Multifunctional Multilayer Nanospheres for Ion Regulation in Lithium Metal Batteries
Abstract
:1. Introduction
2. Results and Discussion
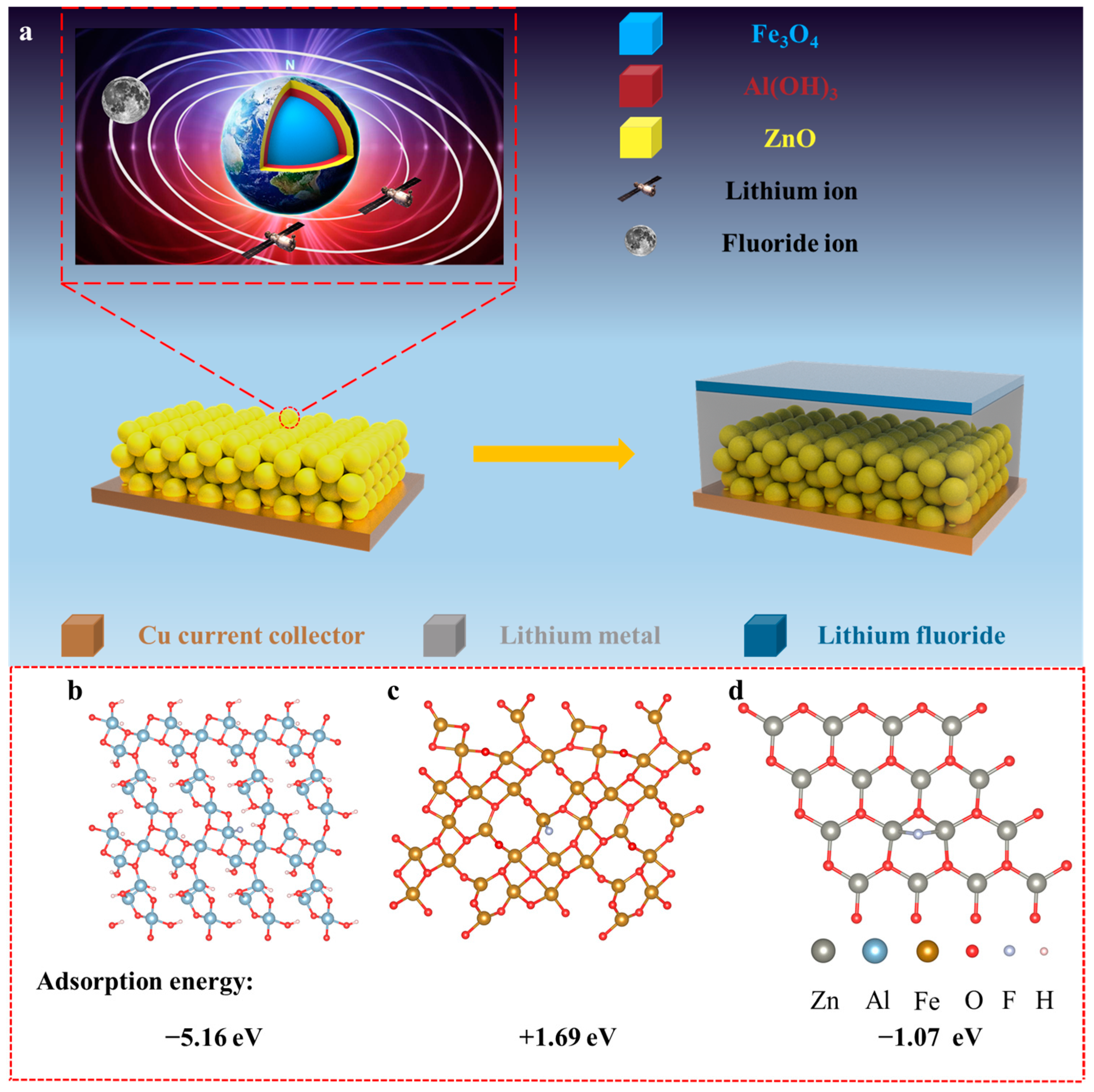
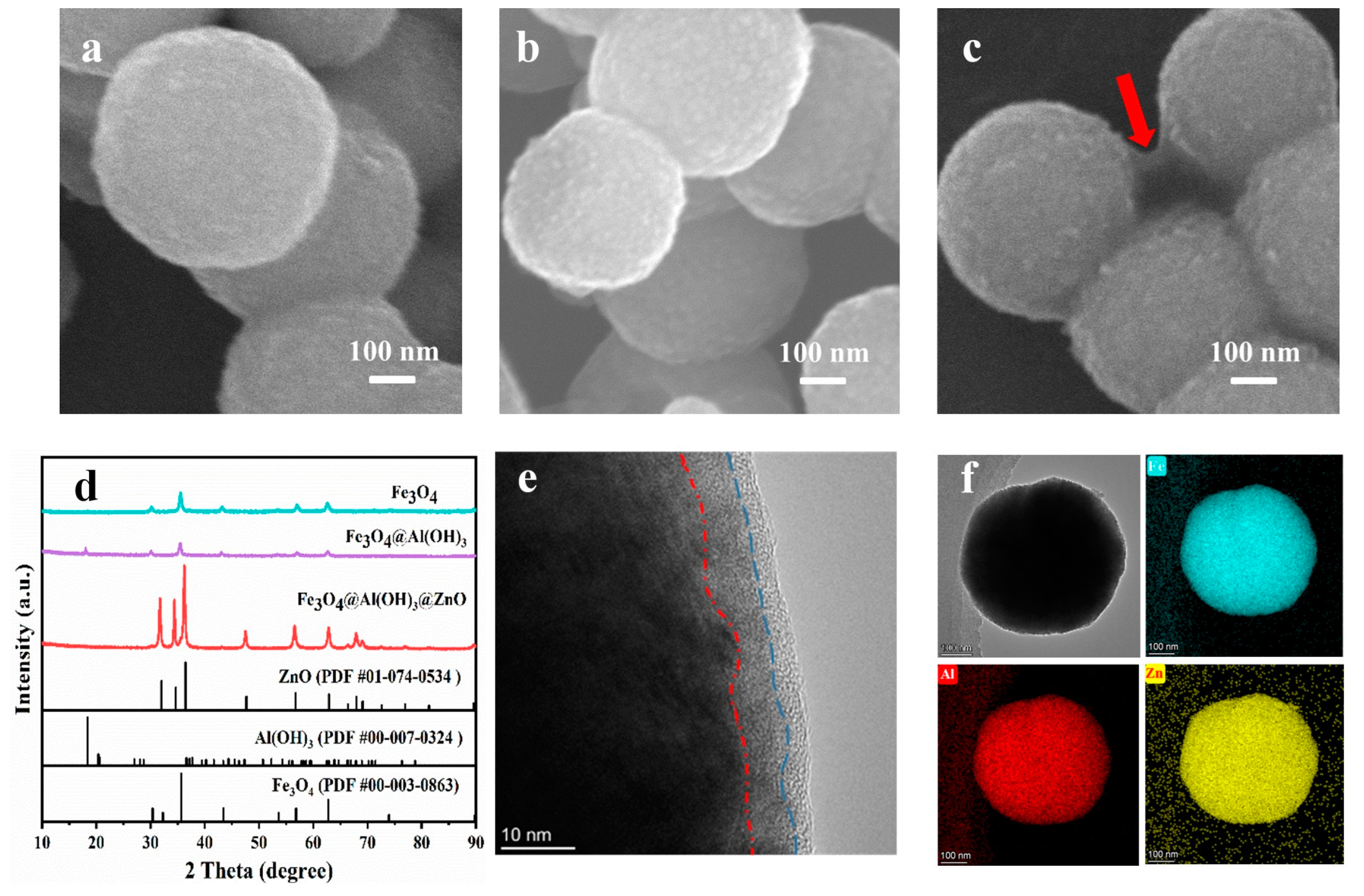
2.1. Morphology and Structure Characterization of Various Nanospheres
2.2. SEM Images of the Nanospheres or Lithium Deposition
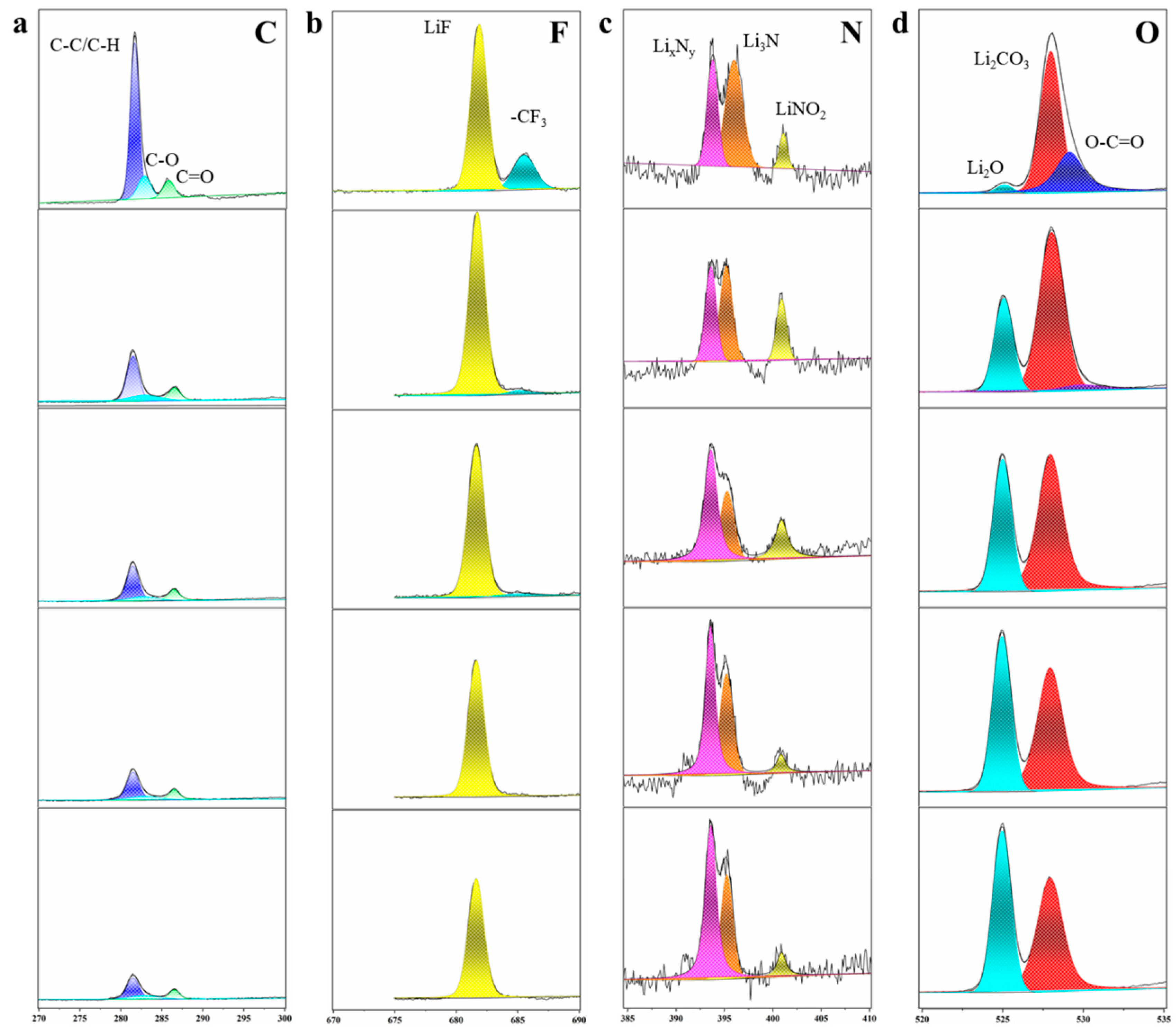
2.3. In Situ XPS Etching Performance
2.4. Electrochemical Performances of Various Nanospheres
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-F.; Xu, R.; Yan, C.; Li, B.-Q.; Yuan, H.; Huang, J.-Q. A review on the failure and regulation of solid electrolyte interphase in lithium batteries. J. Energy Chem. 2021, 59, 306–319. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, X.-Q.; Ding, F.; Huang, J.-Q.; Xu, R.; Chen, X.; Yan, C.; Su, F.-Y.; Chen, C.-M.; Liu, X.; et al. Advanced Electrode Materials in Lithium Batteries: Retrospect and Prospect. Energy Mater. Adv. 2021, 15, 1205324. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, S.; Liu, H.; Cheng, X.; Liu, L.; Xiang, R.; Zhang, Q.; Kaskel, S.; Huang, J. Mechanism understanding for stripping electrochemistry of Li metal anode. Susmat 2021, 1, 506–536. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Louli, A.J.; Eldesoky, A.; Weber, R.; Genovese, M.; Coon, M.; Degooyer, J.; Deng, Z.; White, R.T.; Lee, J.; Rodgers, T.; et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Boyle, D.T.; Jeong, Y.K.; Xu, R.; de Vasconcelos, L.S.; Huang, Z.; Wang, H.; Wang, H.; Huang, W.; et al. Free-standing ultrathin lithium metal–graphene oxide host foils with controllable thickness for lithium batteries. Nat. Energy 2021, 6, 790–798. [Google Scholar] [CrossRef]
- Huang, W.; Yu, Y.; Hou, Z.; Liang, Z.; Zheng, Y.; Quan, Z.; Lu, Y.-C. Dendrite-Free lithium electrode enabled by graphene aerogels with gradient porosity. Energy Storage Mater. 2020, 33, 329–335. [Google Scholar] [CrossRef]
