Zn-Co-Mo-rGO Ultra-Thin Nanosheets Arrays-Based Electrode Materials for Asymmetric Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
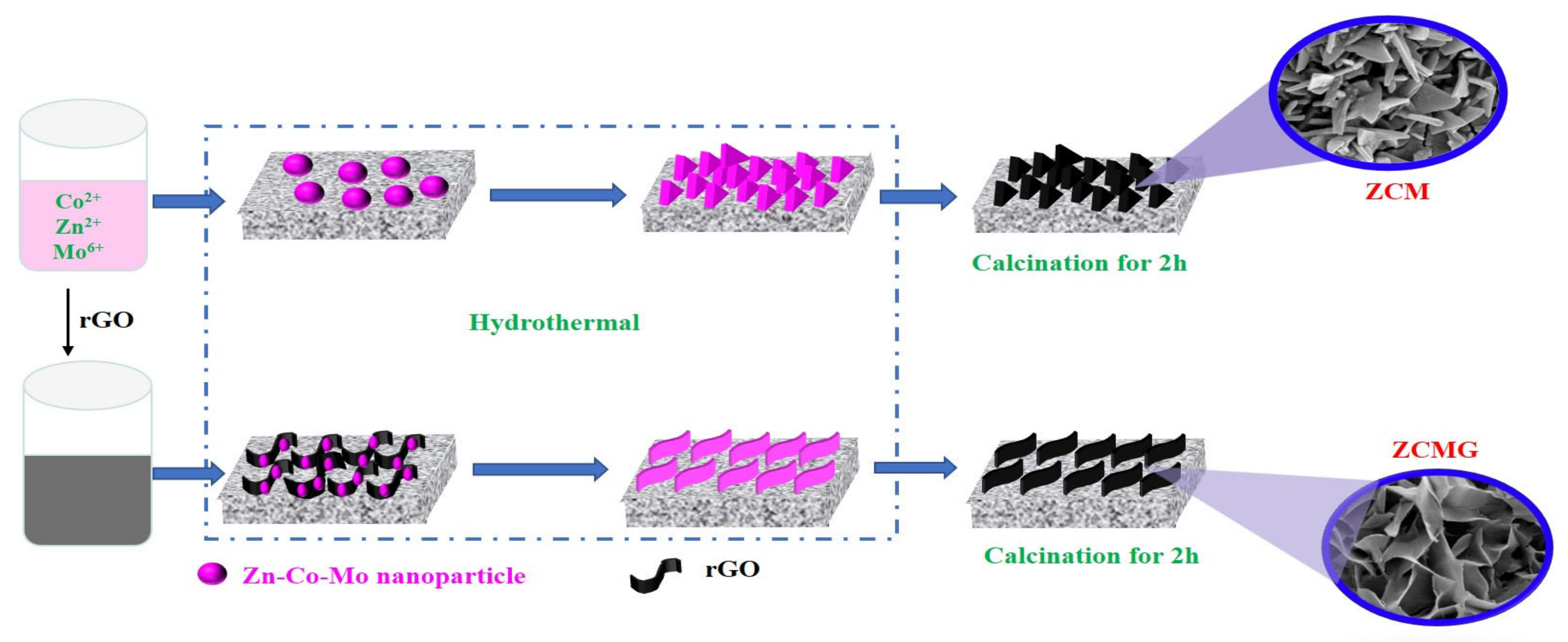
2.2. Synthesis of Electrode Materials
2.3. Structure Characterization
2.4. Electrochemical Measurements
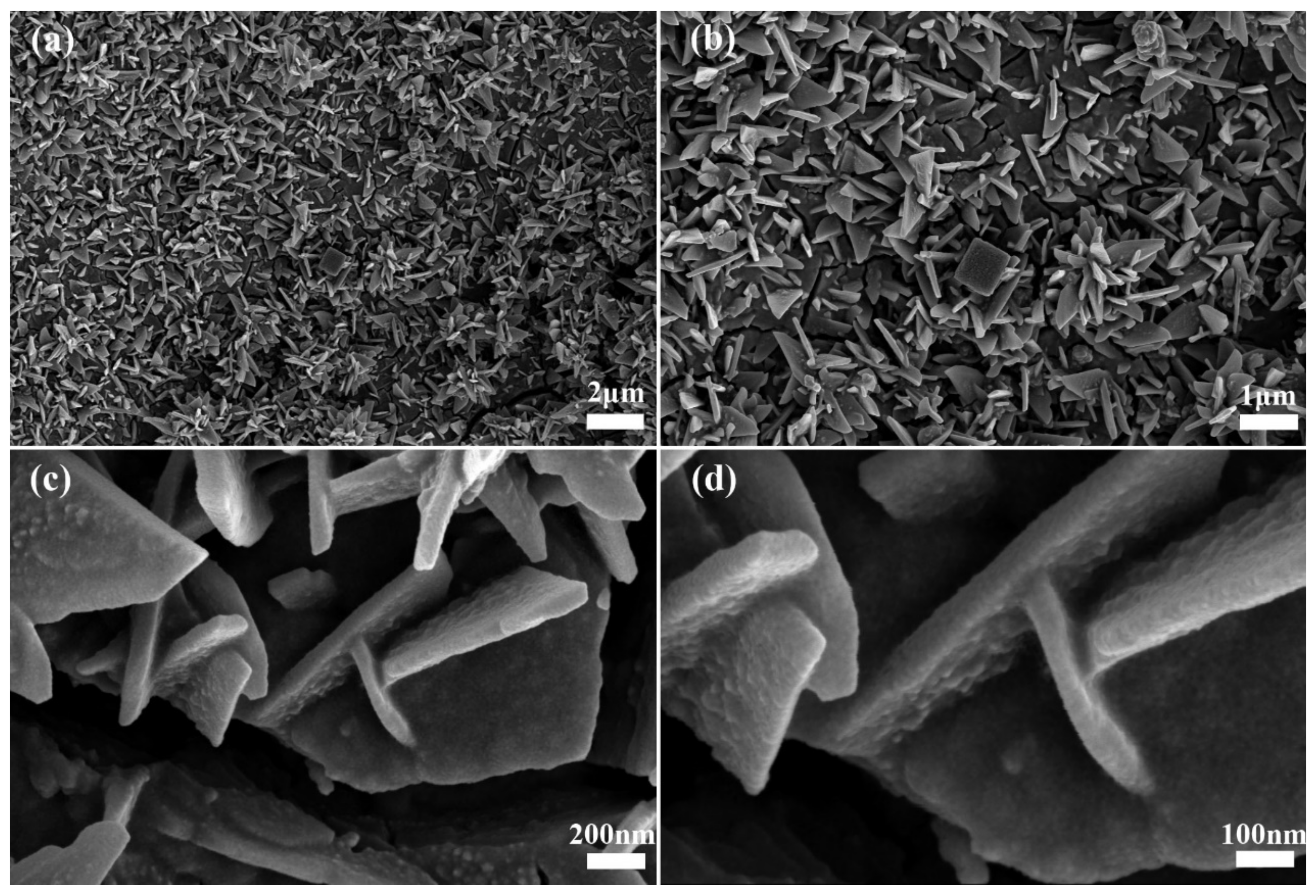
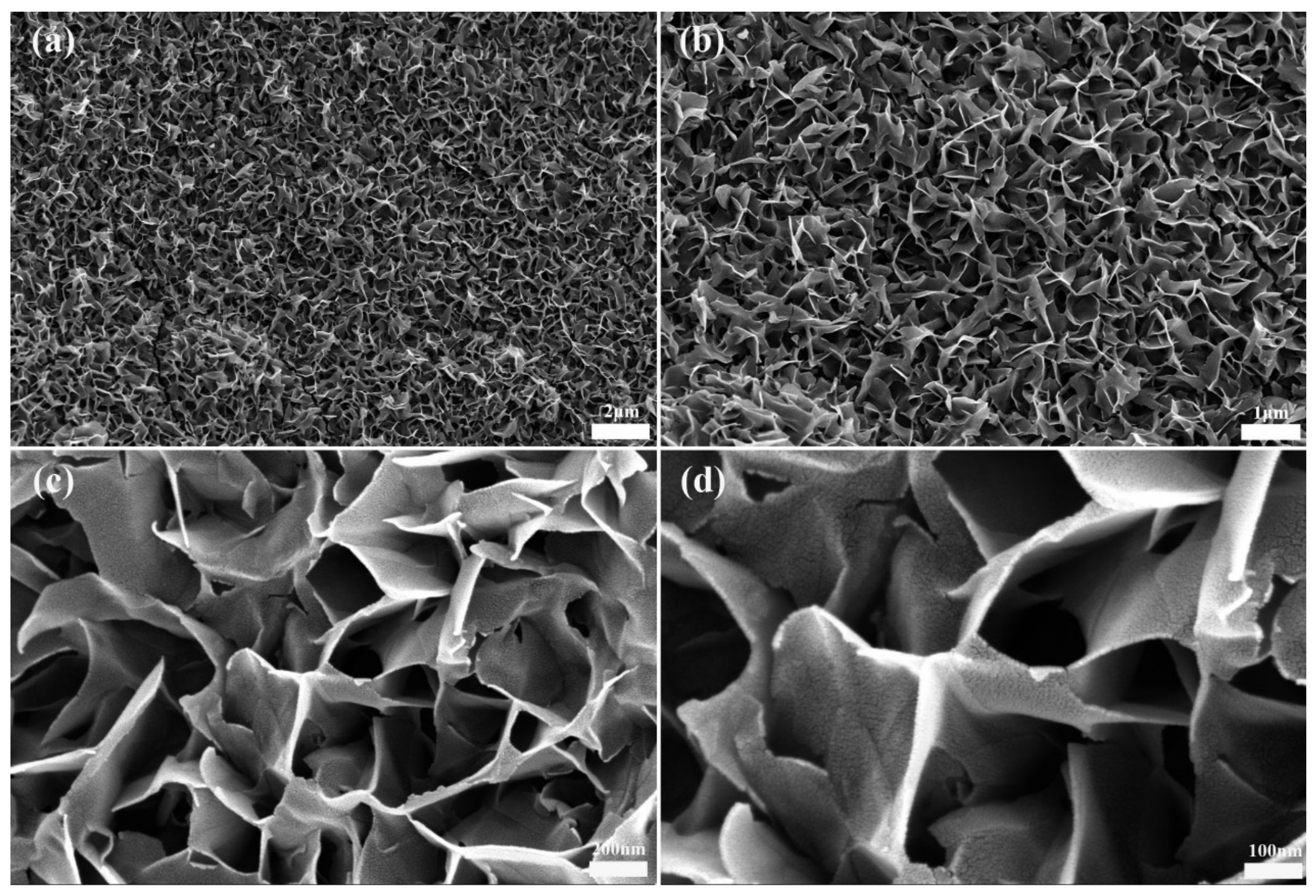
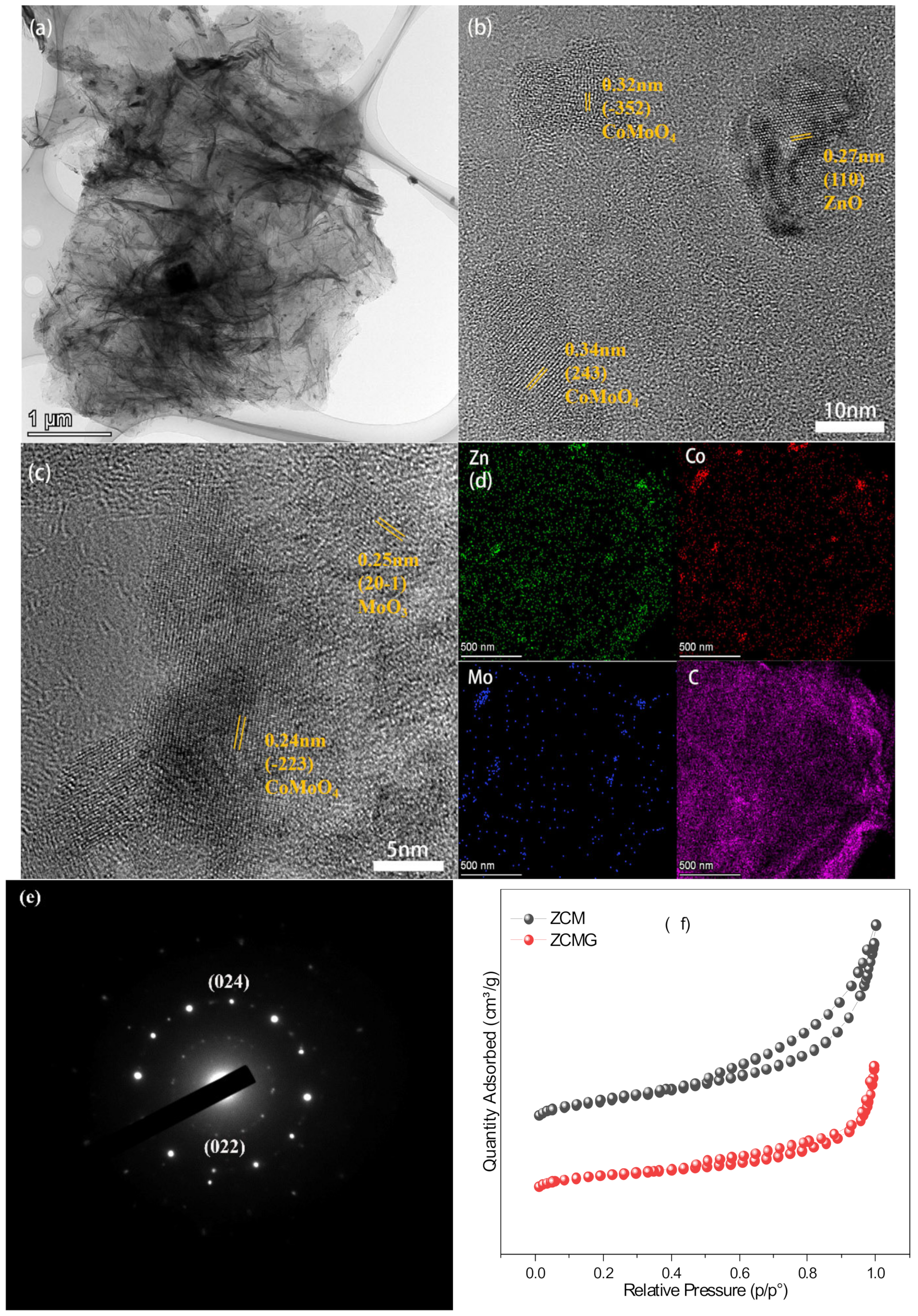
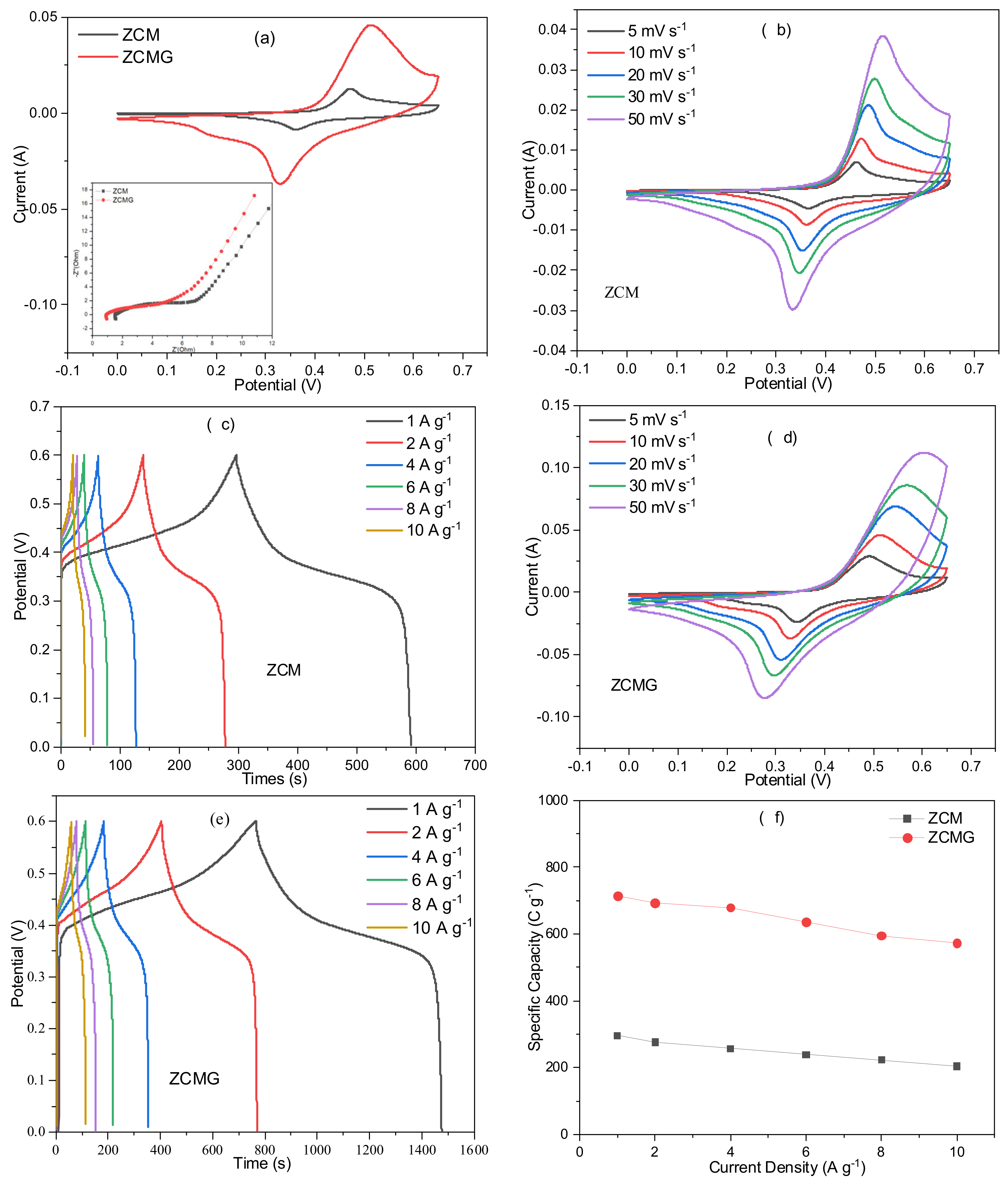
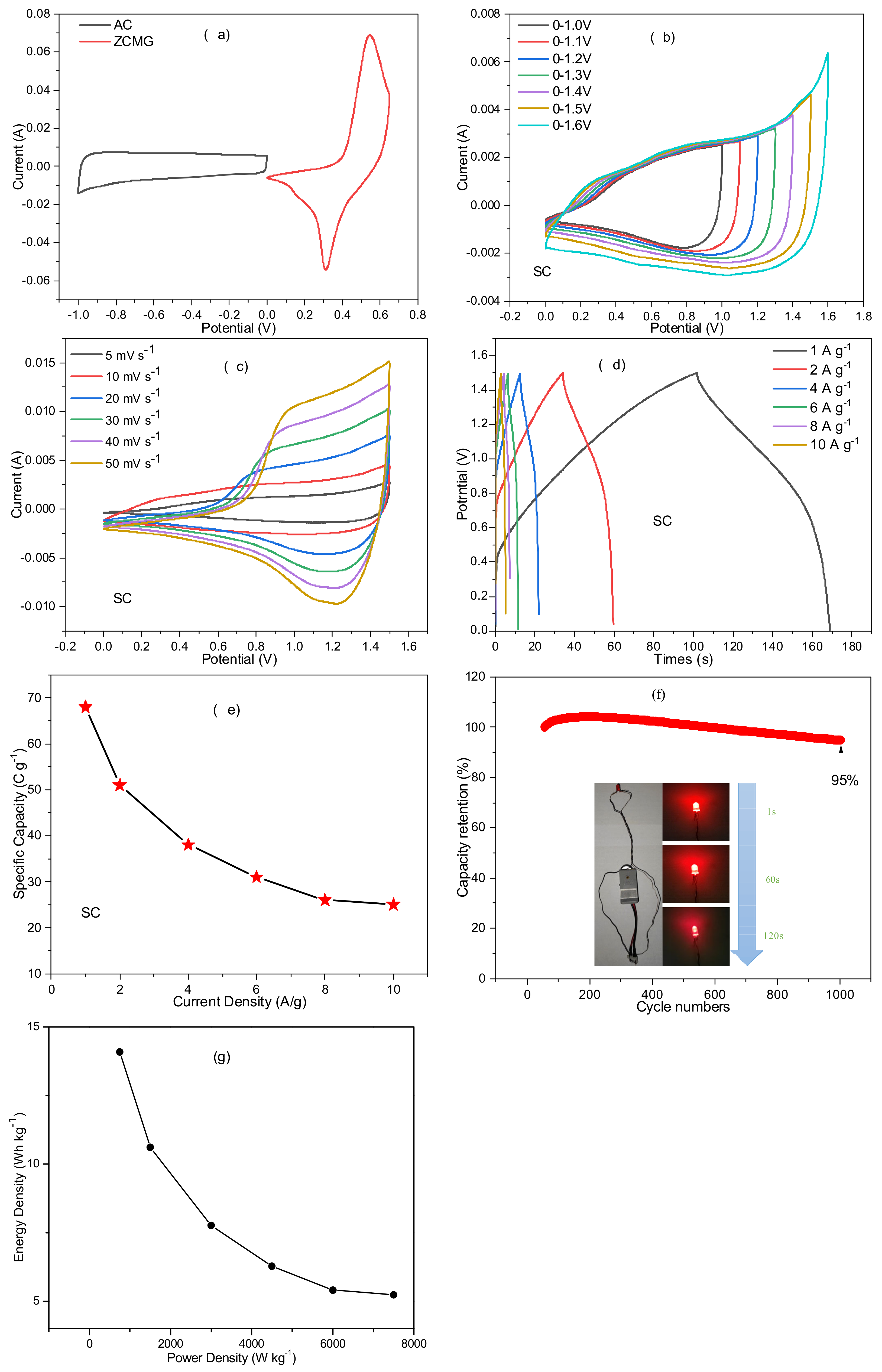
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Luo, Y.; Jia, C.; Zhang, Q.; Luo, G.; Zhao, L.; Boukherroub, R.; Jiang, Z. Facile synthesis of bimetal nickel cobalt phosphate nanostructures for high-performance hybrid supercapacitors. J. Alloys Compd. 2022, 893, 162340. [Google Scholar] [CrossRef]
- Rehman, J.; Eid, K.; Ali, R.; Fan, X.; Murtaza, G.; Faizan, M.; Laref, A.; Zheng, W.; Varma, R.S. Engineering of transition metal sulfide nanostructures as efficient electrodes for high-performance supercapacitors. ACS Appl. Nano Mater. 2022, 5, 6481–6498. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Liu, S.; Yamauchi, Y.; Zhang, F.; Kaneti, Y.V. Ultrathin nanosheet-assembled nickel-based metal–organic framework microflowers for supercapacitor applications. Chem. Commun. 2022, 58, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, L.; Lou, X.; Ding, M.; Jia, C. Fabrication of cobalt-nickel-zinc ternary oxide nanosheet and applications for supercapacitor electrode. Front. Chem. 2018, 6, 597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Ji, X.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; Ren, Y.; He, Y. Nickel/cobalt bimetallic metal-organic frameworks ultrathin nanosheets with enhanced performance for supercapacitors. J. Alloys Compd. 2020, 825, 154069. [Google Scholar] [CrossRef]
