Textile PAN Carbon Fibers Cathode for High-Voltage Seawater Batteries
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
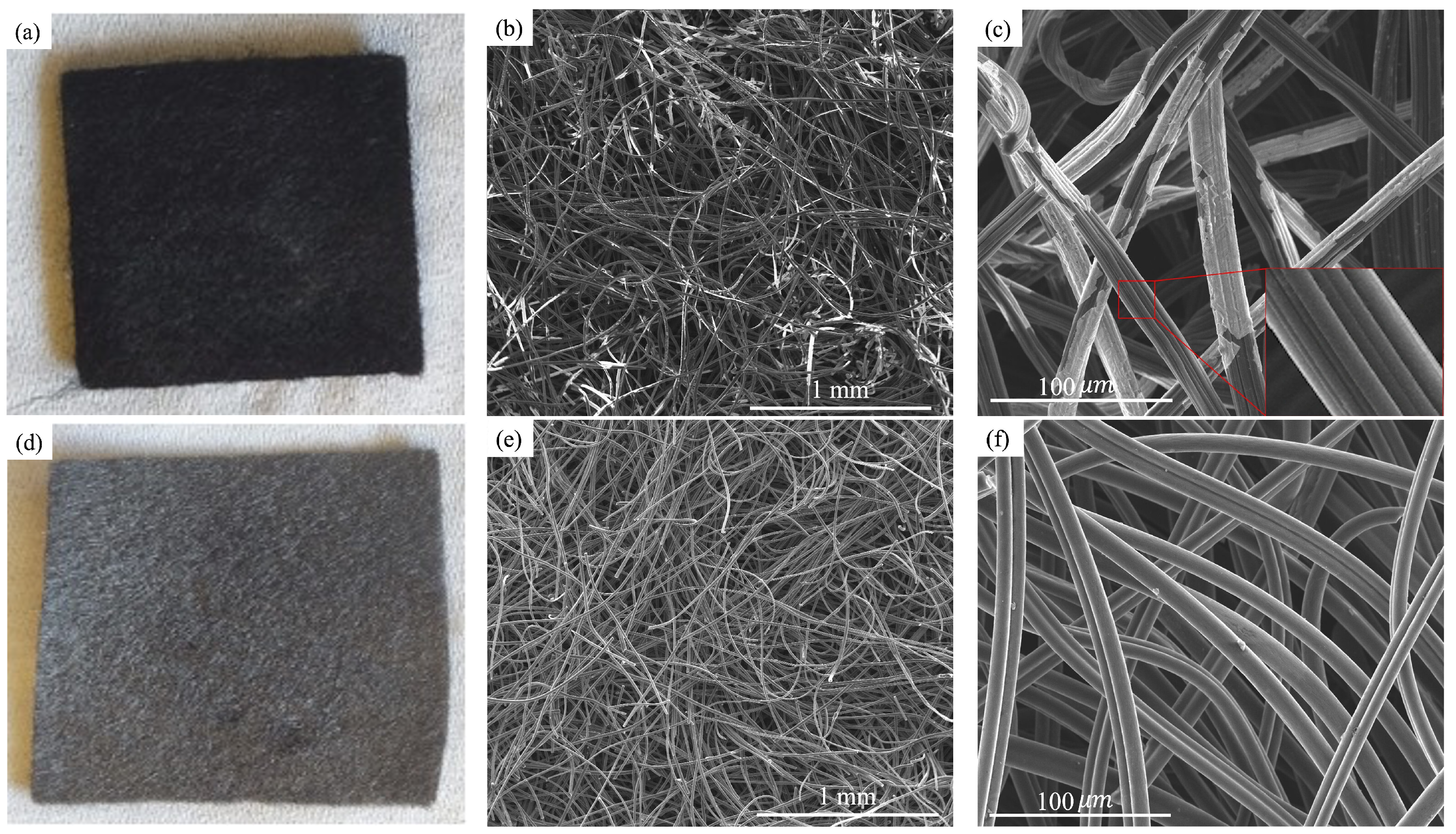
3.1. Morphological and Chemical Characterization
3.2. Wettability
3.3. Cathodes Electrochemical Characterization
Equivalent Circuit
3.4. Full Cell Electrochemical Analysis
3.4.1. Galvanostic Voltage Profiles
3.4.2. Current Variation
3.4.3. Cyclic Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hwang, S.M.; Park, J.S.; Kim, Y.; Go, W.; Han, J.; Kim, Y.; Kim, Y. Rechargeable seawater batteries—From concept to applications. Adv. Mater. 2019, 31, 1804936. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Go, W.; Han, J.; Thuy, L.P.T.; Kishor, K.; Kim, Y.; Kim, Y. Emergence of rechargeable seawater batteries. J. Mater. Chem. A 2019, 7, 22803–22825. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, W.G. Seawater Batteries: Principles, Materials and Technology; Springer Nature: Singapore, 2022. [Google Scholar]
- Han, J.; Hwang, S.M.; Go, W.; Senthilkumar, S.; Jeon, D.; Kim, Y. Development of coin-type cell and engineering of its compartments for rechargeable seawater batteries. J. Power Sources 2018, 374, 24–30. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, J.; Kim, Y.; Kim, Y. Na-ion storage performance of amorphous Sb2S3 nanoparticles: Anode for Na-ion batteries and seawater flow batteries. J. Mater. Chem. A 2016, 4, 17946–17951. [Google Scholar] [CrossRef]
- Manikandan, P.; Kishor, K.; Han, J.; Kim, Y. Advanced perspective on the synchronized bifunctional activities of P2-type materials to implement an interconnected voltage profile for seawater batteries. J. Mater. Chem. A 2018, 6, 11012–11021. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Abirami, M.; Kim, J.; Go, W.; Hwang, S.M.; Kim, Y. Sodium-ion hybrid electrolyte battery for sustainable energy storage applications. J. Power Sources 2017, 341, 404–410. [Google Scholar] [CrossRef]
- Kim, J.K.; Mueller, F.; Kim, H.; Jeong, S.; Park, J.S.; Passerini, S.; Kim, Y. Eco-friendly Energy Storage System: Seawater and Ionic Liquid Electrolyte. ChemSusChem 2016, 9, 42–49. [Google Scholar] [CrossRef]
