Limiting Factors Affecting the Ionic Conductivities of LATP/Polymer Hybrid Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of LATP
Spark Plasma Sintering
2.2. Natural Sintering
2.3. PEO-LATP Solid Composite Electrolyte
2.4. Scanning Electron Microscopy
2.5. Electrochemical Impedance Spectroscopy (EIS)
3. Results
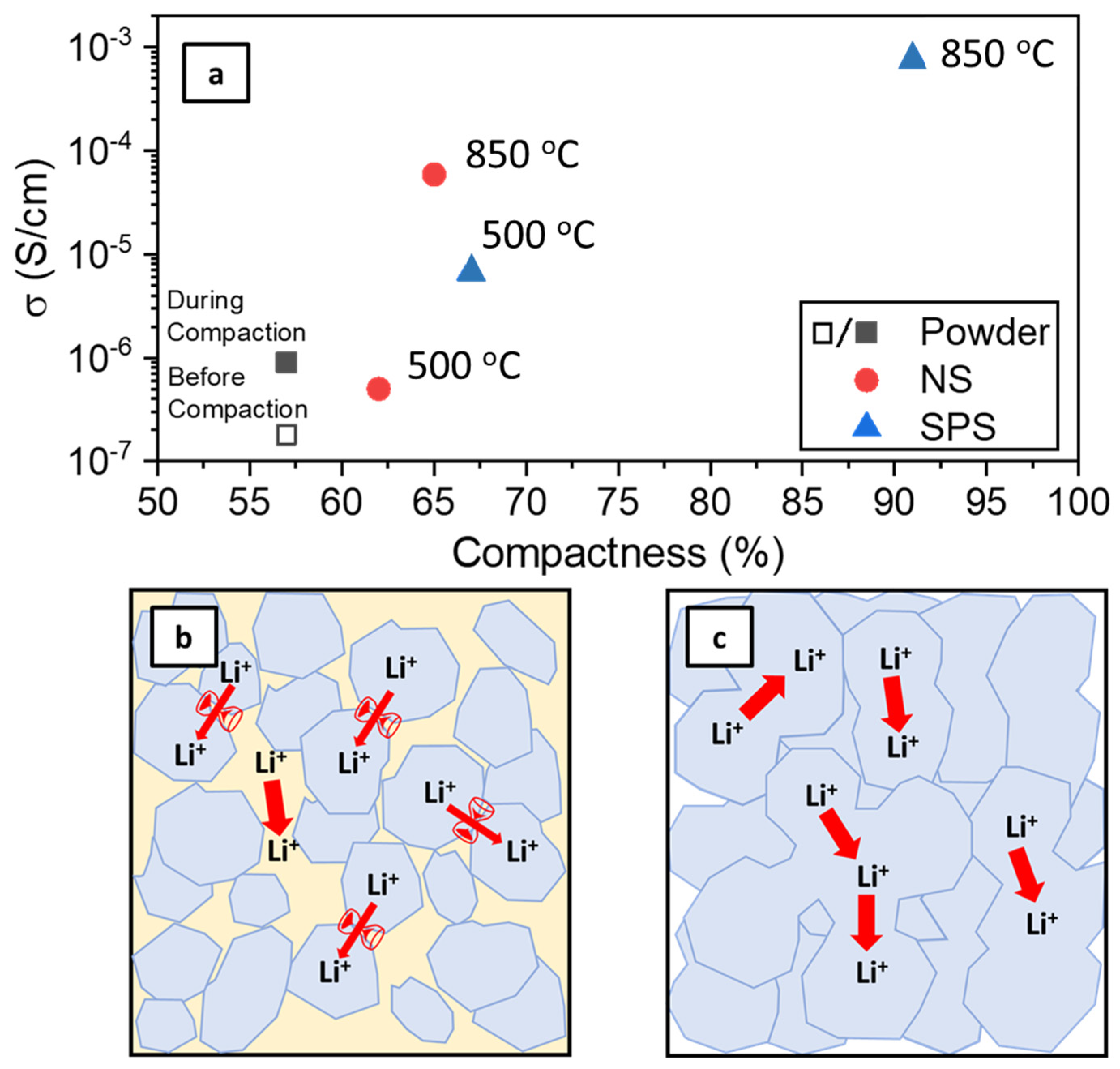
3.1. Evaluating the Benefit of Sintering
3.2. LATP/PEO Solid Composite Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.-F.; Ma, J.; Ling, H.; Kang, F.; He, Y.-B. Progress and Perspective of All-Solid-State Lithium Batteries with High Performance at Room Temperature. Energy Fuels 2020, 34, 13456–13472. [Google Scholar] [CrossRef]
- Doux, J.-M.; Nguyen, H.; Tan, D.H.S.; Banerjee, A.; Wang, X.; Wu, E.A.; Jo, C.; Yang, H.; Meng, Y.S. Stack Pressure Considerations for Room-Temperature All-Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2020, 10, 1903253. [Google Scholar] [CrossRef]
- Doux, J.-M.; Yang, Y.; Tan, D.H.S.; Nguyen, H.; Wu, E.A.; Wang, X.; Banerjee, A.; Meng, Y.S. Pressure effects on sulfide electrolytes for all solid-state batteries. J. Mater. Chem. A 2020, 8, 5049–5055. [Google Scholar]
- Benabed, Y.; Rioux, M.; Rousselot, S.; Hautier, G.; Dollé, M. Assessing the Electrochemical Stability Window of NASICON-Type Solid Electrolytes. Front. Energy Res. 2021, 9, 682008. [Google Scholar] [CrossRef]
- Schell, K.G.; Bucharsky, E.C.; Lemke, F.; Hoffmann, M.J. Effect of calcination conditions on lithium conductivity in Li1.3Ti1.7Al0.3(PO4)3 prepared by sol-gel route. Ionics 2017, 23, 821–827. [Google Scholar] [CrossRef]
- Yan, G.; Yu, S.; Nonemacher, J.F.; Tempel, H.; Kungl, H.; Malzbender, J.; Eichel, R.-A.; Krüger, M. Influence of sintering temperature on conductivity and mechanical behavior of the solid electrolyte LATP. Ceram. Int. 2019, 45, 14697–14703. [Google Scholar] [CrossRef]
- Chaim, R.; Levin, M.; Shlayer, A.; Estournes, C. Sintering and densification of nanocrystalline ceramic oxide powders: A review. Adv. Appl. Ceram. 2008, 107, 159–169. [Google Scholar] [CrossRef]
- Delaizir, G.; Dollé, M.; Rozier, P.; Zhang, X.H. Spark Plasma Sintering: An Easy Way to Make Infrared Transparent Glass-Ceramics. J. Am. Ceram. Soc. 2010, 93, 2495–2498. [Google Scholar] [CrossRef]
- Xu, X.; Wen, Z.; Yang, X.; Chen, L. Dense nanostructured solid electrolyte with high Li-ion conductivity by spark plasma sintering technique. Mater. Res. Bull. 2008, 43, 2334–2341. [Google Scholar] [CrossRef]
- Yamada, H.; Ito, T.; Basappa, R.H. Sintering Mechanisms of High-Performance Garnet-type Solid Electrolyte Densified by Spark Plasma Sintering. Electrochim. Acta 2016, 222, 648–656. [Google Scholar] [CrossRef]