- Dai, M.; Zhao, D.; Wu, X. Research progress on transition metal oxide based electrode materials for asymmetric hybrid capacitors. Chin. Chem. Lett. 2020, 31, 2177–2188. [Google Scholar] [CrossRef]
- Han, D.; Xu, P.; Jing, X.; Wang, J.; Song, D.; Liu, J.; Zhang, M. Facile approach to prepare hollow core–shell NiO microspherers for supercapacitor electrodes. J. Solid State Chem. 2013, 203, 60–67. [Google Scholar] [CrossRef]
- Naeem, F.; Naeem, S.; Zhao, Z.; Shu, G.; Zhang, J.; Mei, Y.; Huang, G. Atomic layer deposition synthesized ZnO nanomembranes: A facile route towards stable supercapacitor electrode for high capacitance. J. Power Sources 2020, 451, 227740. [Google Scholar] [CrossRef]
- Zhang, H.; Geng, S.; Ouyang, M.; Mao, M.; Xie, F.; Riley, D.J. Using metal cation to control the microstructure of cobalt oxide in energy conversion and storage applications. Small 2022, 18, e2106391. [Google Scholar] [CrossRef]
- Wu, F.; Cui, F.; Ma, Q.; Zhang, J.; Qi, X.; Cui, T. Facile construction of hexagonal Co3O4 as the electrode for high-performance supercapacitor. Mater. Lett. 2022, 324, 132791. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, A.; Zhu, Q.; Nie, Z.; Zhang, Y.; Tang, Y.; Liang, S.; Cao, G. Facile synthesis of nanorod-assembled multi-shelled Co3O4 hollow microspheres for high-performance supercapacitors. J. Power Sources 2014, 272, 107–112. [Google Scholar] [CrossRef]
- Rizwan, A.; Ghulam, N.; Faisal, A.; Faiza, N.; Muhammad, I.K.; Tahir, I.; Muhammad, T.; Wajid, A.; Naeem, S.A.; Anum, N.; et al. Controlled growth of Mo-Doped NiO nanowires with enhanced electrochemical performance for supercapacitor applications. Mater. Sci. Eng. B 2022, 284, 115881. [Google Scholar]