- Weber, R.; Genovese, M.; Louli, A.J.; Hames, S.; Martin, C.; Hill, I.G.; Dahn, J.R. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 2019, 4, 683–689. [Google Scholar] [CrossRef]
- Li, Z.; He, Q.; Xu, X.; Zhao, Y.; Liu, X.; Zhou, C.; Ai, D.; Xia, L.; Mai, L. A 3D Nitrogen-Doped Graphene/TiN Nanowires Composite as a Strong Polysulfide Anchor for Lithium-Sulfur Batteries with Enhanced Rate Performance and High Areal Capacity. Adv. Mater. 2018, 30, e1804089. [Google Scholar] [CrossRef]
- Nanda, S.; Gupta, A.; Manthiram, A. Anode-Free Full Cells: A Pathway to High-Energy Density Lithium-Metal Batteries. Adv. Energy Mater. 2021, 11, 2000804. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Liu, Y.; Xing, J.; Zhou, A.; Li, J.; Zou, W.; Zhou, F. LiF headspace affixed metallic Li composite enables Li accommodation on the anode surface with excellent electrochemical performance. Chem. Eng. J. 2022, 430, 132970. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.S.; Yoo, J. An in situ formed LiF protective layer on a Li metal anode with solvent-less cross-linking. Sustain. Energy Fuels 2020, 4, 3282–3287. [Google Scholar] [CrossRef]
- Lang, J.; Long, Y.; Qu, J.; Luo, X.; Wei, H.; Huang, K.; Zhang, H.; Qi, L.; Zhang, Q.; Li, Z.; et al. One-pot solution coating of high quality LiF layer to stabilize Li metal anode. Energy Storage Mater. 2019, 16, 85–90. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Y.; Wang, J.; Zuo, P.; Du, C.; Yin, G.; Gao, Y. Regulating Li deposition by constructing homogeneous LiF protective layer for high-performance Li metal anode. Chem. Eng. J. 2022, 427, 131625. [Google Scholar] [CrossRef]
- Wang, L.; Fu, S.; Zhao, T.; Qian, J.; Chen, N.; Li, L.; Wu, F.; Chen, R. In situ formation of a LiF and Li–Al alloy anode protected layer on a Li metal anode with enhanced cycle life. J. Mater. Chem. A 2020, 8, 1247–1253. [Google Scholar] [CrossRef]