- Dixit, M.B.; Regala, M.; Shen, F.; Xiao, X.; Hatzell, K.B. Tortuosity Effects in Garnet-Type Li7La3Zr2O12 Solid Electrolytes. ACS Appl. Mater. Interfaces 2019, 11, 2022–2030. [Google Scholar] [CrossRef]
- Waetzig, K.; Rost, A.; Heubner, C.; Coeler, M.; Nikolowski, K.; Wolter, M.; Schilm, J. Synthesis and sintering of Li1.3Al0.3Ti1.7(PO4)3 (LATP) electrolyte for ceramics with improved Li+ conductivity. J. Alloys Compd. 2020, 818, 153237. [Google Scholar] [CrossRef]
- Tang, S.; Guo, W.; Fu, Y. Advances in Composite Polymer Electrolytes for Lithium Batteries and Beyond. Adv. Energy Mater. 2021, 11, 2000802. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Ban, X.Y.; Zhang, W.Q.; Chen, N.; Sun, C.W. A High-Performance and Durable Poly(ethylene oxide)-Based Composite Solid Electrolyte for All Solid-State Lithium Battery. J. Phys. Chem. C 2018, 122, 9852–9858. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A Long Cycle Life, All-Solid-State Lithium Battery with a Ceramic–Polymer Composite Electrolyte. ACS Appl. Energy Mater. 2020, 3, 2916–2924. [Google Scholar] [CrossRef]
- Zaman, W.; Hortance, N.; Dixit, M.B.; De Andrade, V.; Hatzell, K.B. Visualizing percolation and ion transport in hybrid solid electrolytes for Li–metal batteries. J. Mater. Chem. A 2019, 7, 23914–23921. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Jiang, D.; Yang, L.; Zhang, J.; Yao, M.; Ji, X.; Wang, G.; Zhang, S. Enhanced transport and favorable distribution of Li-ion in a poly(ionic liquid) based electrolyte facilitated by Li1.3Al0.3Ti1.7(PO4)3 nanoparticles for highly-safe lithium metal batteries. Electrochim. Acta 2021, 368, 137581. [Google Scholar] [CrossRef]
- Foran, G.; Mery, A.; Bertrand, M.; Rousselot, S.; Lepage, D.; Aymé-Perrot, D.; Dollé, M. NMR Study of Lithium Transport in Liquid–Ceramic Hybrid Solid Composite Electrolytes. ACS Appl. Mater. Interfaces 2022, 14, 43226–43236. [Google Scholar] [CrossRef] [PubMed]
- Isaac, J.A.; Devaux, D.; Bouchet, R. Dense inorganic electrolyte particles as a lever to promote composite electrolyte conductivity. Nat. Mater. 2022, 21, 1412–1418. [Google Scholar] [PubMed]
- Paolella, A.; Bertoni, G.; Zhu, W.; Campanella, D.; La Monaca, A.; Girard, G.; Demers, H.; Gheorghe Nita, A.C.; Feng, Z.; Vijh, A.; et al. Unveiling the Cation Exchange Reaction between the NASICON Li1.5Al0.5Ge1.5(PO4)3 Solid Electrolyte and the pyr13TFSI Ionic Liquid. J. Am. Chem. Soc. 2022, 144, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chem. Rev. 2019, 120, 6820–6877. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sakamoto, J. Controlling Ionic Transport through the PEO-LiTFSI/LLZTO Interface. Electrochem. Soc. Interface 2019, 28, 63. [Google Scholar]
