lifespan organic cathode for advanced Na-ion batteries. Chem. Eng. J. 2021, 426, 131251. [Google Scholar] [CrossRef]
- Tuttle, M.R.; Davis, S.T.; Zhang, S. Synergistic Effect of Hydrogen Bonding and π–π Stacking Enables Long Cycle Life in Organic Electrode Materials. ACS Energy Lett. 2021, 6, 643–649. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Shi, Y.; Sun, P.; Wang, Z.; Chen, Z.; Xu, Y. Rational Molecular Design of Benzoquinone-Derived Cathode Materials for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1909597. [Google Scholar] [CrossRef]
- Dai, G.; He, Y.; Niu, Z.; He, P.; Zhang, C.; Zhao, Y.; Zhang, X.; Zhou, H. A Dual-Ion Organic Symmetric Battery Constructed from Phenazine-Based Artificial Bipolar Molecules. Angew. Chem. Int. Ed. 2019, 131, 10007–10011. [Google Scholar] [CrossRef]
- Peng, C.; Ning, G.-H.; Su, J.; Zhong, G.; Tang, W.; Tian, B.; Su, C.; Yu, D.; Zu, L.; Yang, J.; et al. Reversible Multi-Electron Redox Chemistry of π-Conjugated N-Containing Heteroaromatic Molecule-Based Organic Cathodes. Nat. Energy 2017, 2, 17074. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Lin, X.; Pei, Z.; Liu, D.; Zhao, S. Nanostructured hexaazatrinaphthalene based polymers for advanced energy conversion and storage. Chem. Eng. J. 2022, 427, 130995. [Google Scholar] [CrossRef]
- Xu, S.; Wang, G.; Biswal, B.P.; Addicoat, M.; Paasch, S.; Sheng, W.; Zhuang, X.; Brunner, E.; Heine, T.; Berger, R.; et al. A Nitrogen-Rich 2D sp2-Carbon-Linked Conjugated Polymer Framework as a High-Performance Cathode for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 131, 859–863. [Google Scholar] [CrossRef]
- Wang, J.; En, J.C.Z.; Riduan, S.N.; Zhang, Y. Nitrogen-linked hexaazatrinaphthylene polymer as cathode material in lithium-ion battery. Chem. Eur. J. 2020, 26, 2581–2585. [Google Scholar] [CrossRef]
- Wang, J.; Tee, K.; Lee, Y.; Riduan, S.N.; Zhang, Y. Hexaazatriphenylene Derivatives/GO Composites as Organic Cathodes for lithium ion batteries. J. Mater. Chem. A 2018, 6, 2752–2757. [Google Scholar] [CrossRef]
- Sieuw, L.; Jouhara, A.; Quarez, É.; Auger, C.; Gohy, J.-F.; Poizot, P.; Vlad, A. A H-Bond Stabilized Quinone Electrode Material for Li–Organic Batteries: The Strength of Weak Bonds. Chem. Sci. 2019, 10, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Ding, Z.; Ning, G.-H.; Tang, W.; Peng, C.; Liu, B.; Su, J.; Su, C.; Loh, K.P. Amino Group Enhanced Phenazine Derivatives as Electrode Materials for Lithium Storage. Chem. Commun. 2017, 53, 2914–2917. [Google Scholar] [CrossRef]
- Lei, Z.; Yang, Q.; Xu, Y.; Guo, S.; Sun, W.; Liu, H.; Lv, L.-P.; Zhang, Y.; Wang, Y. Boosting Lithium Storage in Covalent Organic Framework via Activation of 14-Electron Redox Chemistry. Nat. Commun. 2018, 9, 576. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tang, M.; Wu, Y.; Chen, Y.; Zhu, S.; Wang, B.; Jiang, C.; Wang, E.; Wang, C. Large π-Conjugated Porous Frameworks as Cathodes for Sodium-Ion Batteries. J. Phys. Chem. Lett. 2018, 9, 3205–3211. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xie, S.; Cao, Z.; Wang, L.; Xu, D.; Zhang, H.; Matz, J.; Dong, P.; Fang, H.; Shen, J.; et al. High-Rate Aqueous Zinc-Organic Battery Achieved by Lowering HOMO/LUMO of Organic Cathode. Energy Storage Mater. 2021, 37, 378–386. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Park, M.J. Long-Life, High-Rate Lithium-Organic Batteries Based on Naphthoquinone Derivatives. Chem. Mater. 2016, 28, 2408–2416. [Google Scholar] [CrossRef]
