C4S Nanosheet: A Potential Anode Material for Potassium-Ion Batteries
Abstract
:1. Introduction
2. Computational Method
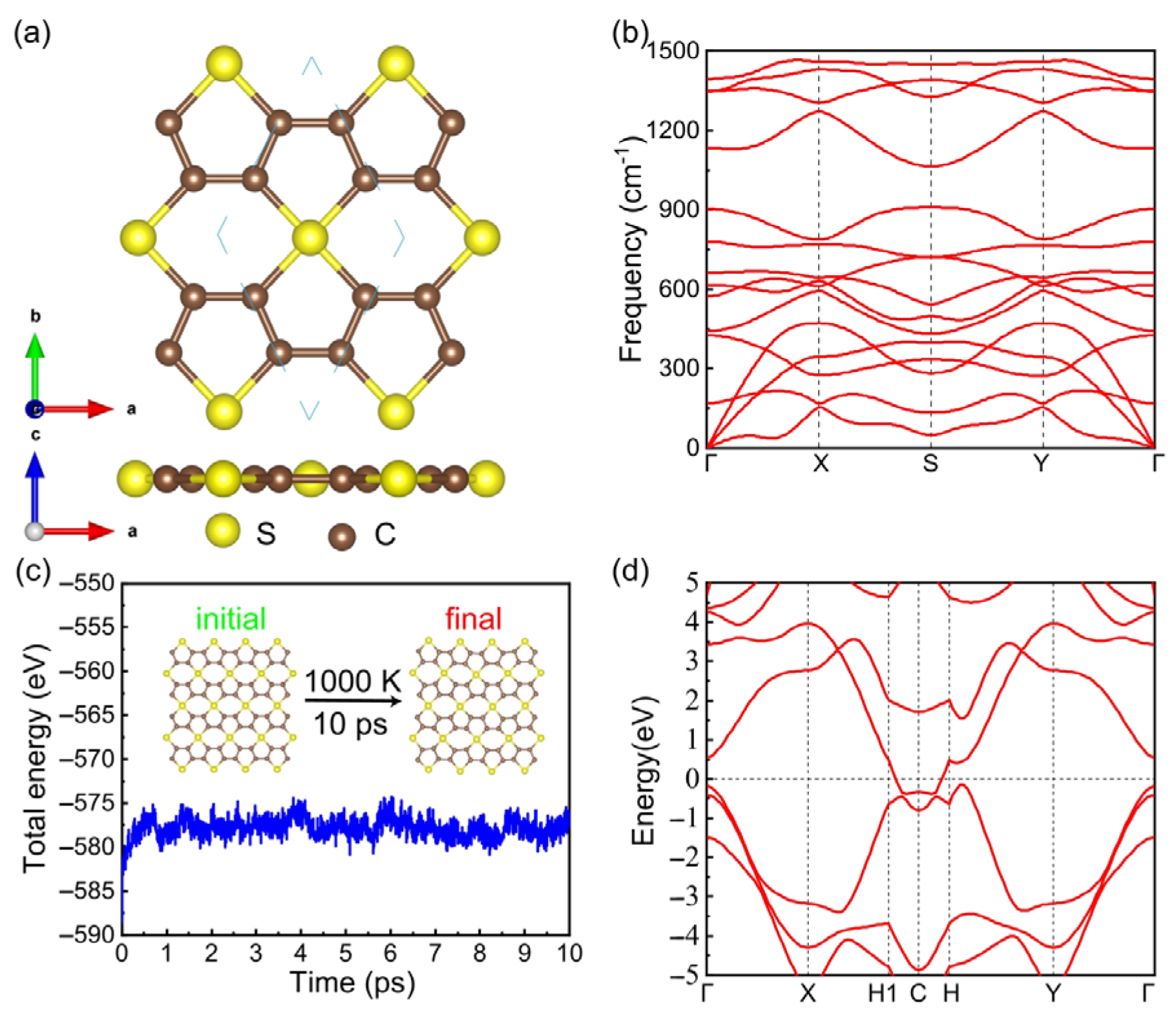
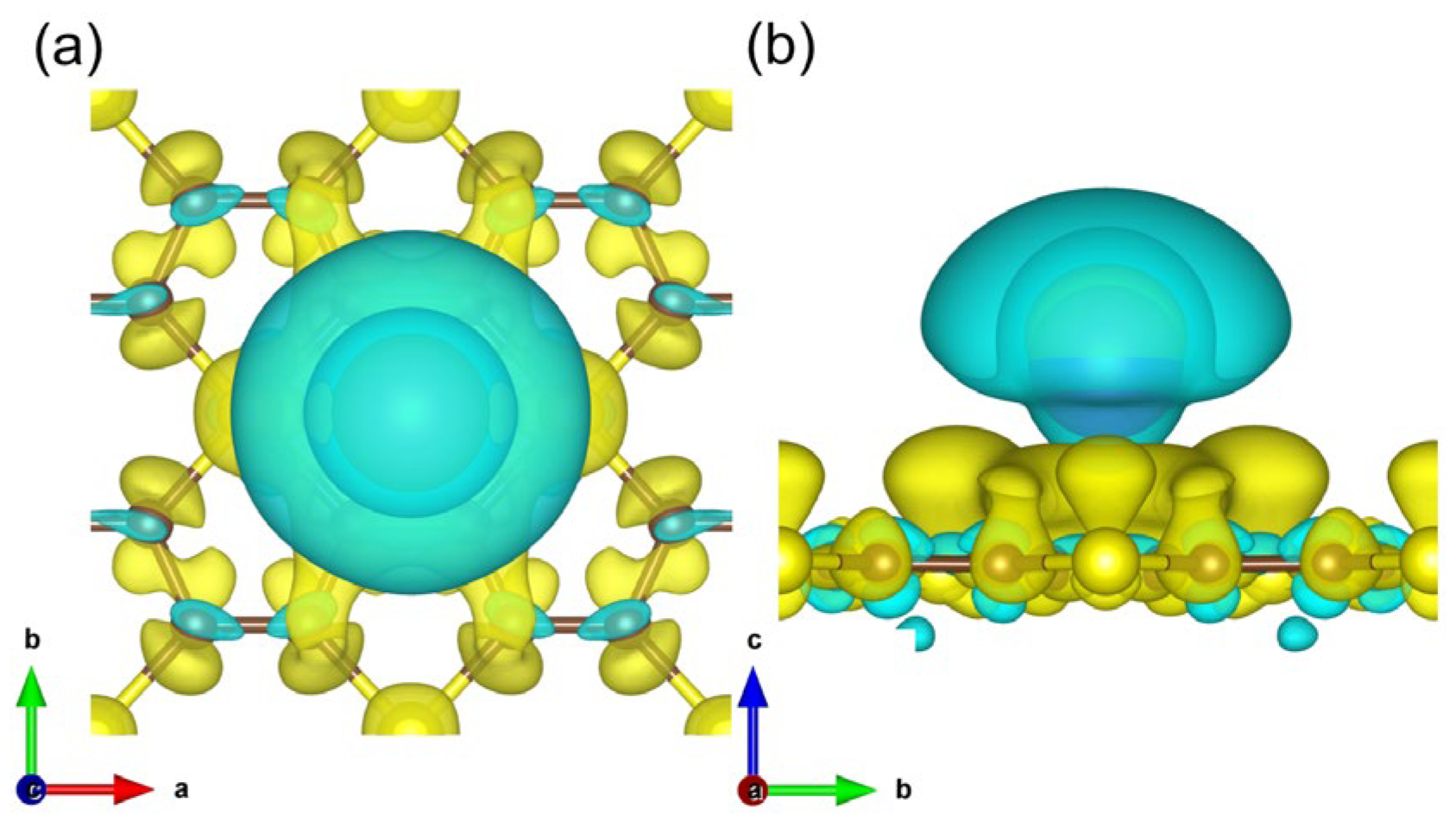
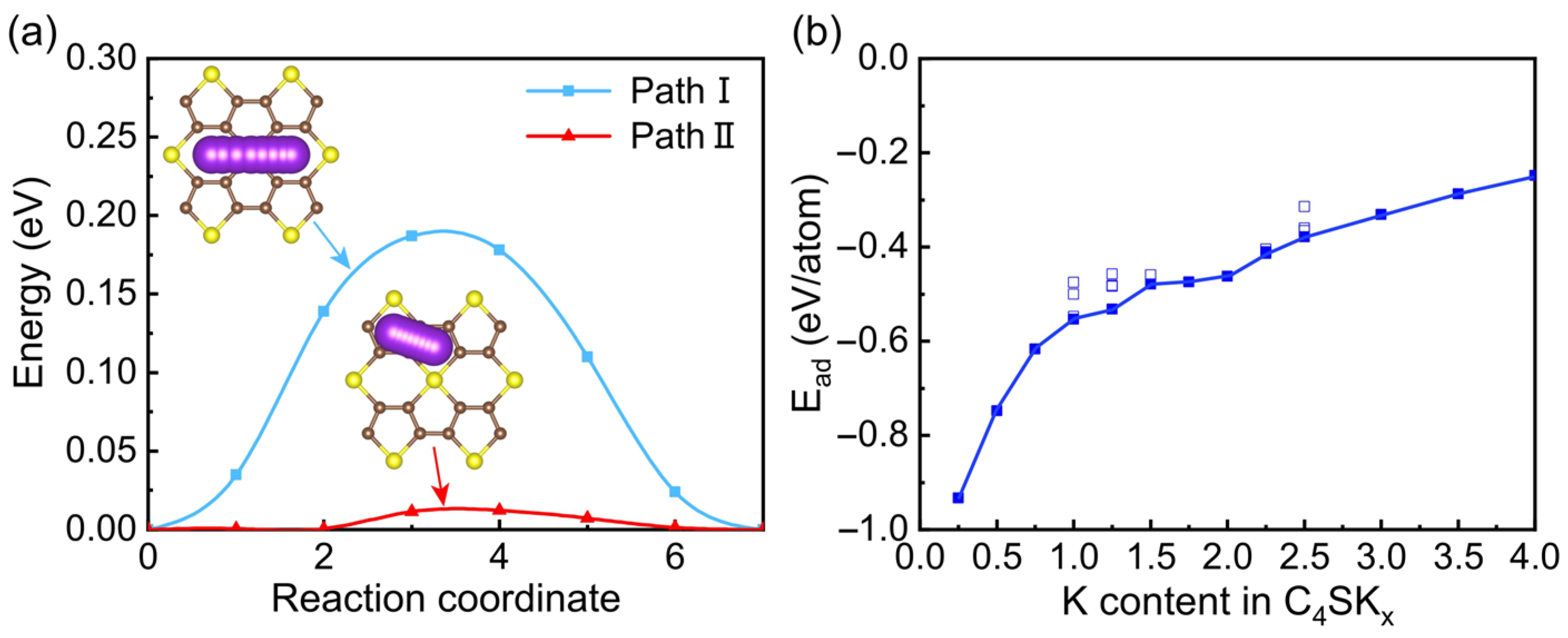
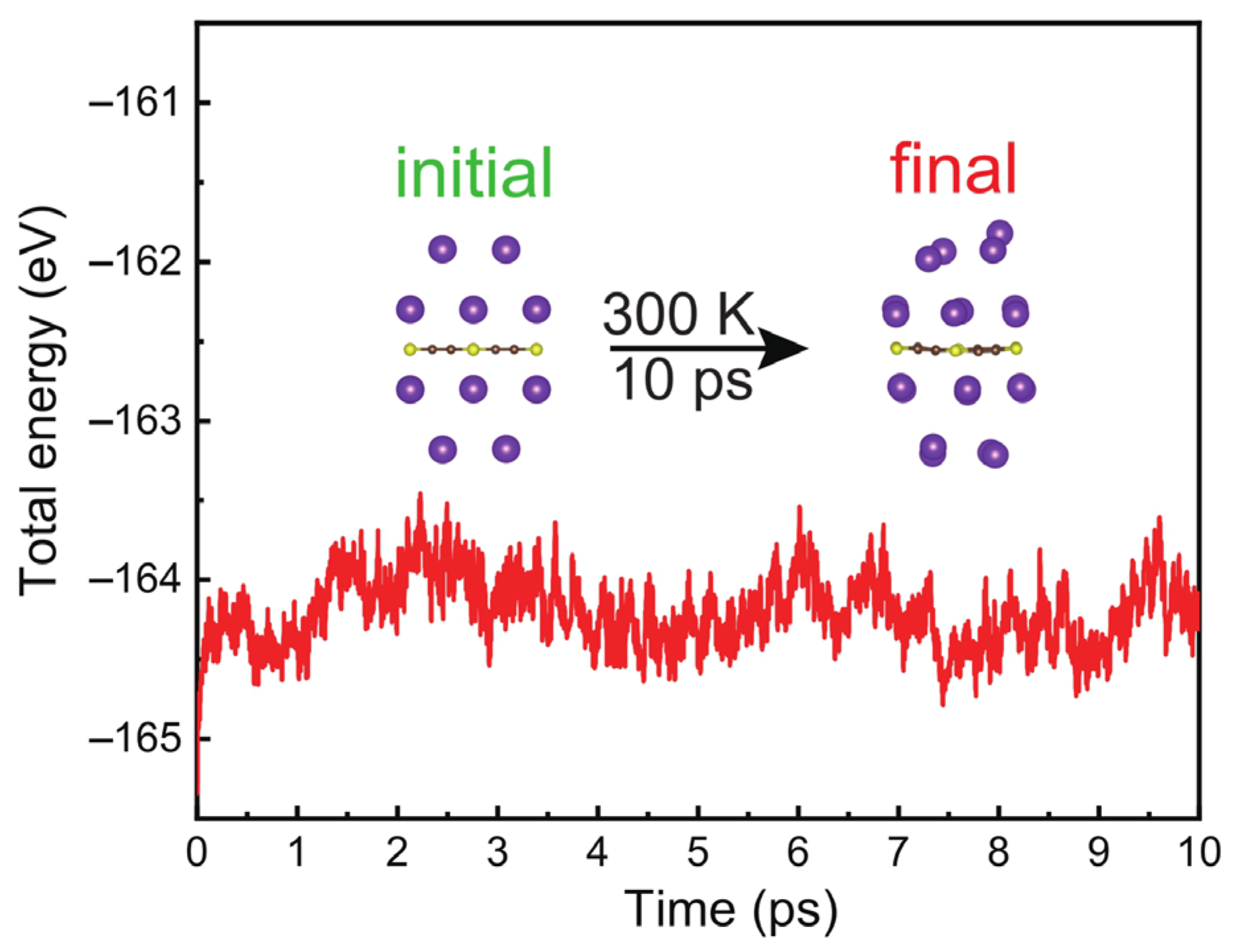
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, T.; Li, G.; Han, Y.; Gao, Y.; Wang, F.; Hu, Z.; Cai, T.; Chu, J.; Song, Z. Oxidized indanthrone as a cost-effective and high-performance organic cathode material for rechargeable lithium batteries. Energy Storage Mater. 2022, 50, 265–273. [Google Scholar] [CrossRef]
- Wu, X.; Markir, A.; Xu, Y.; Zhang, C.; Leonard, D.P.; Shin, W.; Ji, X. A rechargeable battery with an iron metal anode. Adv. Funct. Mater. 2019, 29, 1900911. [Google Scholar] [CrossRef]
- Wang, S.B.; Ran, Q.; Yao, R.Q.; Shi, H.; Wen, Z.; Zhao, M.; Lang, X.Y.; Jiang, Q. Lamella-nanostructured eutectic zinc–aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat. Commun. 2020, 11, 1634. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.C.; Bianchini, M.; Seo, D.H.; Rodriguez-Garcia, J.; Ceder, G. Recent progress and perspective in electrode materials for K-ion batteries. Adv. Energy Mater. 2018, 8, 1702384. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Sergey, C.; Lee, H.; Yoon, J.; Kang, Y.M.; Yoon, W.S. Advances in the cathode materials for lithium rechargeable batteries. Angew. Chem. Int. Ed. 2020, 59, 2578–2605. [Google Scholar] [CrossRef]
- Borah, R.; Hughson, F.; Johnston, J.; Nann, T. On battery materials and methods. Mater. Today Adv. 2020, 6, 100046. [Google Scholar] [CrossRef]
- Jie, Y.; Tan, Y.; Li, L.; Han, Y.; Xu, S.; Zhao, Z.; Cao, R.; Ren, X.; Huang, F.; Lei, Z.; et al. Electrolyte solvation manipulation enables unprecedented room-temperature calcium-metal batteries. Angew. Chem. Int. Ed. 2020, 59, 12689–12693. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Feng, X.; Ren, D.; Li, Y.; Hou, J.; Wu, Y.; Du, J.; Lu, L.; Ouyang, M. Battery eruption triggered by plated lithium on an anode during thermal runaway after fast charging. Energy 2022, 239, 122097. [Google Scholar] [CrossRef]