- Park, J.; Park, J.S.; Senthilkumar, S.; Kim, Y. Hybridization of cathode electrochemistry in a rechargeable seawater battery: Toward performance enhancement. J. Power Sources 2020, 450, 227600. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, C.; Kong, F.; Zhao, Q.; Kong, A.; Shan, Y. Activated biochar derived from peanut shells as the electrode materials with excellent performance in Zinc-air battery and supercapacitance. Waste Manag. 2021, 125, 257–267. [Google Scholar] [CrossRef]
- Kim, K.; Hwang, S.M.; Park, J.S.; Han, J.; Kim, J.; Kim, Y. Highly improved voltage efficiency of seawater battery by use of chloride ion capturing electrode. J. Power Sources 2016, 313, 46–50. [Google Scholar] [CrossRef]
- Suh, D.H.; Park, S.K.; Nakhanivej, P.; Kim, Y.; Hwang, S.M.; Park, H.S. Hierarchically structured graphene-carbon nanotube-cobalt hybrid electrocatalyst for seawater battery. J. Power Sources 2017, 372, 31–37. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Park, S.O.; Kim, J.; Hwang, S.M.; Kwak, S.K.; Kim, Y. Seawater battery performance enhancement enabled by a defect/edge-rich, oxygen self-doped porous carbon electrocatalyst. J. Mater. Chem. A 2017, 5, 14174–14181. [Google Scholar] [CrossRef]
- Jeoung, S.; Sahgong, S.H.; Kim, J.H.; Hwang, S.M.; Kim, Y.; Moon, H.R. Upcycling of nonporous coordination polymers: Controllable-conversion toward porosity-tuned N-doped carbons and their electrocatalytic activity in seawater batteries. J. Mater. Chem. A 2016, 4, 13468–13475. [Google Scholar] [CrossRef]
- Ryu, J.H.; Park, J.; Park, J.; Mun, J.; Im, E.; Lee, H.; Hong, S.Y.; An, K.; Lee, G.; Kim, Y.; et al. Carbothermal shock-induced bifunctional Pt-Co alloy electrocatalysts for high-performance seawater batteries. Energy Storage Mater. 2022, 45, 281–290. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Maia, G. Developing efficient catalysts for the OER and ORR using a combination of Co, Ni, and Pt oxides along with graphene nanoribbons and NiCo2O4. J. Mater. Chem. A 2020, 8, 17691–17705. [Google Scholar] [CrossRef]
- 4 TO ONE. 2022. Available online: https://www.4toone.com/main/ (accessed on 17 March 2023).