- Isaac, J.A.; Mangani, L.R.; Devaux, D.; Bouchet, R. Electrochemical Impedance Spectroscopy of PEO-LATP Model Multilayers: Ionic Charge Transport and Transfer. ACS Appl. Mater. Interfaces 2022, 14, 13158–13168. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.; Rousselot, S.; Aymé-Perrot, D.; Dollé, M. Compatibility assessment of solid ceramic electrolytes and active materials based on thermal dilatation for the development of solid-state batteries. Mater. Adv. 2021, 2, 2989–2999. [Google Scholar] [CrossRef]
- Miara, L.; Windmüller, A.; Tsai, C.-L.; Richards, W.D.; Ma, Q.; Uhlenbruck, S.; Guillon, O.; Ceder, G. About the Compatibility between High Voltage Spinel Cathode Materials and Solid Oxide Electrolytes as a Function of Temperature. ACS Appl. Mater. Interfaces 2016, 8, 26842–26850. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Choi, J.; Anandan, V.; Kim, J.-H. High-Temperature Chemical Stability of Li1.4Al0.4Ti1.6(PO4)3 Solid Electrolyte with Various Cathode Materials for Solid-State Batteries. J. Phys. Chem. C 2020, 124, 14963–14971. [Google Scholar] [CrossRef]
- Wei, X.; Rechtin, J.; Olevsky, E.A. The Fabrication of All-Solid-State Lithium-Ion Batteries via Spark Plasma Sintering. Metals 2017, 7, 372. [Google Scholar] [CrossRef]
- Lascaud, S.; Perrier, M.; Vallee, A.; Besner, S.; Prud’homme, J.; Armand, M. Phase Diagrams and Conductivity Behavior of Poly(ethylene oxide)-Molten Salt Rubbery Electrolytes. Macromolecules 1994, 27, 7469–7477. [Google Scholar] [CrossRef]
- Foran, G.; Mankovsky, D.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. The Impact of Absorbed Solvent on the Performance of Solid Polymer Electrolytes for Use in Solid-State Lithium Batteries. Iscience 2020, 23, 101597. [Google Scholar] [CrossRef] [PubMed]
- Mankovsky, D.; Lepage, D.; Lachal, M.; Caradant, L.; Aymé-Perrot, D.; Dollé, M. Water content in solid polymer electrolytes: The lost knowledge. Chem. Commun. 2020, 56, 10167–10170. [Google Scholar] [CrossRef]
- Famprikis, T.; Kudu, Ö.U.; Dawson, J.A.; Canepa, P.; Fauth, F.; Suard, E.; Zbiri, M.; Dambournet, D.; Borkiewicz, O.J.; Bouyanfif, H.; et al. Under Pressure: Mechanochemical Effects on Structure and Ion Conduction in the Sodium-Ion Solid Electrolyte Na3PS4. J. Am. Chem. Soc. 2020, 142, 18422–18436. [Google Scholar] [CrossRef]
- Bonizzoni, S.; Ferrara, C.; Berbenni, V.; Anselmi-Tamburini, U.; Mustarelli, P.; Tealdi, C. NASICON-type polymer-in-ceramic composite electrolytes for lithium batteries. Phys. Chem. Chem. Phys. 2019, 21, 6142–6149. [Google Scholar] [CrossRef]
