Electrochemical Modelling of Na-MCl2 Battery Cells Based on an Expanded Approximation Method
Abstract
:1. Introduction
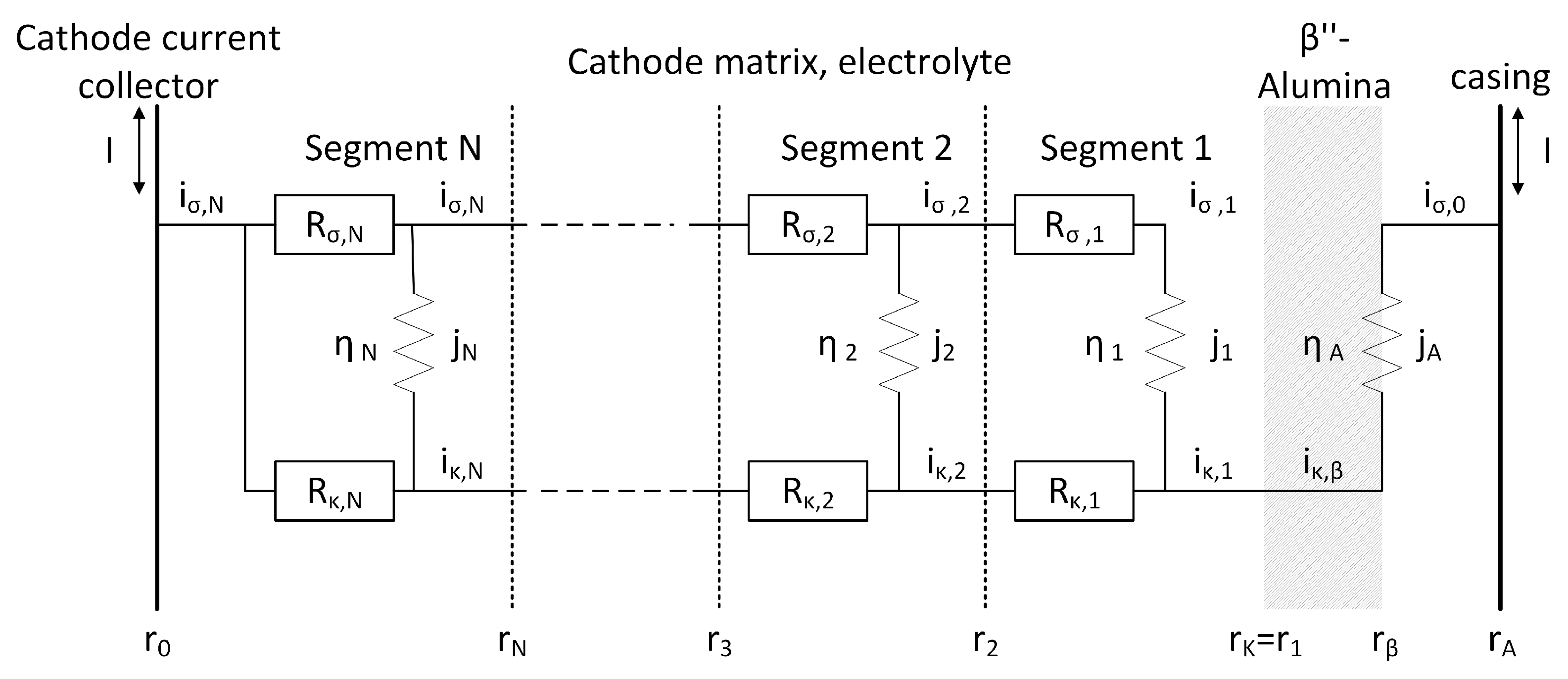
2. Model Development
- 1.
- Ohmic drop due to electronic and ionic conducting structures, e.g., separator and current collector.
- 2.
- Electrode losses/electrode overpotential: Combines electrode processes such as transport processes and charge transfer kinetics.
- represents the ionic resistance of the secondary electrolyte in the cathode segment n, leading to an Ohmic potential loss.
- represents the electrical resistance of the nickel and iron matrix in the cathode segment n, leading to an Ohmic potential loss.