- Xiong, S.; Weng, S.; Tang, Y.; Qian, L.; Xu, Y.; Li, X.; Lin, H.; Xu, Y.; Jiao, Y.; Chen, J. Mo-doped Co3O4 ultrathin nanosheet arrays anchored on nickel foam as a bi-functional electrode for supercapacitor and overall water splitting. J. Colloid Interface Sci. 2021, 602, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, J.; Daniel, B.; Peng, L.; Xu, Y.; Imran, S.; Huang, Y.; Duan, X. Hierarchical 3D electrodes for electrochemical energy storage. Nat. Rev. Mater. 2018, 4, 45–60. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Q.; Yang, Y.; Zang, Q.; Xiao, Z.; Zong, L.; Wang, K. In Situ construction of a heterostructured Zn−Mo−Ni−O−S hollow microflower for high-performance hybrid supercapacitors. ACS Appl. Nano Mater. 2021, 4, 801–809. [Google Scholar] [CrossRef]
- Sumanta, S.; Thi, T.N.; Shim, J.-J. Mesoporous Fe-Ni-Co ternary oxide nanoflake arrays on Ni foam for high-performance supercapacitor applications. J. Ind. Eng. Chem. 2018, 63, 181–190. [Google Scholar]
- Zhu, Y.; Ji, X.; Yin, R.; Hu, Z.; Qiu, X.; Wu, Z.; Liu, Y. Nanorod-assembled NiCo2O4 hollow microspheres assisted by an ionic liquid as advanced electrode materials for supercapacitors. RSC Adv. 2017, 7, 11123–11128. [Google Scholar] [CrossRef] [Green Version]
- Maitra, A.; Das, A.K.; Bera, R.; Karan, S.K.; Paria, S.; Si, S.K.; Khatua, B.B. An approach to fabricate PDMS encapsulated all-solid-state advanced asymmetric supercapacitor device with vertically aligned hierarchical Zn-Fe-Co ternary oxide nanowire and nitrogen doped graphene nanosheet for high power device applications. ACS Appl. Mater. Interfaces 2017, 9, 5947–5958. [Google Scholar] [CrossRef]
- Acharya, J.; Ko, T.H.; Seong, J.; Seo, M.; Khil, M.; Kim, H.; Kim, B.-S. Hybrid electrodes based on Zn−Ni−Co ternary oxide nanowires and nanosheets for ultra-high-rate asymmetric supercapacitors. ACS Appl. Nano Mater. 2020, 3, 8679–8690. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, L.; Hou, L.; Pang, G.; Zhang, X. Green template-free synthesis of mesoporous ternary CoNi-Mn oxide nanowires towards high-performance electrochemical capacitors. Part. Part. Syst. Char. 2014, 31, 778–787. [Google Scholar] [CrossRef]