- Dumont-Botto, E.; Bourbon, C.; Patoux, S.; Rozier, P.; Dolle, M. Synthesis by Spark Plasma Sintering: A new way to obtain electrode materials for lithium ion batteries. J. Power Sources 2011, 196, 2274–2278. [Google Scholar] [CrossRef]
- Heitjans, P.; Indris, S. Diffusion and ionic conduction in nanocrystalline ceramics. J. Physics Condens. Matter 2003, 15, R1257–R1289. [Google Scholar] [CrossRef]
- Bellini, S.; Azzato, G.; Grandinetti, M.; Stellato, V.; De Marco, G.; Sun, Y.; Caravella, A. A Novel Connectivity Factor for Morphological Characterization of Membranes and Porous Media: A Simulation Study on Structures of Mono-Sized Spherical Particles. Appl. Sci. 2018, 8, 573. [Google Scholar] [CrossRef]
- Caravella, A.; Bellini, S.; Azzato, G.; De Marco, G.; Sun, Y. 4-Tortuosity evaluation for characterization of transport phenomena in pure-crystalline metal lattices and porous media. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Gallucci, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–122. [Google Scholar]
- Wiedenmann, D.; Keller, L.; Holzer, L.; Stojadinović, J.; Münch, B.; Suarez, L.; Fumey, B.; Hagendorfer, H.; Brönnimann, R.; Modregger, P.; et al. Three-dimensional pore structure and ion conductivity of porous ceramic diaphragms. AIChE J. 2013, 59, 1446–1457. [Google Scholar] [CrossRef]
- Tjaden, B.; Brett, D.J.L.; Shearing, P.R. Tortuosity in electrochemical devices: A review of calculation approaches. Int. Mater. Rev. 2018, 63, 47–67. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, X.; Cheng, G. Numerical simulation for tortuosity of porous media. Microporous Mesoporous Mater. 2013, 173, 37–42. [Google Scholar] [CrossRef]
- Tjaden, B.; Lane, J.; Withers, P.J.; Bradley, R.S.; Brett, D.J.L.; Shearing, P.R. The application of 3D imaging techniques, simulation and diffusion experiments to explore transport properties in porous oxygen transport membrane support materials. Solid State Ionics 2016, 288, 315–321. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Qin, Y.; Ji, S.; Huo, Y.; Wang, Z.; Liu, Z.; Shen, J.; Liu, J. Recent Progress in Organic–Inorganic Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Chem. Eur. J. 2020, 26, 1720–1736. [Google Scholar] [CrossRef]
- Siyal, S.H.; Li, M.; Li, H.; Lan, J.-L.; Yu, Y.; Yang, X. Ultraviolet irradiated PEO/LATP composite gel polymer electrolytes for lithium-metallic batteries (LMBs). Appl. Surf. Sci. 2019, 494, 1119–1126. [Google Scholar] [CrossRef]