- Jiang, J.; Ou, Y.; Lu, S.; Shen, C.; Li, B.; Liu, X.; Jiang, Y.; Zhao, B.; Zhang, J. In-situ construction of Li-Mg/LiF conductive layer to achieve an intimate lithium-garnet interface for all-solid-state Li metal battery. Energy Storage Mater. 2022, 50, 810–818. [Google Scholar] [CrossRef]
- Jiang, P.; Cao, J.; Wei, B.; Qian, G.; Wang, S.; Shi, Y.; Du, G.; Lu, X.; Ouyang, C.; Cao, F.; et al. LiF involved interphase layer enabling thousand cycles of LAGP-based solid-state Li metal batteries with 80% capacity retention. Energy Storage Mater. 2022, 48, 145–154. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.; Bai, Y.; Li, Y.; Chen, G.; Wang, Z.; Wu, C. Regulating Li deposition by constructing LiF-rich host for dendrite-free lithium metal anode. Energy Storage Mater. 2019, 16, 411–418. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, G.; Wang, J.; Li, K.; Ma, J.; Zhang, X. In Situ Designing a Gradient Li+ Capture and Quasi-Spontaneous Diffusion Anode Protection Layer toward Long-Life Li−O2 Batteries. Adv. Mater. 2020, 32, 2004157. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, N.; Zhang, Z.; Wan, H.; Lin, H.; Liu, M.; Shen, C.; He, H.; Guo, X.; Zhang, J.-G.; et al. Stabilizing Li/electrolyte interface with a transplantable protective layer based on nanoscale LiF domains. Nano Energy 2017, 39, 662–672. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Chen, X.; Xu, R.; Cheng, X.-B.; Peng, H.-J.; Zhang, R.; Huang, J.-Q.; Zhang, Q. Columnar Lithium Metal Anodes. Angew. Chem. Int. Ed. 2017, 56, 14207–14211. [Google Scholar] [CrossRef] [PubMed]
- Shadike, Z.; Lee, H.; Borodin, O.; Cao, X.; Fan, X.; Wang, X.; Lin, R.; Bak, S.-M.; Ghose, S.; Xu, K.; et al. Identification of LiH and nanocrystalline LiF in the solid–electrolyte interphase of lithium metal anodes. Nat. Nanotechnol. 2021, 16, 549–554. [Google Scholar] [CrossRef]