- Vaalma, C.; Giffin, G.A.; Buchholz, D.; Passerini, S. Non-aqueous K-ion battery based on layered K0.3MnO2 and hard carbon/carbon black. J. Electrochem. Soc. 2016, 163, A1295. [Google Scholar] [CrossRef]
- Hounjet, L.J. Comparing lithium- and sodium-ion batteries for their applicability within energy storage systems. Energy Storage 2022, 4, e309. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, C.; Zhao, H.; Lei, Y. Recent Advances in 2D Heterostructures as Advanced Electrode Materials for Potassium-Ion Batteries. Small Struct. 2022, 3, 2100221. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Tang, Y.; Ji, X.; Jia, C.; Wang, H. Advancements and challenges in potassium ion batteries: A comprehensive review. Adv. Funct. Mater. 2020, 30, 1909486. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H. Designing carbon anodes for advanced potassium-ion batteries: Materials, modifications, and mechanisms. Adv. Powder Mater. 2022, 1, 100057. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Wu, Z.; Manaig, D.; Freschi, D.J.; Wang, Z.; Liu, J. Enhanced potassium storage performance for K-Te batteries via electrode design and electrolyte salt chemistry. ACS Appl. Mater. Interfaces 2021, 13, 16345–16354. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef]
- Yin, H.; Han, C.; Liu, Q.; Wu, F.; Zhang, F.; Tang, Y. Recent advances and perspectives on the polymer electrolytes for sodium/potassium-ion batteries. Small 2021, 17, 2006627. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, H.; Zhou, M.; Zhang, C.; Wu, Y.; Li, W.; Dong, Y.; Lei, Y. Ammonium vanadium bronze as a potassium-ion battery cathode with high rate capability and cyclability. Small Methods 2019, 3, 1800349. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Z.; Dinh, K.N.; Chen, N.; Chen, G.; Du, F.; Yan, Q. Layered oxide cathode for potassium-ion battery: Recent progress and prospective. Small 2020, 16, 2002700. [Google Scholar] [CrossRef]