- Yu, X.; Li, J.; Manthiram, A. Rational Design of a Laminated Dual-Polymer/Polymer–Ceramic Composite Electrolyte for High-Voltage All-Solid-State Lithium Batteries. ACS Mater. Lett. 2020, 2, 317–324. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Electrode–Electrolyte Interfaces in Lithium–Sulfur Batteries with Liquid or Inorganic Solid Electrolytes. Acc. Chem. Res. 2017, 50, 2653–2660. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.Y.; Lin, D.C.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Liu, L.; Chu, L.; Jiang, B.; Li, M. Li1.4Al0.4Ti1.6(PO4)3 nanoparticle-reinforced solid polymer electrolytes for all-solid-state lithium batteries. Solid State Ionics 2019, 331, 89–95. [Google Scholar] [CrossRef]
- Pan, K.; Zhang, L.; Qian, W.; Wu, X.; Dong, K.; Zhang, H.; Zhang, S. Flexible Ceramic/Polymer Hybrid Solid Electrolyte for Solid-State Lithium Metal Batteries. Adv. Mater. 2020, 32, e2000399. [Google Scholar] [CrossRef]
- Guo, J.; Berbano, S.S.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Randall, C.A. Cold Sintering Process of Composites: Bridging the Processing Temperature Gap of Ceramic and Polymer Materials. Adv. Funct. Mater. 2016, 26, 7115–7121. [Google Scholar] [CrossRef]
- Stoeva, Z.; Imrie, C.T.; Ingram, M.D. Effect of pressure on ion transport in amorphous and semi-crystalline polymer electrolytes. Phys. Chem. Chem. Phys. 2003, 5, 395–399. [Google Scholar]
- Roman, H.E.; Bunde, A.; Dieterich, W. Conductivity of dispersed ionic conductors: A percolation model with two critical points. Phys. Rev. B 1986, 34, 3439–3445. [Google Scholar] [CrossRef]
- Choi, B.-K.; Kim, Y.-W.; Shin, K.-H. Effects of ceramic fillers on the electrical properties of (PEO)16LiClO4 electrolytes. J. Power Sources 1997, 68, 357–360. [Google Scholar] [CrossRef]
- Din, M.M.U.; Häusler, M.; Fischer, S.M.; Ratzenböck, K.; Chamasemani, F.F.; Hanghofer, I.; Henninge, V.; Brunner, R.; Slugovc, C.; Rettenwander, D. Role of Filler Content and Morphology in LLZO/PEO Membranes. Front. Energy Res. 2021, 9, 711610. [Google Scholar] [CrossRef]
- Johansson, P.; Ratner, M.A.; Shriver, D.F. The Influence of Inert Oxide Fillers on Poly(ethylene oxide) and Amorphous Poly(ethylene oxide) Based Polymer Electrolytes. J. Phys. Chem. B 2001, 105, 9016–9021. [Google Scholar] [CrossRef]
- Shin, J.H.; Passerini, S. PEO-LiN(SO2CF2CF3)2 Polymer Electrolytes. J. Electrochem. Soc. 2004, 151, A238. [Google Scholar] [CrossRef]
- Zagórski, J.; López del Amo, J.M.; Cordill, M.J.; Aguesse, F.; Buannic, L.; Llordés, A. Garnet–Polymer Composite Electrolytes: New Insights on Local Li-Ion Dynamics and Electrodeposition Stability with Li Metal Anodes. ACS Appl. Energy Mater. 2019, 2, 1734–1746. [Google Scholar] [CrossRef]
