A historical overview of lithium batteries is provided by [
1]. In the article he discusses the evolution of lithium batteries and their applications over time. The issues and challenges facing rechargeable lithium batteries are discussed by [
2]. They highlight the importance of developing new materials and technologies to improve the performance and safety of these batteries. The challenges of developing better batteries and the importance of finding sustainable ways to provide power for modern lifestyles are described by [
3]. They also highlight the need for new materials and technologies to improve battery performance and safety. Lithium batteries are common nowadays. The main steps that led to the success and industrial production in a relatively short time have been described by [
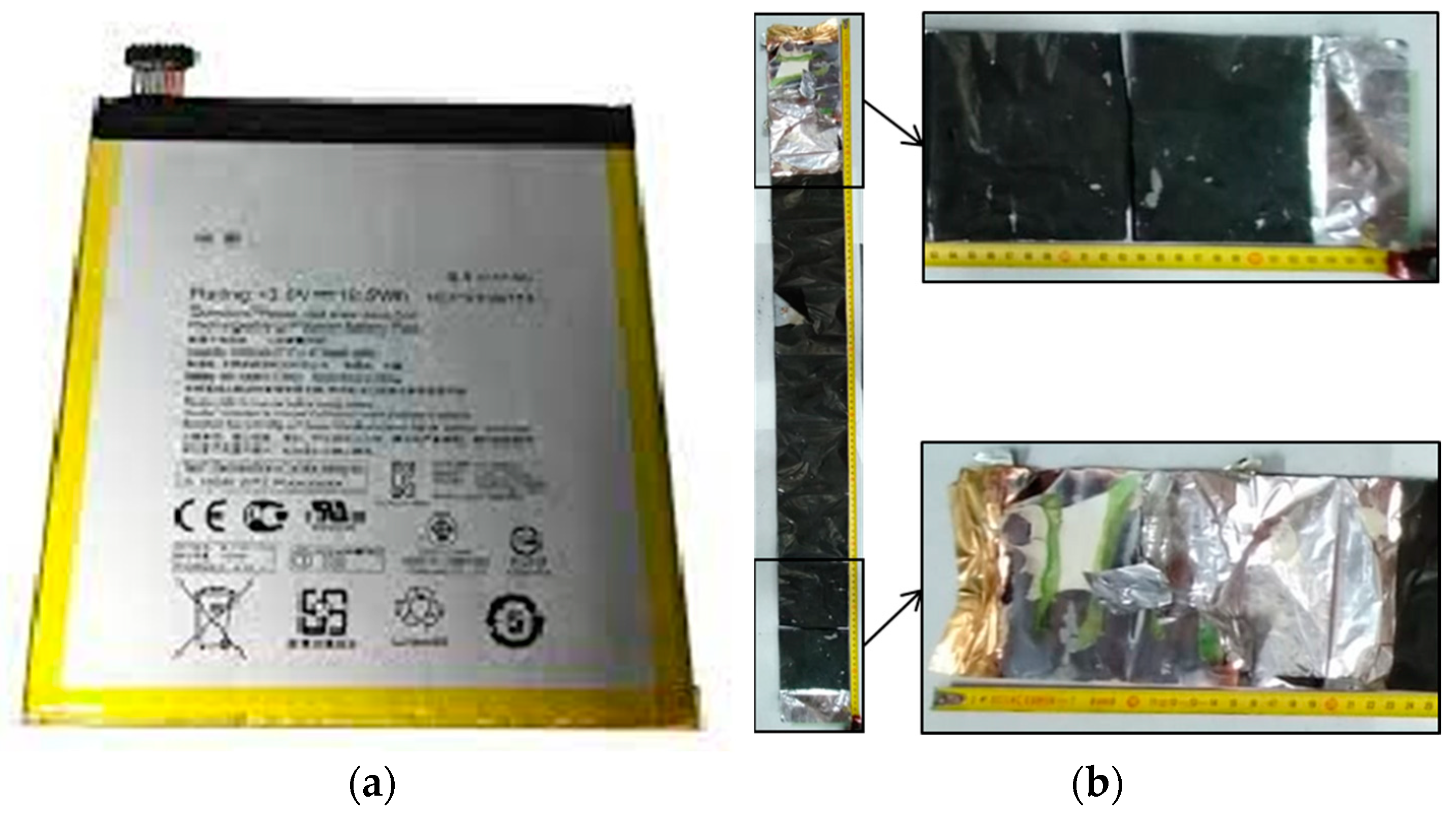
4]. Lithium batteries are marketed in a wide range of shapes and sizes. They mainly come in configurations: cylindrical, button, pouch and prismatic. The battery used in this study is a LiPo and falls into the single prismatic cell type. The long-term durability and safety features of Li-ion batteries are major limitations for their performance [
5]. Li-ion batteries typically operate within the temperature range of 0–40 °C. Excessive or uneven heat generation in the cells can cause cell degradation and cell breakdown. Cell degradation results in a significant reduction in open circuit voltage and electric capacity, while cell failure can result in thermal runaway or even a fire hazard [
6].