- Dhir, S.; Wheeler, S.; Capone, I.; Pasta, M. Outlook on K-ion batteries. Chem 2020, 6, 2442–2460. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, C.; Wang, F.; Hou, S.; Liou, S.C.; Qing, T.; Li, Q.; Zheng, J.; Cui, C.; Wang, C. An organic anode for high temperature 303 potassium-ion batteries. Adv. Energy Mater. 2019, 9, 1802986. [Google Scholar] [CrossRef]
- Xu, J.; Dou, S.; Cui, X.; Liu, W.; Zhang, Z.; Deng, Y.; Hu, W.; Chen, Y. Potassium-based electrochemical energy storage devices: Development status and future prospect. Energy Storage Mater. 2021, 34, 85–106. [Google Scholar] [CrossRef]
- Fan, S.S.; Liu, H.P.; Liu, Q.; Ma, C.S.; Yi, T.F. Comprehensive insights and perspectives into the recent progress of electrode materials for non-aqueous K-ion battery. J. Mater. 2020, 6, 431–454. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Lu, S.; Xiang, Y. Carbon anode materials: A detailed comparison between Na-ion and K-ion batteries. Adv. Energy Mater. 2021, 11, 2003640. [Google Scholar] [CrossRef]
- Liu, G.; Cao, Z.; Zhou, L.; Zhang, J.; Sun, Q.; Hwang, J.Y.; Cavallo, L.; Wang, L.; Sun, Y.K.; Ming, J. Additives Engineered Nonflammable Electrolyte for Safer Potassium Ion Batteries. Adv. Funct. Mater. 2020, 30, 2001934. [Google Scholar] [CrossRef]
- Naylor, A.J.; Carboni, M.; Valvo, M.; Younesi, R. Interfacial reaction mechanisms on graphite anodes for K-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 45636–45645. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Z.; Yan, K.; Guo, X.; Wan, S.; He, F.; Sun, B.; Wang, G. The rise of prussian blue analogs: Challenges and opportunities for high-performance cathode materials in potassium-ion batteries. Small Struct. 2021, 2, 2000054. [Google Scholar] [CrossRef]