- Son, M.; Park, S.; Kim, N.; Angeles, A.T.; Kim, Y.; Cho, K.H. Simultaneous energy storage and seawater desalination using rechargeable seawater battery: Feasibility and future directions. Adv. Sci. 2021, 8, 2101289. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.A.d.; Matsushima, J.T.; Rezende, M.C.; Gonçalves, E.S.; Marcuzzo, J.S.; Baldan, M.R. Production and characterization of activated carbon fiber from textile PAN fiber. J. Aerosp. Technol. Manag. 2017, 9, 423–430. [Google Scholar] [CrossRef]
- Saldanha Marcuzzo, J.; Otani, C. Fibra de Carbono Ativada-Produção Ultrarrápida a Partir da PAN Têxtil: Uma Abordagem da Produção e Caracterização de Fibra de Carbono Ativada a Partir de Matéria Prima Têxtil, 1st ed.; Novas Edições Acadêmicas: Saarbrücken, Germany, 2015; OCLC: 913015440. [Google Scholar]
- Marcuzzo, J.; Cuña, A.; Tancredi, N.; Polidoro, H.; Otani, S.; Otani, C. Microporous Activated Carbono Fiber Felt Produced from Brasilian Textile Pan Fiber; X Encontro Brasileiro sobre Adsorção: Guarujá, Brazil, 2014. [Google Scholar]
- Ko, F.K.; Yang, H. Functional nanofibre: Enabling material for the next generations smart textiles. J. Fiber Bioeng. Inform. 2008, 1, 81–92. [Google Scholar]
- Mirzaeian, M.; Hall, P.J. Characterizing capacity loss of lithium oxygen batteries by impedance spectroscopy. J. Power Sources 2010, 195, 6817–6824. [Google Scholar] [CrossRef]
- Guanhua, N.; Qian, S.; Meng, X.; Hui, W.; Yuhang, X.; Weimin, C.; Gang, W. Effect of NaCl-SDS compound solution on the wettability and functional groups of coal. Fuel 2019, 257, 116077. [Google Scholar] [CrossRef]
- Kim, C.H.; Pyun, S.I.; Kim, J.H. An investigation of the capacitance dispersion on the fractal carbon electrode with edge and basal orientations. Electrochim. Acta 2003, 48, 3455–3463. [Google Scholar] [CrossRef]
- Chang, B.Y. Conversion of a constant phase element to an equivalent capacitor. J. Electrochem. Sci. Technol. 2020, 11, 318–321. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, S.W.; Yim, T.; Kim, J.G.; Choi, J.W.; Kim, J.H.; Park, M.S.; Kim, Y.J. A new strategy for integrating abundant oxygen functional groups into carbon felt electrode for vanadium redox flow batteries. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Pandey, L.; Sarkar, S.; Arya, A.; Sharma, A.; Panwar, A.; Kotnala, R.; Gaur, A. Fabrication of activated carbon electrodes derived from peanut shell for high-performance supercapacitors. Biomass Convers. Biorefinery 2021, 1–10. [Google Scholar] [CrossRef]
- Mogensen, R. Realization of Sodium-ion Batteries: From Electrode to Electrolyte Materials. Ph.D. Thesis, Acta Universitatis Upsaliensis, Uppsala, Sweden, 1941. [Google Scholar]
- El-Deen, A.G.; Choi, J.H.; Khalil, K.A.; Almajid, A.A.; Barakat, N.A. A TiO2 nanofiber/activated carbon composite as a novel effective electrode material for capacitive deionization of brackish water. RSC Adv. 2014, 4, 64634–64642. [Google Scholar] [CrossRef]




| Cathode | Contact Angle (°) | Absorption Time (s) | |
|---|---|---|---|
| CCF | Unused | 141.9 | No Absorption |
| Used | Unmeasurable | 0.25 | |
| ACF | Unused | 133.1 | 137.6 |
| Used | 134.9 | 42.1 | |
| Cathode | Capacitance (mF) | Increase (%) | |
|---|---|---|---|
| CCF | 26.9 | - | |
| 1 Layer | 186.0 | 590.5 | |
| ACF | 2 Layers | 1920.0 | 7026.5 |
| 3 Layers | 2521.6 | 9258.7 | |
| CCC | Parameters | |||||
|---|---|---|---|---|---|---|
| RE () | RCT () | CPEDL (S · sn) | CPED (S · sn) | |||
| Y0 | n | Y0 | n | |||
| CCF | 6.366 | 22.33 | 665.0 × 10−6 | 0.556 | 2.320 × 10−3 | 0.882 |
| ACF1 | 3.805 | 15.64 | 504.3 × 10−6 | 0.583 | 849.3 × 10−6 | 0.623 |
| ACF2 | 5.000 | 45.00 | 390.3 × 10−6 | 0.666 | 400.0 × 10−3 | 0.840 |
| ACF3 | 5.835 | 50.80 | 2.054 × 10−3 | 0.462 | 334.7 × 10−3 | 0.776 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.; Salgueiro, T.; Marcuzzo, J.; Arruda, E.; Ventura, J.; Oliveira, J. Textile PAN Carbon Fibers Cathode for High-Voltage Seawater Batteries. Batteries 2023, 9, 178. https://doi.org/10.3390/batteries9030178
Ferreira J, Salgueiro T, Marcuzzo J, Arruda E, Ventura J, Oliveira J. Textile PAN Carbon Fibers Cathode for High-Voltage Seawater Batteries. Batteries. 2023; 9(3):178. https://doi.org/10.3390/batteries9030178
Chicago/Turabian StyleFerreira, João, Tiago Salgueiro, Jossano Marcuzzo, Eduardo Arruda, João Ventura, and Joana Oliveira. 2023. "Textile PAN Carbon Fibers Cathode for High-Voltage Seawater Batteries" Batteries 9, no. 3: 178. https://doi.org/10.3390/batteries9030178
APA StyleFerreira, J., Salgueiro, T., Marcuzzo, J., Arruda, E., Ventura, J., & Oliveira, J. (2023). Textile PAN Carbon Fibers Cathode for High-Voltage Seawater Batteries. Batteries, 9(3), 178. https://doi.org/10.3390/batteries9030178