- Cylindrical cell geometry with the cathode space divided into 100 segments;
- as an additional active compound besides ;
- Electron transfer in the metal matrix of the cathode;
- A constant current charging and discharging cycle;
- Heat generation.
- In this case, only is converted, because in this voltage range the iron reduction is thermodynamically not preferred.
- The electrochemical reaction follows Equation (1).
- In this case, both and are converted, because in this voltage range iron reduction is also thermodynamically preferred.
Modelling Heat Generation
3. Results
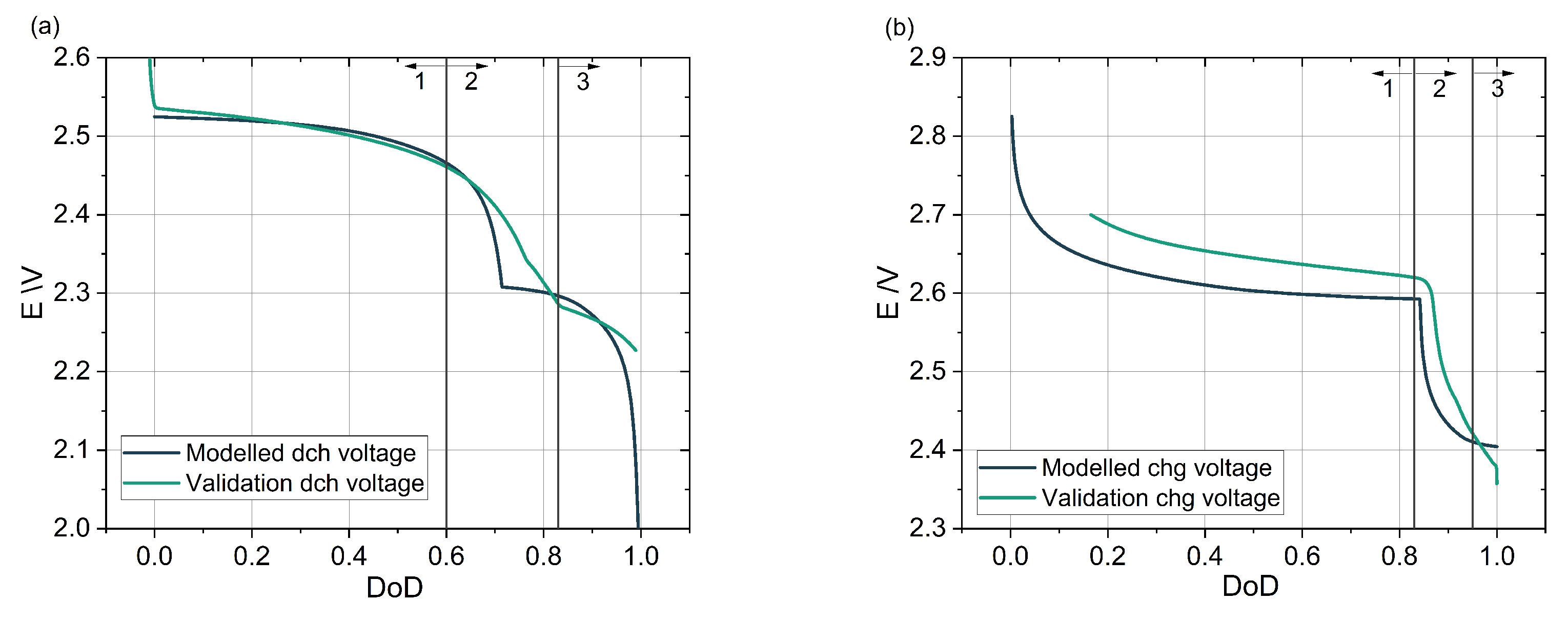
3.1. Cell Voltage for Discharge and Charge Cycle
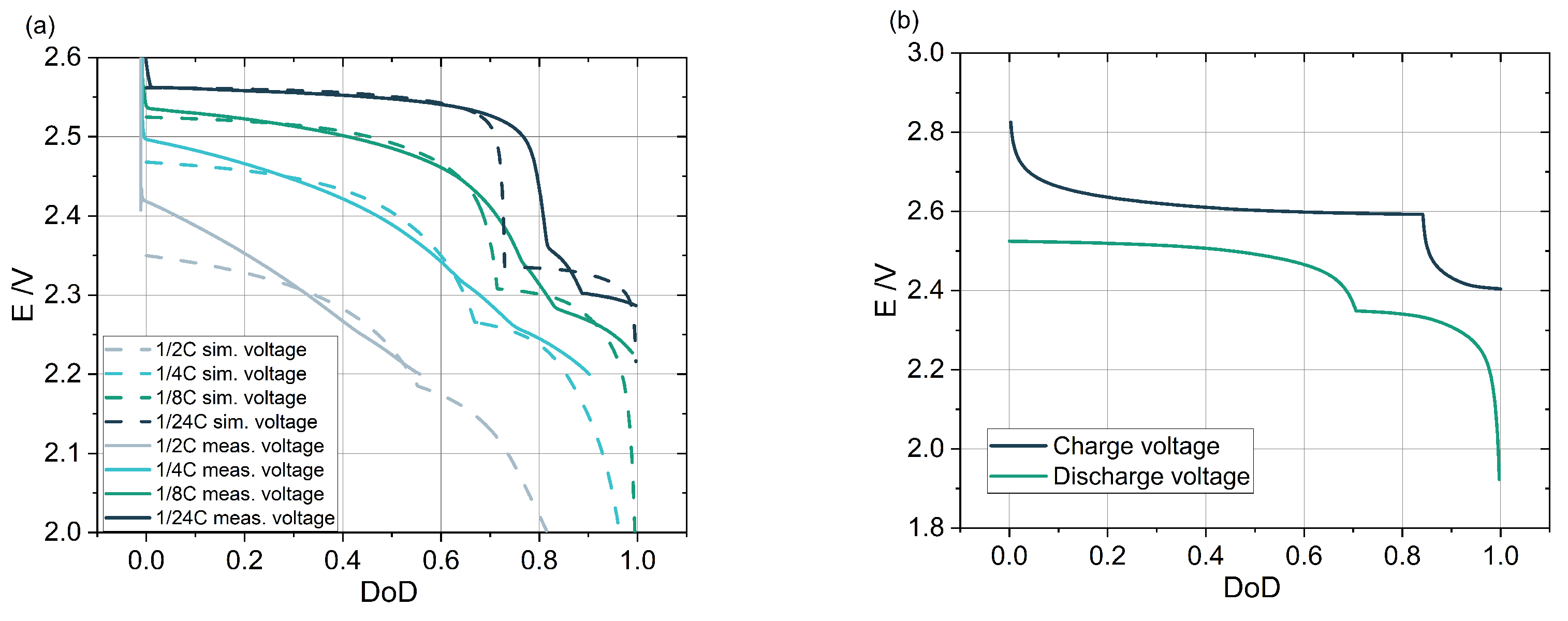
3.2. Impact of C-Rate on the Discharge Voltage
3.3. Voltages Losses during Cell Cycling
3.4. Hysteresis Effect
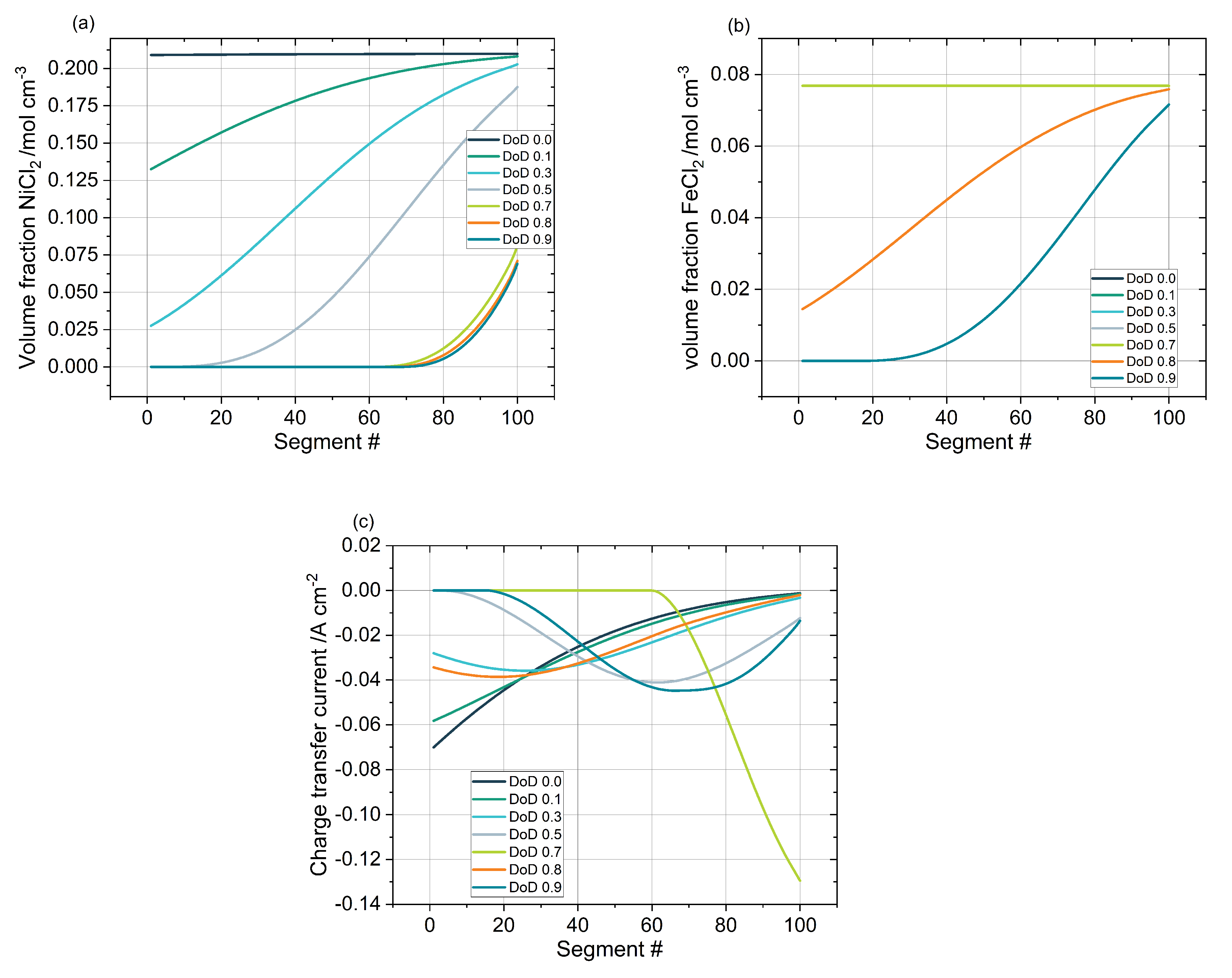
3.5. Material and Volume Distribution
3.6. Heat Generation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tamilselvi, S.; Gunasundari, S.; Karuppiah, N.; Razak RK, A.; Madhusudan, S.; Nagarajan, V.M.; Sathish, T.; Shamim, M.Z.M.; Saleel, C.A.; Afzal, A. A Review on Battery Modelling Techniques. Sustainability 2021, 13, 10042. [Google Scholar] [CrossRef]
- Rivera-Barrera, J.; Muñoz-Galeano, N.; Sarmiento-Maldonado, H. SoC Estimation for Lithium-ion Batteries: Review and Future Challenges. Electronics 2017, 6, 102. [Google Scholar] [CrossRef] [Green Version]
- Boi, M.; Battaglia, D.; Salimbeni, A.; Damiano, A. A Non-Linear Electrical Model for Iron Doped Sodium Metal Halides Batteries. In Proceedings of the 2018 IEEE Energy Conversion Congress and Exposition (ECCE), Piscataway, NJ, USA, 23–27 September 2018; pp. 2039–2046. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Liaw, B.Y.; Zhang, J. On state-of-charge determination for lithium-ion batteries. J. Power Sources 2017, 348, 281–301. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zou, C.; Zhang, C.; Li, Y. Technological Developments in Batteries: A Survey of Principal Roles, Types, and Management Needs. IEEE Power Energy Mag. 2017, 15, 20–31. [Google Scholar] [CrossRef]