- He, X.; Wang, R.; Yin, H.; Zhang, Y.; Chen, W.; Huang, S. 1T-MoS2 monolayer as a promising anode material for (Li/Na/Mg)-ion batteries. Appl. Surf. Sci. 2022, 584, 152537. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, Y.; Li, W.; Fu, Q.; Wu, M.; Manske, E.; Kröger, J.; Lei, Y. Insights into the crystallinity of layer-structured transition metal dichalcogenides on potassium ion battery performance: A case study of molybdenum disulfide. Small 2019, 15, 1900497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, H.; Sun, L.; Xu, F.; Ouyang, Y.; Rosei, F. Progress and perspectives of 2D materials as anodes for potassium-ion batteries. Energy Storage Mater. 2021, 38, 354–378. [Google Scholar] [CrossRef]
- Sibari, A.; Kerrami, Z.; Kara, A.; Hamedoun, M.; Benyoussef, A.; Mounkachi, O.; Benaissa, M. Adsorption and diffusion on a phosphorene monolayer: A DFT study. J. Solid State Electrochem. 2018, 22, 11–16. [Google Scholar] [CrossRef]
- Xu, Y.; Bahmani, F.; Zhou, M.; Li, Y.; Zhang, C.; Liang, F.; Kazemi, S.H.; Kaiser, U.; Meng, G.; Lei, Y. Enhancing potassium-ion battery performance by defect and interlayer engineering. Nanoscale Horiz. 2019, 4, 202–207. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The chemistry and promising applications of graphene and porous graphene materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Wang, B.; Ruan, T.; Chen, Y.; Jin, F.; Peng, L.; Zhou, Y.; Wang, D.; Dou, S. Graphene-based composites for electrochemical energy storage. Energy Storage Mater. 2020, 24, 22–51. [Google Scholar] [CrossRef]
- Chen, K.T.; Chong, S.; Yuan, L.; Yang, Y.C.; Tuan, H.Y. Conversion-alloying dual mechanism anode: Nitrogen-doped carbon-coated Bi2Se3 wrapped with graphene for superior potassium-ion storage. Energy Storage Mater. 2021, 39, 239–249. [Google Scholar] [CrossRef]