- Hussain, I.; Mohamed, S.G.; Ali, A.; Abbas, N.; Ammar, S.M. Uniform growth of Zn-Mn-Co ternary oxide nanoneedles for high-performance energy-storage applications. J. Electroanal. Chem. 2019, 837, 39–47. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Fan, M.; Ma, T.; Chen, H.; Wang, H. Two-dimensional nanosheets constituted trimetal Ni-Co-Mn sulfide nanoflower-like structure for high-performance hybrid supercapacitors. Appl. Surf. Sci. 2021, 565, 150482. [Google Scholar] [CrossRef]
- Su, W.; Miao, R.; Tao, B.; Miao, F. High-performance symmetric supercapacitor based on flower-like zinc-cobalt-molybdenum hybrid metal oxide. Ionics 2019, 25, 5419–5427. [Google Scholar] [CrossRef]
- Shmatko, V.; Leontyeva, D.; Nevzorova, N.; Smirnova, N.; Brzhezinskaya, M.; Yalovega, G. Interaction between NiOx and MWСNT in NiOx/MWСNTs composite: XANES and XPS study. J. Electron. Spectrosc. 2017, 220, 76–80. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Shen, F.; Tian, D.; Huang, M.; He, J.; Zou, J.; Zhao, L.; Zeng, Y. Trichoderma bridges waste biomass and ultra-high specific surface area carbon to achieve a high-performance supercapacitor. J. Power Sources 2021, 497, 229880. [Google Scholar] [CrossRef]
- Mohammad, B.; Parisa, S.; Majid, S.; Mohammad, H.; Di Bartolomeo, A. ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloys Compd. 2021, 860, 158497. [Google Scholar]
- Zhang, X.; Zhang, J.; Kong, S.; Zhu, K.; Yan, J.; Ye, K.; Wang, G.; Cheng, K.; Zhou, L.; Cao, D. A novel calendula-like MnNb2O6 anchored on graphene sheet as high-performance intercalation pseudocapacitive anode for lithium-ion capacitors. J. Mater. Chem. A 2019, 7, 2855–2863. [Google Scholar] [CrossRef]