- Xu, R.; Han, F.; Ji, X.; Fan, X.; Tu, J.; Wang, C. Interface engineering of sulfide electrolytes for all-solid-state lithium batteries. Nano Energy 2018, 53, 958–966. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, K.; Wu, H.; Ma, S.; Li, J.; Cui, Y.; Dong, Z.; Cui, Y.; Li, C. LiF Splitting Catalyzed by Dual Metal Nanodomains for an Efficient Fluoride Conversion Cathode. ACS Nano 2019, 13, 2490–2500. [Google Scholar] [CrossRef]
- Huang, Y.; Li, R.; Weng, S.; Zhang, H.; Zhu, C.; Lu, D.; Sun, C.; Huang, X.; Deng, T.; Fan, L.; et al. Eco-friendly electrolytes via a robust bond design for high-energy Li metal batteries. Energy Environ. Sci. 2022, 15, 4349–4361. [Google Scholar] [CrossRef]
- Qi, S.; Wang, H.; He, J.; Liu, J.; Cui, C.; Wu, M.; Li, F.; Feng, Y.; Ma, J. Electrolytes enriched by potassium perfluorinated sulfonates for lithium metal batteries. Sci. Bull. 2021, 66, 685–693. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Fan, H.; Zhao, Y.; Su, Y.; Liu, H.; Li, X.; Su, Y.; Yuan, H.; Pan, T.; et al. Stable Solid Electrolyte Interphase In Situ Formed on Magnesium-Metal Anode by using a Perfluorinated Alkoxide-Based All-Magnesium Salt Electrolyte. Adv. Mater. 2022, 34, 2203783. [Google Scholar] [CrossRef]
- Luo, J.; Bi, Y.; Zhang, L.; Zhang, X.; Liu, T.L. A Stable, Non-Corrosive Perfluorinated Pinacolatoborate Mg Electrolyte for Rechargeable Mg Batteries. Angew. Chem. Int. Ed. 2019, 58, 6967–6971. [Google Scholar] [CrossRef]
- Qiu, F.; Ren, S.; Zhang, X.; He, P.; Zhou, H. A high efficiency electrolyte enables robust inorganic–organic solid electrolyte interfaces for fast Li metal anode. Sci. Bull. 2021, 66, 897–903. [Google Scholar] [CrossRef]
- Qiu, F.; Li, X.; Deng, H.; Wang, D.; Mu, X.; He, P.; Zhou, H. A Concentrated Ternary-Salts Electrolyte for High Reversible Li Metal Battery with Slight Excess Li. Adv. Energy Mater. 2019, 9, 1803372. [Google Scholar] [CrossRef]
