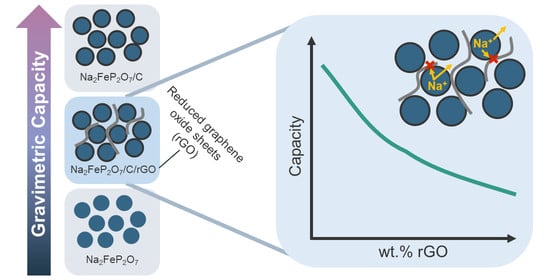
The Impact of Graphene in Na2FeP2O7/C/Reduced Graphene Oxide Composite Cathode for Sodium-Ion Batteries
Abstract
:1. Introduction
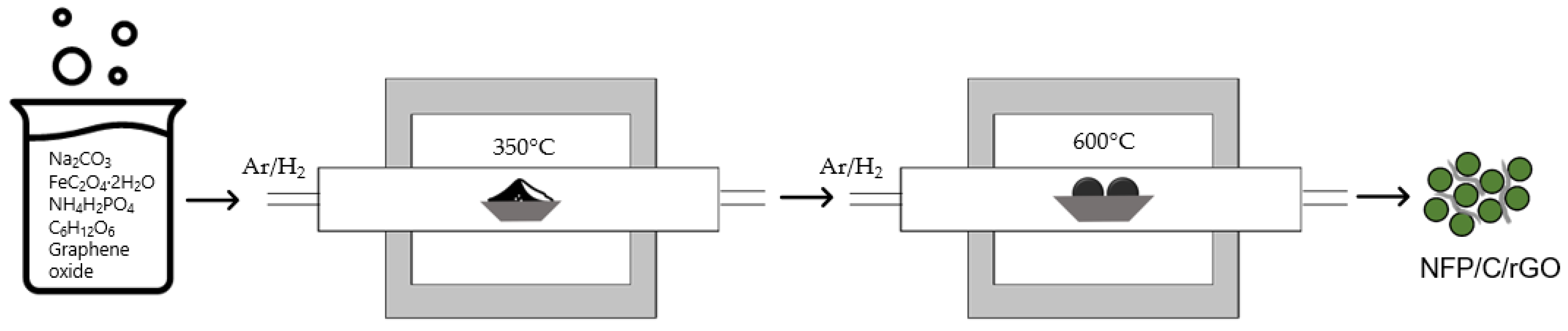
2. Materials and Methods
3. Results
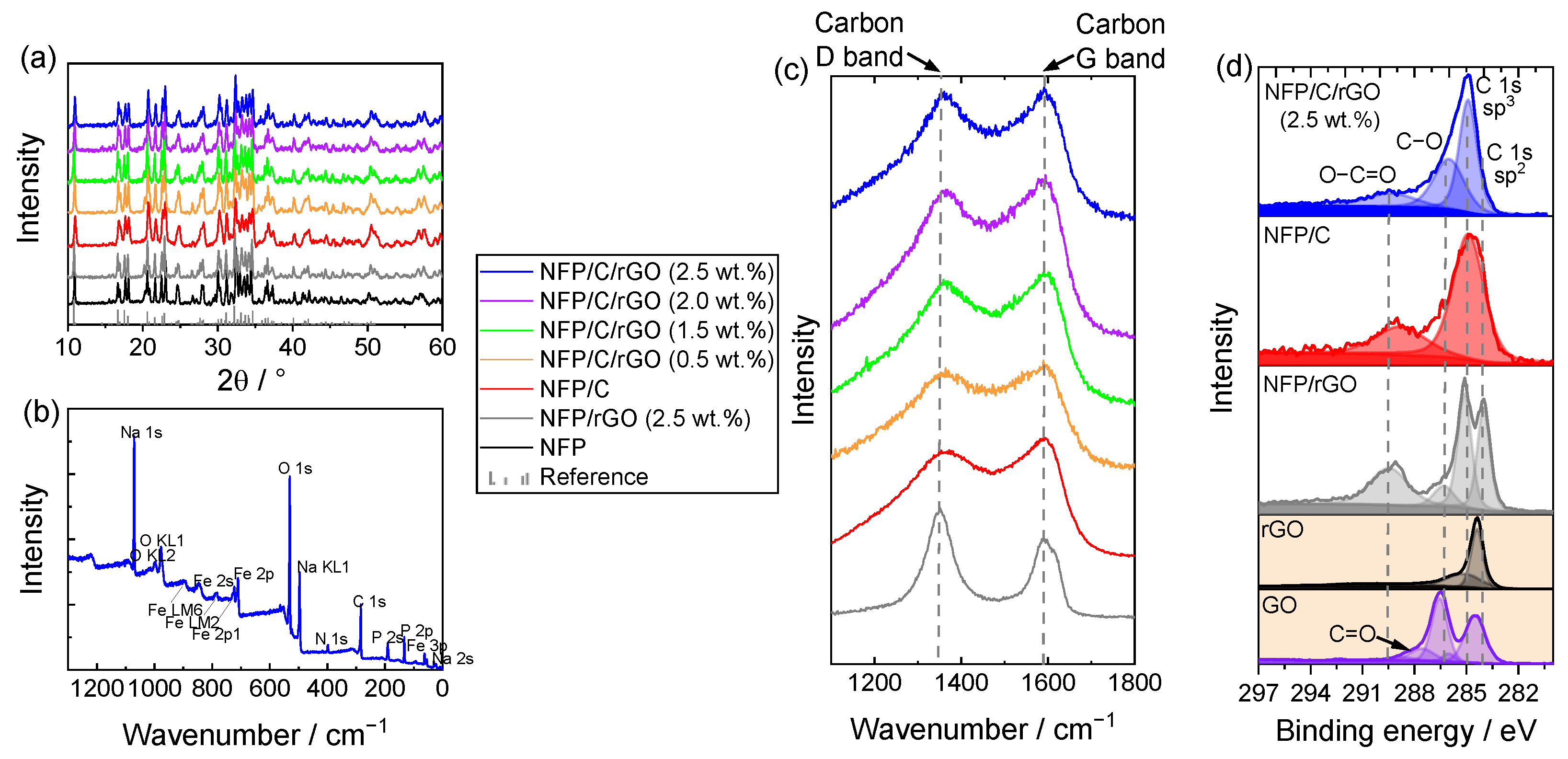