- Yuan, J.; Yao, D.; Jiang, L.; Tao, Y.; Che, J.; He, G.; Chen, H. Mn-doped NiMoO4 mesoporous nanorods/reduced graphene oxide composite for high-performance all-solid-state supercapacitor. ACS Appl. Energy Mater. 2020, 3, 1794–1803. [Google Scholar] [CrossRef]
- Kulurumotlakatla, D.K.; Yedluri, A.K.; Kim, H.-J. Hierarchical NiCo2S4 nanostructure as highly efficient electrode material for high-performance supercapacitor applications. J. Energy Storage 2020, 31, 101069. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO4 honeycombs on nickel foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef]
- Anil, K.Y.; Dasha, K.K.; Kim, H.-J. Facile preparation of a highly efficient NiZn2O4–NiO nanoflower composite grown on Ni foam as an advanced battery-type electrode material for high-performance electrochemical supercapacitors. Dalton Trans. 2020, 49, 3622–3629. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Ojha, G.P.; Kim, B.S.; Pant, B.; Park, M. Modish designation of hollow-tubular rGO-NiMoO4@Ni-Co-S hybrid core-shell electrodes with multichannel superconductive pathways for high-performance asymmetric aupercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 17487–17500. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Liao, T.; Sun, Z. Two-dimensional metal oxide nanosheets for rechargeable batteries. J. Energy Chem. 2018, 27, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, V.; Srinivasarao, U.; Ramachandran, R.; Saranya, M.; Nirmala Grace, A. Synthesis of tin oxide/graphene (SnO2/G) nanocomposite and its electrochemical properties for supercapacitor applications. Mater. Res. Bull. 2016, 84, 145–151. [Google Scholar] [CrossRef]