- Musielak, M.; Gagor, A.; Zawisza, B.; Talik, E.; Sitko, R. Graphene oxide/carbon nanotube membranes for highly efficient removal of metal ions from water. ACS Appl. Mater. Interfaces 2019, 11, 28582–28590. [Google Scholar] [CrossRef]
- Mansouri, Z.; Sibari, A.; Al-Shami, A.; Lahbabi, S.; El Kenz, A.; Benyoussef, A.; El Fatimy, A.; Mounkachi, O. Graphene/Phosphorene nano-heterostructure as a potential anode material for (K/Na)-ion batteries: Insights from DFT and AIMD. Comput. Mater. Sci. 2022, 202, 110936. [Google Scholar] [CrossRef]
- Dou, K.; Ma, Y.; Zhang, T.; Huang, B.; Dai, Y. Prediction of two-dimensional PC6 as a promising anode material for potassium-ion batteries. Phys. Chem. Chem. Phys. 2019, 21, 26212–26218. [Google Scholar] [CrossRef] [PubMed]
- Bhauriyal, P.; Mahata, A.; Pathak, B. Graphene-like carbon–nitride monolayer: A potential anode material for Na- and K-ion batteries. J. Phys. Chem. C 2018, 122, 2481–2489. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Zhu, L.; Lu, S.; Yin, K.; Li, Q.; Wang, H.; Zhang, L.; Ma, Y. Materials discovery via CALYPSO methodology. J. Phys. Condens. Mater. 2015, 27, 203203. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y.; Liu, H.; Miao, M.S.; Ma, Y. Self-assembled ultrathin nanotubes on diamond (100) surface. Nat. Commun. 2014, 5, 3666. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Parr, R.G. Density functional theory of atoms and molecules. In Proceedings of the Horizons of Quantum Chemistry: Proceedings of the Third International Congress of Quantum Chemistry, Kyoto, Japan, 29 October–3 November 1979; Springer: Berlin/Heidelberg, Germany, 1980; pp. 5–15. [Google Scholar]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Bucko, T.; Hafner, J.; Lebegue, S.; Angyan, J.G. Improved description of the structure of molecular and layered crystals: Ab initio DFT calculations with van der Waals corrections. J. Phys. Chem. A 2010, 114, 11814–11824. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Togo, A.; Oba, F.; Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 2008, 78, 134106. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Born, M.; Huang, K.; Lax, M. Dynamical theory of crystal lattices. Am. J. Phys. 1955, 23, 474. [Google Scholar] [CrossRef]