- Liu, W.; Lin, D.; Sun, J.; Zhou, G.; Cui, Y. Improved Lithium Ionic Conductivity in Composite Polymer Electrolytes with Oxide-Ion Conducting Nanowires. ACS Nano 2016, 10, 11407–11413. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Shi, F.; Lin, D.; Liu, Y.; Liu, W.; Pei, A.; Gong, Y.; Wang, H.; Liu, K.; et al. Vertically Aligned and Continuous Nanoscale Ceramic–Polymer Interfaces in Composite Solid Polymer Electrolytes for Enhanced Ionic Conductivity. Nano Lett. 2018, 18, 3829–3838. [Google Scholar] [CrossRef]
- Brivio, C.; Musolino, V.; Alet, P.J.; Merlo, M.; Hutter, A.; Ballif, C. Application-independent protocol for predicting the efficiency of lithium-ion battery cells in operations. J. Energy Storage 2018, 15, 415–422. [Google Scholar] [CrossRef]
- Krasnikova, I.V.; Pogosova, M.A.; Sanin, A.O.; Stevenson, K.J. Toward Standardization of Electrochemical Impedance Spectroscopy Studies of Li-Ion Conductive Ceramics. Chem. Mater. 2020, 32, 2232–2241. [Google Scholar] [CrossRef]
- Ohno, S.; Bernges, T.; Buchheim, J.; Duchardt, M.; Hatz, A.-K.; Kraft, M.A.; Kwak, H.; Santhosha, A.L.; Liu, Z.; Minafra, N.; et al. How Certain Are the Reported Ionic Conductivities of Thiophosphate-Based Solid Electrolytes? An Interlaboratory Study. ACS Energy Lett. 2020, 5, 910–915. [Google Scholar] [CrossRef]
- Bae, J.; Li, Y.; Zhang, J.; Zhou, X.; Zhao, F.; Shi, Y.; Goodenough, J.B.; Yu, G. A 3D Nanostructured Hydrogel-Framework-Derived High-Performance Composite Polymer Lithium-Ion Electrolyte. Angew. Chem. Int. Ed. 2018, 57, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Grundish, N.S.; Goodenough, J.B.; Khani, H. Designing composite polymer electrolytes for all-solid-state lithium batteries. Curr. Opin. Electrochem. 2021, 30, 100828. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méry, A.; Rousselot, S.; Lepage, D.; Aymé-Perrot, D.; Dollé, M. Limiting Factors Affecting the Ionic Conductivities of LATP/Polymer Hybrid Electrolytes. Batteries 2023, 9, 87. https://doi.org/10.3390/batteries9020087
Méry A, Rousselot S, Lepage D, Aymé-Perrot D, Dollé M. Limiting Factors Affecting the Ionic Conductivities of LATP/Polymer Hybrid Electrolytes. Batteries. 2023; 9(2):87. https://doi.org/10.3390/batteries9020087
Chicago/Turabian StyleMéry, Adrien, Steeve Rousselot, David Lepage, David Aymé-Perrot, and Mickael Dollé. 2023. "Limiting Factors Affecting the Ionic Conductivities of LATP/Polymer Hybrid Electrolytes" Batteries 9, no. 2: 87. https://doi.org/10.3390/batteries9020087
APA StyleMéry, A., Rousselot, S., Lepage, D., Aymé-Perrot, D., & Dollé, M. (2023). Limiting Factors Affecting the Ionic Conductivities of LATP/Polymer Hybrid Electrolytes. Batteries, 9(2), 87. https://doi.org/10.3390/batteries9020087