- Sudworth, J.L.; Galloway, R.C. Secondary Batteries—High Temperature Systems|Sodium–Nickel Chloride. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 312–323. [Google Scholar] [CrossRef]
- Moseley, P.T.; Rand, D.A. High-Temperature Sodium Batteries for Energy Storage. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Moseley, P.T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 253–268. [Google Scholar] [CrossRef]
- Zhu, R. Characterization of Positive Electrodes in Sodium-Metal Chloride Batteries. Ph.D. Dissertation, Columbia University, New York, NY, USA, 2016. [Google Scholar]
- Musio, M.; Damiano, A. A Non-Linear Dynamic Electrical Model of Sodium-Nickel Chloride Batteries. In Proceedings of the 4th International Conference on Renewable Energy Research and Applications, Palermo, Italy, 22–25 November 2015. [Google Scholar]
- Hueso, K.B.; Armand, M.; Rojo, T. High temperature sodium batteries: Status, challenges and future trends. Energy Environ. Sci. 2013, 6, 734. [Google Scholar] [CrossRef]
- Sudoh, M.; Newman, J. Mathematical Modeling of the Sodium/Iron Chloride Battery. J. Electrochem. Soc. 1990, 137, 876–883. [Google Scholar] [CrossRef]
- Pollard, R.; Newman, J. Transport Equations for a Mixture of Two Binary Molten Salts in a Porous Electrode. J. Electrochem. Soc. 1979, 126, 1713. [Google Scholar] [CrossRef]
- Eroglu, D.; West, A.C. Modeling of reaction kinetics and transport in the positive porous electrode in a sodium–iron chloride battery. J. Power Sources 2012, 203, 211–221. [Google Scholar] [CrossRef]
- Christin, R.; Cugnet, M.; Zanon, N.; Crugnola, G.; Mailley, P. Multi-Physics Modeling of a Na-NiCl2 Commercial Cell. ECS Trans. 2015, 66, 3–15. [Google Scholar] [CrossRef]
- Orchard, S.W.; Weaving, J.S. Modelling of the sodium-ferrous chloride electrochemical cell. J. Appl. Electrochem. 1993, 23, 1214–1222. [Google Scholar] [CrossRef]