- Gao, J.; Li, B.; Tan, J.; Chow, P.; Lu, T.M.; Koratkar, N. Aging of transition metal dichalcogenide monolayers. ACS Nano 2016, 10, 2628–2635. [Google Scholar] [CrossRef]
- Lei, S.; Chen, X.; Xiao, B.; Zhang, W.; Liu, J. Excellent electrolyte wettability and high energy density of B2S as a two-dimensional Dirac anode for non-lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 28830–28840. [Google Scholar] [CrossRef]
- Poole, R. Cohesive energy of the alkali metals. Am. J. Phys. 1980, 48, 536–538. [Google Scholar] [CrossRef]
- Khan, S.; Mushtaq, M.; Berdiyorov, G.R.; Tit, N. Relevance of metal (Ca versus Mn) embedded C2N for energy-storage applications: Atomic-scale study. Int. J. Hydrogen Energy 2021, 46, 2445–2463. [Google Scholar] [CrossRef]
- Nakada, K.; Ishii, A. Migration of adatom adsorption on graphene using DFT calculation. Solid State Commun. 2011, 151, 13–16. [Google Scholar] [CrossRef]
- Sannyal, A.; Ahn, Y.; Jang, J. First-principles study on the two-dimensional siligene (2D SiGe) as an anode material of an alkali metal ion battery. Comput. Mater. Sci. 2019, 165, 121–128. [Google Scholar] [CrossRef]
- Liang, B.; Ma, N.; Wang, Y.; Wang, T.; Fan, J. N-functionalized Ti2B MBene as high-performance anode materials for sodium-ion batteries: A DFT study. Appl. Surf. Sci. 2022, 599, 153927. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Liu, Z.; Pan, M.; Huang, Z.; Pan, L.; Han, L.; Zhang, K.; Zhao, Y.; Deng, H. Theoretically evaluating two-dimensional tetragonal Si2Se2 and SiSe2 nanosheets as anode materials for alkali metal-ion batteries. Phys. Chem. Chem. Phys. 2022, 24, 26241–26253. [Google Scholar] [CrossRef]