- Barlow, S.; Zhang, Q.; Kaafarani, B.R.; Risko, C.; Amy, F.; Chan, C.K.; Domercq, B.; Starikova, Z.A.; Antipin, M.Y.; Timofeeva, T.V.; et al. Synthesis, Ionisation Potentials and Electron Affinities of Hexaazatrinaphthylene Derivatives. Chem. Eur. J. 2007, 13, 3537–3547. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, L.; Zhao, Q.; Li, F.; Chen, J. An Insoluble Benzoquinone-Based Organic Cathode for Use in Rechargeable Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2017, 129, 12735–12739. [Google Scholar] [CrossRef]
- Lyu, H.; Jafta, C.J.; Popovs, I.; Meyer, H.M.; Hachtel, J.A.; Huang, J.; Sumpter, B.G.; Dai, S.; Sun, X.-G. A Dicyanobenzoquinone Based Cathode Material for Rechargeable Lithium and Sodium Ion Batteries. J. Mater. Chem. A 2019, 7, 17888–17895. [Google Scholar] [CrossRef]
- Zhang, J.-N.; Li, Q.; Wang, Y.; Zheng, J.; Yu, X.; Li, H. Dynamic Evolution of Cathode Electrolyte Interphase (CEI) on High Voltage LiCoO2 Cathode and Its Interaction with Li Anode. Energy Storage Mater. 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Ciucci, F. Modeling Electrochemical Impedance Spectroscopy. Curr. Opin. Electrochem. 2019, 13, 132–139. [Google Scholar] [CrossRef]
- Amin, K.; Zhang, J.; Zhou, H.-Y.; Lu, R.; Zhang, M.; Ashraf, N.; YueLi, C.; Mao, L.; Faul, C.F.J.; Wei, Z. Surface Controlled Pseudo-Capacitive Reactions Enabling Ultra-Fast Charging and Long-Life Organic lithium ion batteries. Sustain. Energy Fuels 2020, 4, 4179–4185. [Google Scholar] [CrossRef]
- Wang, X.; Hao, H.; Liu, J.; Huang, T.; Yu, A. A Novel Method for Preparation of Macroposous Lithium Nickel Manganese Oxygen as Cathode Material for lithium-ion Batteries. Electrochim. Acta 2011, 56, 4065–4069. [Google Scholar] [CrossRef]
- Luo, W.; Allen, M.; Raju, V.; Ji, X. An Organic Pigment as a High-Performance Cathode for Sodium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400554. [Google Scholar] [CrossRef]
- Kim, H.; Seo, D.-H.; Yoon, G.; Goddard, W.A.I.; Lee, Y.S.; Yoon, W.-S.; Kang, K. The Reaction Mechanism and Capacity Degradation Model in Lithium Insertion Organic Cathodes, Li2C6O6, Using Combined Experimental and First Principle Studies. J. Phys. Chem. Lett. 2014, 5, 3086–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Lee, M.; Lee, B.; Seo, D.-H.; Park, C.B.; Kang, K. Biologically Inspired Pteridine Redox Centres for Rechargeable Batteries. Nat. Commun. 2014, 5, 5335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Zhao, Y.; Sun, B.; Sun, Z.; Li, C.; Han, Y.; Xu, L.; Ge, Z.; Ren, Y.; Zhang, M.; et al. A 2D Covalent Organic Framework as a High-Performance Cathode Material for Lithium-Ion Batteries. Nano Energy 2020, 70, 104498. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Lai, C.; Li, R.; Xu, H.; Tan, D.H.S.; Zhang, K.; Yu, W.; Fjeldberg, O.; Lin, M.; et al. Dense-Stacking Porous Conjugated Polymer as Reactive-Type Host for High-Performance Lithium Sulfur Batteries. Angew. Chem. Int. Ed. 2021, 60, 11359–11369. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Jin, X.; Zhang, B.; Wang, X.; Liu, D. An Insoluble Amino-Functionalized Hexaazatriphenylene as Stable Organic Cathode in Lithium-Ion Batteries. Batteries 2023, 9, 85. https://doi.org/10.3390/batteries9020085
Xu P, Jin X, Zhang B, Wang X, Liu D. An Insoluble Amino-Functionalized Hexaazatriphenylene as Stable Organic Cathode in Lithium-Ion Batteries. Batteries. 2023; 9(2):85. https://doi.org/10.3390/batteries9020085
Chicago/Turabian StyleXu, Pengfei, Xiao Jin, Biao Zhang, Xin Wang, and Dong Liu. 2023. "An Insoluble Amino-Functionalized Hexaazatriphenylene as Stable Organic Cathode in Lithium-Ion Batteries" Batteries 9, no. 2: 85. https://doi.org/10.3390/batteries9020085