To achieve this goal, simulation can be used, which allows you to predict system performance as an alternative to experimental tests, which in addition to a high cost, may have some limitations: in some cases, it may be difficult to calculate physical quantities, such as temperature in the heart of the component.
There are different types of simulation models for batteries, the main categories are analytical models, equivalent circuit models (ECM) and electrochemical models [
8]. Analytical models are the simplest and fastest, but also the least accurate. They use mathematical equations to describe the battery behavior [
9]. ECM are based on electrical components that mimic the battery dynamics, such as resistors, capacitors and voltage sources. They can capture the nonlinear effects of temperature, state of charge (SOC) and state of health on the battery performance. They are intended for dynamic and long-run simulation studies [
10]. Electrochemical models describe the physical and chemical processes that occur inside the battery, such as diffusion, reaction and transport. They are the most accurate and detailed, but also the most complex and computationally intensive. They are suitable for battery design and analysis at the molecular level [
8]. Several studies have examined the thermal behavior of Li-ion batteries using various methods and models. For example, a 3-D CFD model was developed by [
11] to analyze the natural convection effects on the batteries. A Li-ion battery module was investigated both experimentally and computationally by [
12] for its thermal management. A mathematical model and infrared images were used by [
13] to estimate the transient temperature distributions of a large-sized 20Ah-LiFePO4 prismatic battery at various discharge rates. A similar approach was used by [
14] to study the transient temperature and voltage distributions of 18650 cylindrical Li-ion battery at different C-rates with an air-cooling method. Later, they developed a numerical model using commercial code. Two cooling methods for Li-ion battery cells used in electric vehicles are compared by [
15]: immersion cooling and cold-plate based cooling for different discharge rates and for different coolant flow rates by performing 3D CFD simulations using an experimentally validated numerical methodology. A combination of CFD simulations and optical measurements was employed by [
16] to explore the distribution of the vanadium electrolyte and an alternative fluid substitute in a carbon felt electrode in three dimensions. They obtained qualitatively consistent results. Different cell arrangement structures and various air-cooling strategies were evaluated by [
7] for their effects on the thermal performance of a battery module. They used a 3D CFD method and a lumped model of a single cell in the simulation. [
17] Furthermore, a calibration of the model was performed based on the experimental tests to obtain more accurate results. A study on the thermal characteristics of Li-ion batteries is described by [
18]. The authors created a 3D electrochemical-thermal-flow coupling model. Through the analysis of simulation results, they explored the thermal characteristics of Li-ion batteries for electric vehicles. The thermal characterization of a commercially available Li-ion battery is discussed by [
19]. The study measures the heat generation of the battery at different operating conditions and charge and discharge rates. The study found some important parameters that have a significant influence on total generated heat. The heat generation during charging and discharging of a battery and how it affects temperature behaviors is discussed by [
20]. The study shows that enthalpy heating and heat of mixing, generally neglected, should be included to accurately and completely describe the heat generation. An electrochemical-thermal model including these additional terms is experimentally validated against calorimetric measurements. An electrochemical-thermal model for a commercial Li-ion battery to simulate the distribution of electrochemical and thermal variables 3D through the electrode layers in the pouch cell is presented by [
21]. The model is validated against experimental voltage and temperature distribution data. A physics-based electrochemical model for the study of Li-ion batteries is presented by [
22]. The article discusses two challenges to apply Newman’s models in the electrochemical-thermal coupled battery simulation. A 3D thermal modeling of a single Li-ion battery cell and a 50 V Li-ion battery pack composed of 14 prismatic batteries using an ECM for subscale electrochemical modeling was developed by [
23]. The study aimed to predict the thermal behavior of the battery pack under fast discharging and abusive conditions. A 3D electrochemical-thermal coupled model was developed by [
24] for a 30 Ah ternary cathode Li-ion battery. The model was used to study the internal electrochemical processes and thermal characteristics and results were compared with experimental data showing a good agreement. An insight into the discharge behavior of Li-ion batteries based on investigations conducted by researchers was given by [
25]. The Dual-Potential Multi-Scale Multi-Domain (MSMD) Battery Model was used to analyze the discharge behavior of Li-ion batteries. The development of 3D-FEA models to simulate the electro-thermal behavior of Li-ion battery cells with various geometries is described by [
26]. They focus on the thermal behavior of Li-ion battery cells with different geometries, electrical loads and cooling conditions. Modeling results and performance prediction for a Li-ion battery are presented and compared to experimental data for several different experimental cell configurations on both charge and discharge by [
27] and different system designs are explored using the mathematical model. A model for the galvanostatic charge and discharge of a lithium cell with a lithium anode, solid polymer separator and insertion cathode is described by [
17]. The model uses concentrated solution theory and is general enough to include a wide range of polymeric separator materials, lithium salts and composite insertion cathodes. The insertion of lithium into the active cathode material is simulated using superposition, greatly simplifying numerical calculations. A study that investigates the nail penetration process in a large-format Li-ion cell using a 3D multiscale electrochemical-thermal coupled model is described by [
28]. The study reveals strong coupling of the cell thermal response and electrochemical behavior, which is influenced substantially by key parameters. A 3D electrochemical-thermal coupled model is used by [
29] to scrutinize the internal short circuit process in a large-format Li-ion cell and a parametric study was carried out.