- Shukla, V.; Araujo, R.B.; Jena, N.K.; Ahuja, R. The curious case of two dimensional Si2BN: A high-capacity battery anode material. Nano Energy 2017, 41, 251–260. [Google Scholar] [CrossRef]
- Share, K.; Cohn, A.P.; Carter, R.; Rogers, B.; Pint, C.L. Role of nitrogen-doped graphene for improved high-capacity potassium ion battery anodes. ACS Nano 2016, 10, 9738–9744. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, J.; Lin, H. Metallic two-dimensional P2C3: A promising flexible anode for high-performance potassium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126536. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y. Ab initio prediction of two-dimensional Si3C enabling high specific capacity as an anode material for Li/Na/K-ion batteries. J. Mater. Chem. A 2020, 8, 4274–4282. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Y.; Ma, Y.; Jing, T.; Wei, W.; Huang, B. Ab initio prediction and characterization of Mo2C monolayer as anodes for lithium-ion and sodium-ion batteries. J. Phys. Chem. Lett. 2016, 7, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Mizuno, F. Boron-doped graphene as a promising anode for Na-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 10419–10424. [Google Scholar] [CrossRef] [PubMed]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Lu, E.; Zhu, K.; Hu, X. C4S Nanosheet: A Potential Anode Material for Potassium-Ion Batteries. Batteries 2023, 9, 288. https://doi.org/10.3390/batteries9060288
Lu S, Lu E, Zhu K, Hu X. C4S Nanosheet: A Potential Anode Material for Potassium-Ion Batteries. Batteries. 2023; 9(6):288. https://doi.org/10.3390/batteries9060288
Chicago/Turabian StyleLu, Shaohua, Enhao Lu, Kai Zhu, and Xiaojun Hu. 2023. "C4S Nanosheet: A Potential Anode Material for Potassium-Ion Batteries" Batteries 9, no. 6: 288. https://doi.org/10.3390/batteries9060288
APA StyleLu, S., Lu, E., Zhu, K., & Hu, X. (2023). C4S Nanosheet: A Potential Anode Material for Potassium-Ion Batteries. Batteries, 9(6), 288. https://doi.org/10.3390/batteries9060288