- Peng, Z.; Cao, X.; Gao, P.; Jia, H.; Ren, X.; Roy, S.; Li, Z.; Zhu, Y.; Xie, W.; Liu, D.; et al. High-Power Lithium Metal Batteries Enabled by High-Concentration Acetonitrile-Based Electrolytes with Vinylene Carbonate Additive. Adv. Funct. Mater. 2020, 30, 2001285. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Mei, D.; Han, K.S.; Engelhard, M.H.; Zhao, W.; Xu, W.; Liu, J.; Zhang, J. High-Voltage Lithium-Metal Batteries Enabled by Localized High-Concentration Electrolytes. Adv. Mater. 2018, 30, e1706102. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, X.; Liu, Y.; Wang, Q.; Lin, C.; Chen, S.; Zhang, Y. Ultra-stable Li||LiFePO4 batteries via advanced designing of localized high concentration electrolyte. J. Colloid Interface Sci. 2022, 628, 14–23. [Google Scholar] [CrossRef] [PubMed]
- May, R.; Hestenes, J.C.; Munich, N.A.; Marbella, L.E. Fluorinated ether decomposition in localized high concentration electrolytes. J. Power Sources 2023, 553, 232299. [Google Scholar] [CrossRef]
- Zheng, Y.; Soto, F.A.; Ponce, V.; Seminario, J.M.; Cao, X.; Zhang, J.-G.; Balbuena, P.B. Localized high concentration electrolyte behavior near a lithium–metal anode surface. J. Mater. Chem. A 2019, 7, 25047–25055. [Google Scholar] [CrossRef]
- Chang, C.; Yao, Y.; Li, R.; Cong, Z.; Li, L.; Guo, Z.H.; Hu, W.; Pu, X. Stable lithium metal batteries enabled by localized high-concentration electrolytes with sevoflurane as a diluent. J. Mater. Chem. A 2022, 10, 9001–9009. [Google Scholar] [CrossRef]
- Zhang, G.; Deng, X.; Li, J.; Wang, J.; Shi, G.; Yang, Y.; Chang, J.; Yu, K.; Chi, S.-S.; Wang, H.; et al. A bifunctional fluorinated ether co-solvent for dendrite-free and long-term lithium metal batteries. Nano Energy 2022, 95, 107014. [Google Scholar] [CrossRef]
- Gao, P.; Wu, H.; Zhang, X.; Jia, H.; Kim, J.; Engelhard, M.H.; Niu, C.; Xu, Z.; Zhang, J.; Xu, W. Optimization of Magnesium-Doped Lithium Metal Anode for High Performance Lithium Metal Batteries through Modeling and Experiment. Angew. Chem. Int. Ed. 2021, 60, 16506–16513. [Google Scholar] [CrossRef]