- Von Srbik, M.T.; Marinescu, M.; Martinez-Botas, R.F.; Offer, G.J. A physically meaningful equivalent circuit network model of a lithium-ion battery accounting for local electrochemical and thermal behaviour, variable double layer capacitance and degradation. J. Power Sources 2016, 325, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Vilathgamuwa, M.; Farrell, T.; Choi, S.S.; Tran, N.T.; Teague, J. A physics-based distributed-parameter equivalent circuit model for lithium-ion batteries. Electrochim. Acta 2019, 299, 451–469. [Google Scholar] [CrossRef]
- Merla, Y.; Wu, B.; Yufit, V.; Martinez-Botas, R.F.; Offer, G.J. An easy-to-parameterise physics-informed battery model and its application towards lithium-ion battery cell design, diagnosis, and degradation. J. Power Sources 2018, 384, 66–79. [Google Scholar] [CrossRef]
- Bloom, I.; Nelson, P.A.; Redey, L.; Orth, S.K.; Hammer, C.L.; Skocypec, R.S.; Dees, D.W.; Hash, M.C.; Vissers, D.R. Design Considerations For The Development Of Advanced Sodium/metal-chloride Cells. In Proceedings of the 25th Intersociety Energy Conversion Engineering Conference, Reno, NV, USA, 1990; pp. 341–347. [Google Scholar] [CrossRef]
- Vallance, M.; Hall, D.B. Finite Element Analysis of a Sodium-Metal Halide Electrochemical Cell. Meet. Abstr. 2010, MA2010-01, 267. Available online: https://iopscience.iop.org/article/10.1149/MA2010-01/3/267/pdf (accessed on 14 May 2022). [CrossRef]
- Rexed, I.; Behm, M.; Lindbergh, G. Modelling of ZEBRA Batteries Royal Institut of Technology KTH Stockholm, School of Chemical Science and Engineering, Applied Electrochemistry. In Proceedings of the 61th Annual Meeting of the International Society of Electrochgemistry, Nice, France, 26 September–1 October 2010. [Google Scholar]
- Zhu, R.; Vallance, M.; Rahimian, S.K.; West, A.C. Galvanostatic Intermittent Titration Study of the Positive Electrode of a Na|Ni(Fe)-Chloride Cell. J. Electrochem. Soc. 2015, 162, A2051–A2057. [Google Scholar] [CrossRef] [Green Version]
- Bracco, S.; Delfino, F.; Trucco, A.; Zin, S. Electrical storage systems based on Sodium/Nickel chloride batteries: A mathematical model for the cell electrical parameter evaluation validated on a real smart microgrid application. J. Power Sources 2018, 399, 372–382. [Google Scholar] [CrossRef]