- Aadithya, J.; Tamil, S.; Craig, J.N.; Sudipta, S. Scalable ternary hierarchical microspheres composed of PANI/rGO/CeO2 for high performance supercapacitor applications. Carbon 2019, 151, 192–202. [Google Scholar]
- Poochai, C.; Sriprachuabwong, C.; Odtipinta, J.; Lohitkarn, J.; Pasakon, P.; Primpray, V.; Maeboonruan, N.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. Alpha-MnO2 nanofibers/nitrogen and sulfur-co-doped reduced graphene oxide for 4.5 V quasi-solid state supercapacitors using ionic liquid-based polymer electrolyte. J. Colloid Interface Sci. 2021, 583, 734–745. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Al, A.; Pallavolu, M.R.; Rao, S.S.; Nallapureddy, R.R.; Ramakrishna, S. Multiple structural defects in poor crystalline nickel-doped tungsten disulfide nanorods remarkably enhance supercapacitive performance. Int. J. Energ. Res. 2022, 46, 14227–14239. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kumar, K.D.; Kim, H.-J. Reagents assisted ZnCo2O4 nanomaterial for supercapacitor application. Electrochim. Acta 2020, 330, 135261. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Zhang, P.; He, H.; Peng, Y.; Wu, Y. Engineering RuO2 on CuCo2O4/CuO nanoneedles as multifunctional electrodes for the hybrid supercapacitors and water oxidation catalysis. J. Alloys Compd. 2020, 832, 154962. [Google Scholar] [CrossRef]
- Cao, Y.; An, L.; Liao, L.; Liu, X.; Ji, T.; Zou, R.; Yang, J.; Qin, Z.; Hu, J. Hierarchical core/shell structures of ZnO nanorod@CoMoO4 nanoplates used as a high-performance electrode for supercapacitors. RSC Adv. 2016, 6, 3020–3024. [Google Scholar] [CrossRef]
- Yuan, Y.; Jia, H.; Liu, Z.; Wang, L.; Sheng, J.; Fei, W. A highly conductive Ni(OH)2 nano-sheet wrapped CuCo2S4 nano-tube electrode with a core-shell structure for high performance supercapacitors. Dalton Trans. 2021, 50, 8476–8486. [Google Scholar] [CrossRef] [PubMed]