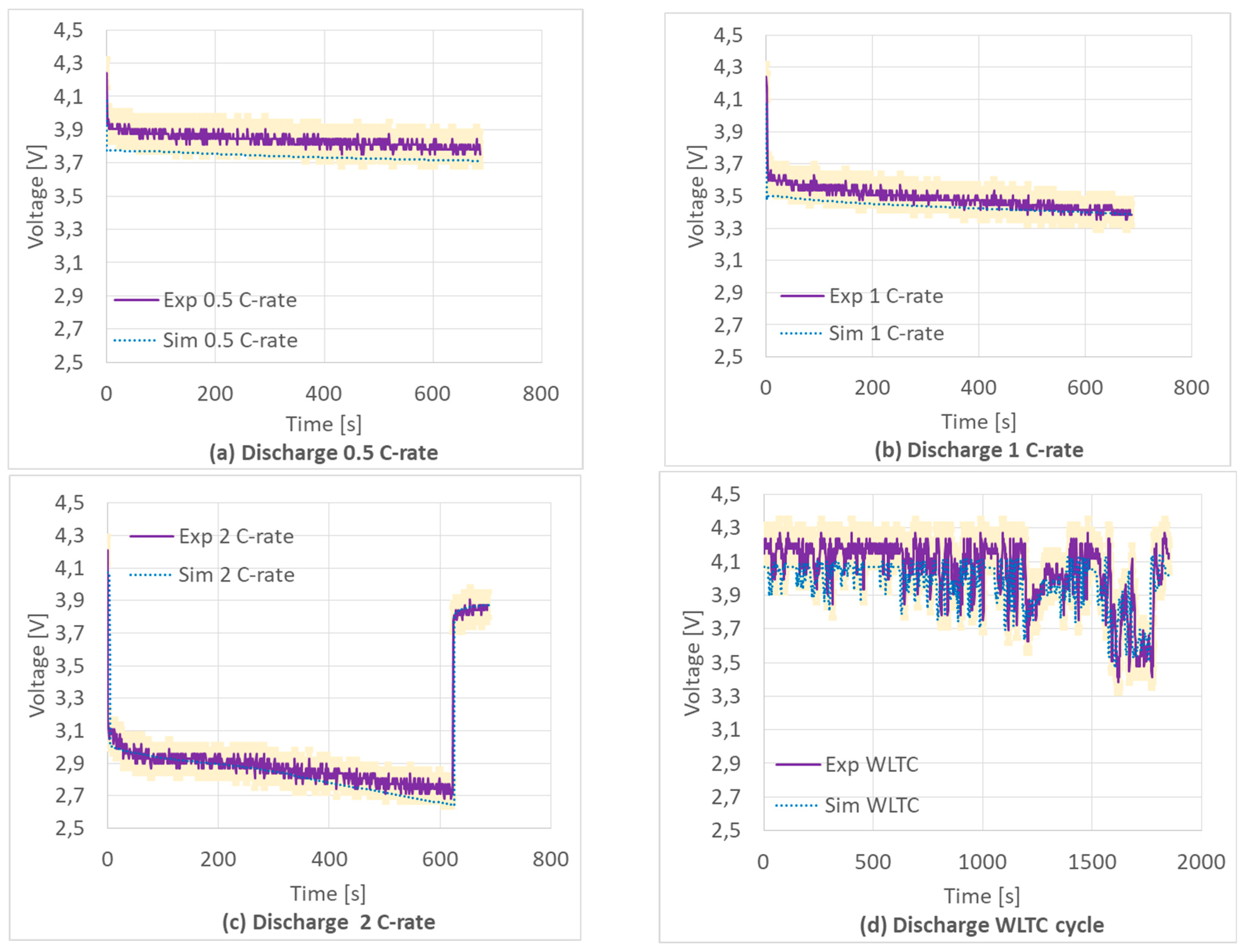
In this paper, a numerical and experimental validation of a prismatic Li-ion battery is carried out using the Newman’s pseudo-2D approach also called Newman’s approach in commercial code. To date, studies on CFD modeling of Li-ion batteries are few and only a part of these have been developed with an electrochemical approach. This approach can accurately capture Li-ion migration in the cell. The electrochemical models require many parameters to be set which are different according to the type of cell. These parameters are difficult to find in the literature and depend on the type of battery and on the materials used for the cells. Through this work they are highlighted and experimentally validated so that they can be used for the simulation and optimization of other batteries.