- Trasatti, S. Electrokinetics. In Encyclopedia of Electrochemical Power Sources; Newnes: Waltham, MA, USA, 2009; pp. 22–31. [Google Scholar]
- Panero, S. Kinetics. In Encyclopedia of Electrochemical Power Sources; Newnes: Waltham, MA, USA, 2009; pp. 14–22. [Google Scholar]
- Richter, M.; Dittrich, R.; Zindel, A.; Nousch, L.; Lehmann, M.; Franke, M.; Eißmann, N.; Hutsch, T.; Cerdas, F.; Zellmer, S.; et al. Development and Environmental Assessment of a Phase Change Material Based Thermal Management System for Na/NiCl2 Batteries. Batteries 2022, 8, 197. [Google Scholar] [CrossRef]
- Gu, W.B.; Wang, C.Y. Thermal-Electrochemical Modeling of Battery Systems. J. Electrochem. Soc. 2000, 147, 2910. [Google Scholar] [CrossRef]
- Christin, R. Modélisation Multiphysique de Cellules Sodium Chlorure de Nickel. Ph.D. Dissertation, L’Université Grenoble Alpes, Grenoble, France, 2015. [Google Scholar]
- Büttner, N.; Skadell, K.; Schüßler, B.; Nousch, L.; Richter, M.; Schulz, M.; Michaelis, A. Internal Resistance Analysis of Na/NiCl 2 Cells using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2022, 169, 090506. [Google Scholar] [CrossRef]
- Bernardi, D. A General Energy Balance for Battery Systems. J. Electrochem. Soc. 1985, 132, 5. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, V.; Wang, C.Y. Analysis of Electrochemical and Thermal Behavior of Li-Ion Cells. J. Electrochem. Soc. 2003, 150, A98–A106. [Google Scholar] [CrossRef] [Green Version]
- Rijssenbeek, J.; Gao, Y.; Zhong, Z.; Croft, M.; Jisrawi, N.; Ignatov, A.; Tsakalakos, T. In situ X-ray diffraction of prototype sodium metal halide cells: Time and space electrochemical profiling. J. Power Sources 2011, 196, 2332–2339. [Google Scholar] [CrossRef]
- Zinth, V.; Seidlmayer, S.; Zanon, N.; Crugnola, G.; Schulz, M.; Gilles, R.; Hofmann, M. In Situ Spatially Resolved Neutron Diffraction of a Sodium Metal Halide Battery. J. Electrochem. Soc. 2015, 162, A384–A391. [Google Scholar] [CrossRef]