- Barqi, J.; Masoudpanah, S.M.; Hasheminiasari, M.; Liu, X. Nanoribbon-like NiCo2O4/reduced graphene oxide nanocomposite for high-performance hybrid supercapacitor. J. Alloys Compd. 2023, 930, 167509. [Google Scholar] [CrossRef]
- Subbukalai, V.; Lee, S.-H.; Sadayappan, N.; Ryu, K. Cu-Zn-Co oxide nanoflakes on Ni-foam as a binder free electrode for energy storage applications. Materials Letters. Mater. Lett. 2018, 219, 143–147. [Google Scholar]
- Han, C.; Xu, X.; Mu, H.; Tian, Q.; Li, Q.; Liu, Y.; Zhang, X.; Zhao, Z.; Su, X. Construction of hierarchical sea urchin-like manganese substituted nickel cobaltite@tricobalt tetraoxide core-shell microspheres on nickel foam as binder-free electrodes for high performance supercapacitors. J. Colloid Interface Sci. 2021, 596, 89–99. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Hu, P.; Song, J.; Xiao, L. Asymmetric hybrid capacitor based on Co3O4 nanowire electrode. Ionics 2020, 26, 6289–6295. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, D.; Li, X.; Sun, H.; Li, L.; Bai, P.; Xing, W.; Xue, Q.; Yan, Z. Electrostatic self-assembly of sandwich-like CoAl-LDH/polypyrrole/graphene nanocomposites with wnhanced capacitive performance. ACS Appl. Mater. Interfaces 2017, 9, 31699–31709. [Google Scholar] [CrossRef]
- Yan, S.; Han, Z.; Sun, J.; Yang, X.; Hu, X.; Li, C.; Cao, B. Controllable ZnFe2O4/reduced graphene oxide hybrid for high-performance supercapacitor electrode. Electrochim. Acta 2018, 268, 20–26. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P. Binary nickel ferrite oxide (NiFe2O4) nanoparticles coated on reduced graphene oxide as stable and high-performance asymmetric supercapacitor electrode material. Int. J. Hydrogen Energy 2020, 45, 27482–27491. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, W.; Yang, M.; Zhang, Y.; Meng, F. Preparation and study of porous MnCo2O4@NiO nanosheets for high-performance supercapacitor. Ceram. Int. 2019, 45, 20451–20457. [Google Scholar] [CrossRef]