- Beltran, S.P.; Cao, X.; Zhang, J.-G.; El-Khoury, P.Z.; Balbuena, P.B. Influence of diluent concentration in localized high concentration electrolytes: Elucidation of hidden diluent-Li+ interactions and Li+ transport mechanism. J. Mater. Chem. A 2021, 9, 17459–17473. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, M.; Shen, C.; Cui, L.; Yang, W.; Zhang, C.; Chen, X.; Mai, L.; Zhao, Y.; Xu, X. Three-dimensional SEI framework induced by ion regulation in toroidal magnetic field for lithium metal battery. Cell Rep. Phys. Sci. 2022, 3, 101080. [Google Scholar] [CrossRef]
- Xiu-Lan, Y.U.; Wang, Z.C.; Han, Y.X.; Zeng, F.W.; Zhang, Q. Extraction of Rare Earths from Baogang Tailings by Carbochlorination Reaction Taking AlCl3 as Defluorinating Agent; Chinese Rare Earths: Shenyang, China, 2006. [Google Scholar]
- Shao, M.; Ning, F.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Preparation of Fe3O4@SiO2@Layered Double Hydroxide Core–Shell Microspheres for Magnetic Separation of Proteins. J. Am. Chem. Soc. 2012, 134, 1071–1077. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiao, M.; Shen, C.; Ma, H.; Cui, L.; Yang, W.; Zhao, T.; Zhao, Y.; Xu, X. Multifunctional Multilayer Nanospheres for Ion Regulation in Lithium Metal Batteries. Batteries 2023, 9, 149. https://doi.org/10.3390/batteries9030149
Li Y, Xiao M, Shen C, Ma H, Cui L, Yang W, Zhao T, Zhao Y, Xu X. Multifunctional Multilayer Nanospheres for Ion Regulation in Lithium Metal Batteries. Batteries. 2023; 9(3):149. https://doi.org/10.3390/batteries9030149
Chicago/Turabian StyleLi, Yan, Manjie Xiao, Chunli Shen, Haoqing Ma, Lianmeng Cui, Wei Yang, Tianhao Zhao, Yan Zhao, and Xu Xu. 2023. "Multifunctional Multilayer Nanospheres for Ion Regulation in Lithium Metal Batteries" Batteries 9, no. 3: 149. https://doi.org/10.3390/batteries9030149
APA StyleLi, Y., Xiao, M., Shen, C., Ma, H., Cui, L., Yang, W., Zhao, T., Zhao, Y., & Xu, X. (2023). Multifunctional Multilayer Nanospheres for Ion Regulation in Lithium Metal Batteries. Batteries, 9(3), 149. https://doi.org/10.3390/batteries9030149