- Neelakanta, P.S. Handbook of Electromagnetic Materials: Monolithic and Composite Versions and Their Applications; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Newman, J.; Tiedemann, W. Porous-electrode theory with battery applications. AIChE Journal 1975, 21, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Lu, X.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L. Cell degradation of a Na–NiCl2 (ZEBRA) battery. J. Mater. Chem. A 2013, 1, 14935. [Google Scholar] [CrossRef]




| Features | 1990 Sudoh et al. [11] | 1990 Boom et al. [19] | 1993 Orchard et al. [15] | 2008 Vallance et al. [20] | 2010 Rexed et al. [21] | 2012 Eroglu et al. [13] | 2015 Christin [14] | 2016 Zhu et al. [22] | 2018 Bracco et al. [23] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Operation mode | Discharge | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Charge | Yes | No | No | No | Yes | Yes | No | Yes | No | |
| Cell chemistry | Fe | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Ni | No | Yes | No | No | Yes | No | Yes | Yes | No | |
| Cell geometry | Planar | No | No | Yes | No | No | No | No | No | No |
| Radial | Yes | Yes | No | No | No | Yes | No | Yes | Yes | |
| Cloverleaf | No | No | No | Yes | No | No | Yes | No | No | |
| Processes | Porosity | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Na Transport | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| MCl lattice | Yes | No | No | Yes | Yes | Yes | Yes | No | No | |
| Heat formation | No | No | No | No | No | No | Yes | No | No | |
| Validation | No | Yes | Yes | Yes | No | No | Yes | No | Yes | |
| Symbol | Unit | Description |
|---|---|---|
| J | Gibbs free energy change | |
| F | A s mol | Faraday constant |
| R | J K mol | Universal gas constant |
| T | K | Temperature |
| z | Number of electrons transferred | |
| V | Open circuit v t V oltage | |
| E | V | Cell voltage |
| V | Overpotential | |
| V | Cathode overpotential | |
| V | Anode overpotential | |
| V | Overpotential in segment n | |
| I | A | Current |
| A | Ionic current in segment n | |
| A | Electronic current in segment n | |
| A cm | Anodic standard exchange current density | |
| A cm | Anodic exchange current density | |
| A cm | Charge exchange current density of active material M | |