| Electrode Materials | Specific Capacitance (F g−1) /Specific Capacity (C g−1) | Current Density | Ref. |
|---|---|---|---|
| CuZnCo | 430 F g−1 | 1 A g−1 | [44] |
| CoNiMn | 612 F g−1 | 0.5 A g−1 | [20] |
| Ni0.5Mn0.5Co2O4@Co3O4 | 931 F g−1 | 1 A g−1 | [45] |
| Co3O4/rGO | 1152 F g−1 | 1 A g−1 | [46] |
| CoAl-LDH/graphene | 864 F g−1 | 1 A g−1 | [47] |
| ZnFe2O4/rGO | 352.9 F g−1 | 1 A g−1 | [48] |
| NiFe2O4/rGO | 584.63 F g−1 | 1 A g−1 | [49] |
| MnCo2O4@NiO | 508.3 F g−1 | 1 A g−1 | [50] |
| Zn-Co-Mo | 384 F g−1 | 60 mA cm−2 | [23] |
| ZCMG | 713 C g−1 | 1 A g−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; He, S.; Xiang, Y.; Peng, X.; Xiong, L.; Wu, J. Zn-Co-Mo-rGO Ultra-Thin Nanosheets Arrays-Based Electrode Materials for Asymmetric Supercapacitor. Batteries 2023, 9, 158. https://doi.org/10.3390/batteries9030158
Liu S, He S, Xiang Y, Peng X, Xiong L, Wu J. Zn-Co-Mo-rGO Ultra-Thin Nanosheets Arrays-Based Electrode Materials for Asymmetric Supercapacitor. Batteries. 2023; 9(3):158. https://doi.org/10.3390/batteries9030158
Chicago/Turabian StyleLiu, Shuang, Siwei He, Yanhong Xiang, Xiaochun Peng, Lizhi Xiong, and Jianhua Wu. 2023. "Zn-Co-Mo-rGO Ultra-Thin Nanosheets Arrays-Based Electrode Materials for Asymmetric Supercapacitor" Batteries 9, no. 3: 158. https://doi.org/10.3390/batteries9030158
APA StyleLiu, S., He, S., Xiang, Y., Peng, X., Xiong, L., & Wu, J. (2023). Zn-Co-Mo-rGO Ultra-Thin Nanosheets Arrays-Based Electrode Materials for Asymmetric Supercapacitor. Batteries, 9(3), 158. https://doi.org/10.3390/batteries9030158