| A cm | Sum of exchange current densities | |
| A cm | Standard exchange current density | |
| A cm | Standard exchange current density of active material M | |
| Local depth of discharge | ||
| Separator resistance | ||
| Ionic resistance | ||
| Electronic resistance | ||
| cm | Standard conductivity | |
| cm | Ionic conductivity in segment n at timestep j | |
| cm | Standard conductivity | |
| cm | Electronic conductivity in segment n | |
| Cathode porosity | ||
| Metal matrix porosity | ||
| Totuosity | ||
| Charge transfer coefficient | ||
| cm | Length of each segment | |
| cm | Exchange area | |
| q | W cm | Volumetric heat generation rate |
| V | Potential in solid phase | |
| V | Potential in the electrolyte | |
| mol cm | Molar volume of active material M in segment n at timestep j | |
| Subscripts | ||
| j | Time step index | |
| n | Segment number | |
| N | Total number of segments | |
| M | Metal indexing (,) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büttner, N.; Purr, F.; Sangrós Giménez, C.; Richter, M.; Nousch, L.; Zellmer, S.; Michaelis, A. Electrochemical Modelling of Na-MCl2 Battery Cells Based on an Expanded Approximation Method. Batteries 2023, 9, 200. https://doi.org/10.3390/batteries9040200
Büttner N, Purr F, Sangrós Giménez C, Richter M, Nousch L, Zellmer S, Michaelis A. Electrochemical Modelling of Na-MCl2 Battery Cells Based on an Expanded Approximation Method. Batteries. 2023; 9(4):200. https://doi.org/10.3390/batteries9040200
Chicago/Turabian StyleBüttner, Nils, Foelke Purr, Clara Sangrós Giménez, Maria Richter, Laura Nousch, Sabrina Zellmer, and Alexander Michaelis. 2023. "Electrochemical Modelling of Na-MCl2 Battery Cells Based on an Expanded Approximation Method" Batteries 9, no. 4: 200. https://doi.org/10.3390/batteries9040200
APA StyleBüttner, N., Purr, F., Sangrós Giménez, C., Richter, M., Nousch, L., Zellmer, S., & Michaelis, A. (2023). Electrochemical Modelling of Na-MCl2 Battery Cells Based on an Expanded Approximation Method. Batteries, 9(4), 200. https://doi.org/10.3390/batteries9040200