3.1. Structure, Composition and Morphology
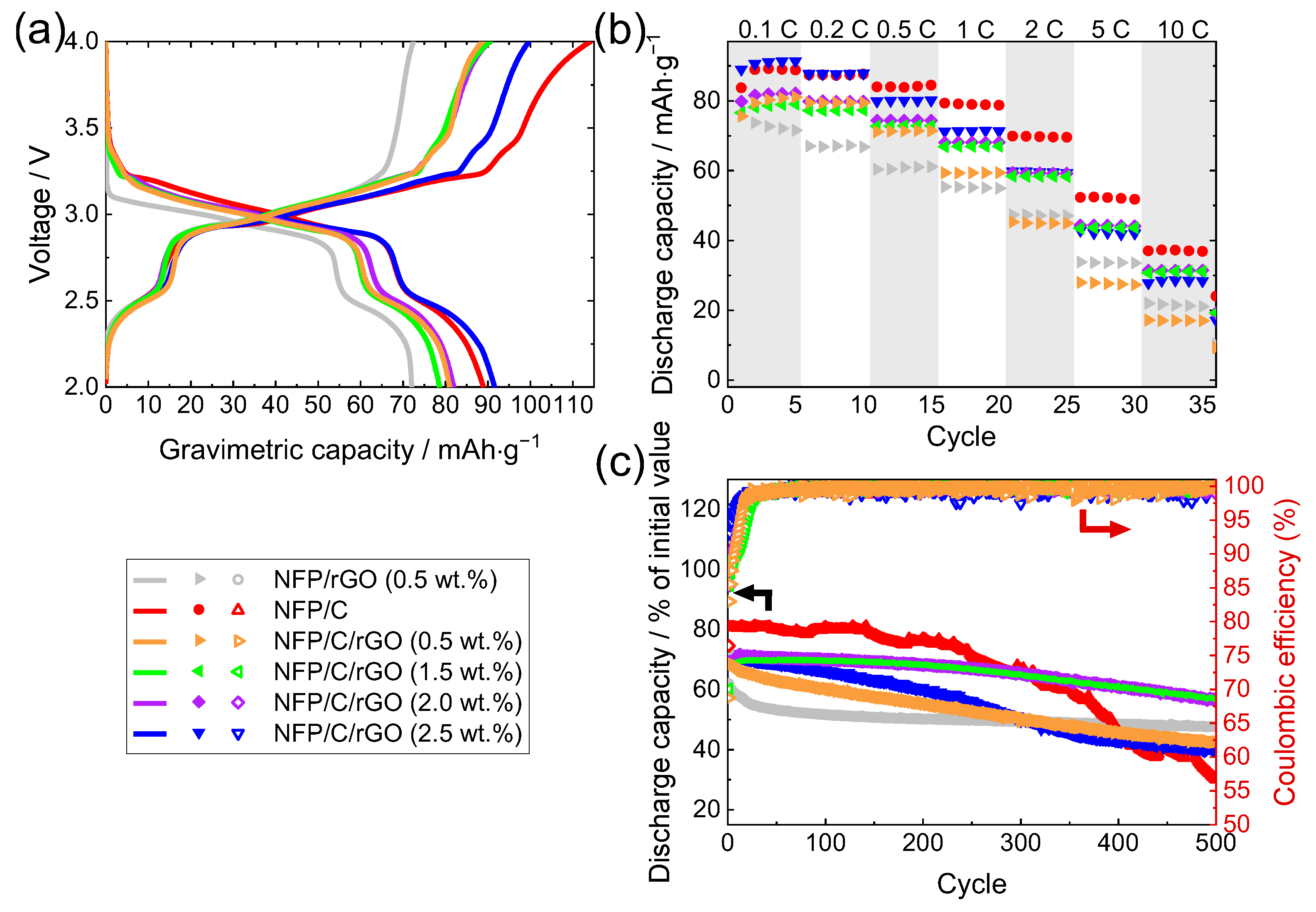
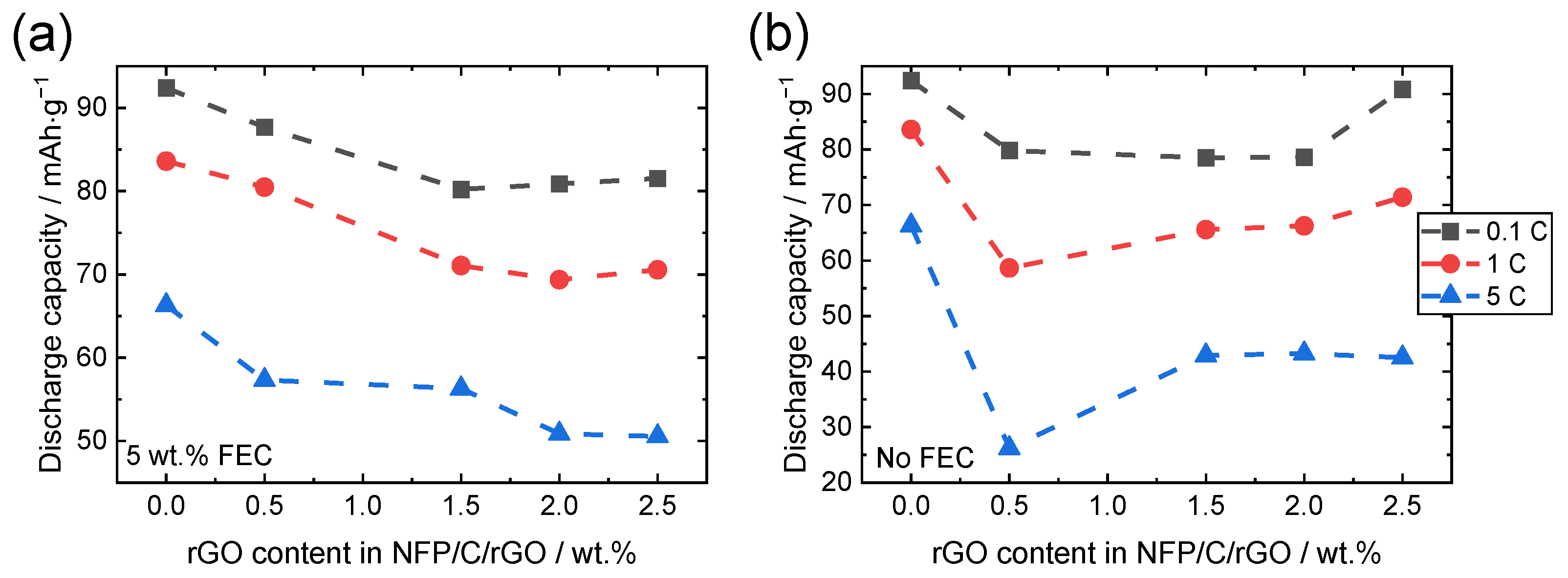
3.2. Electrochemical Measurements
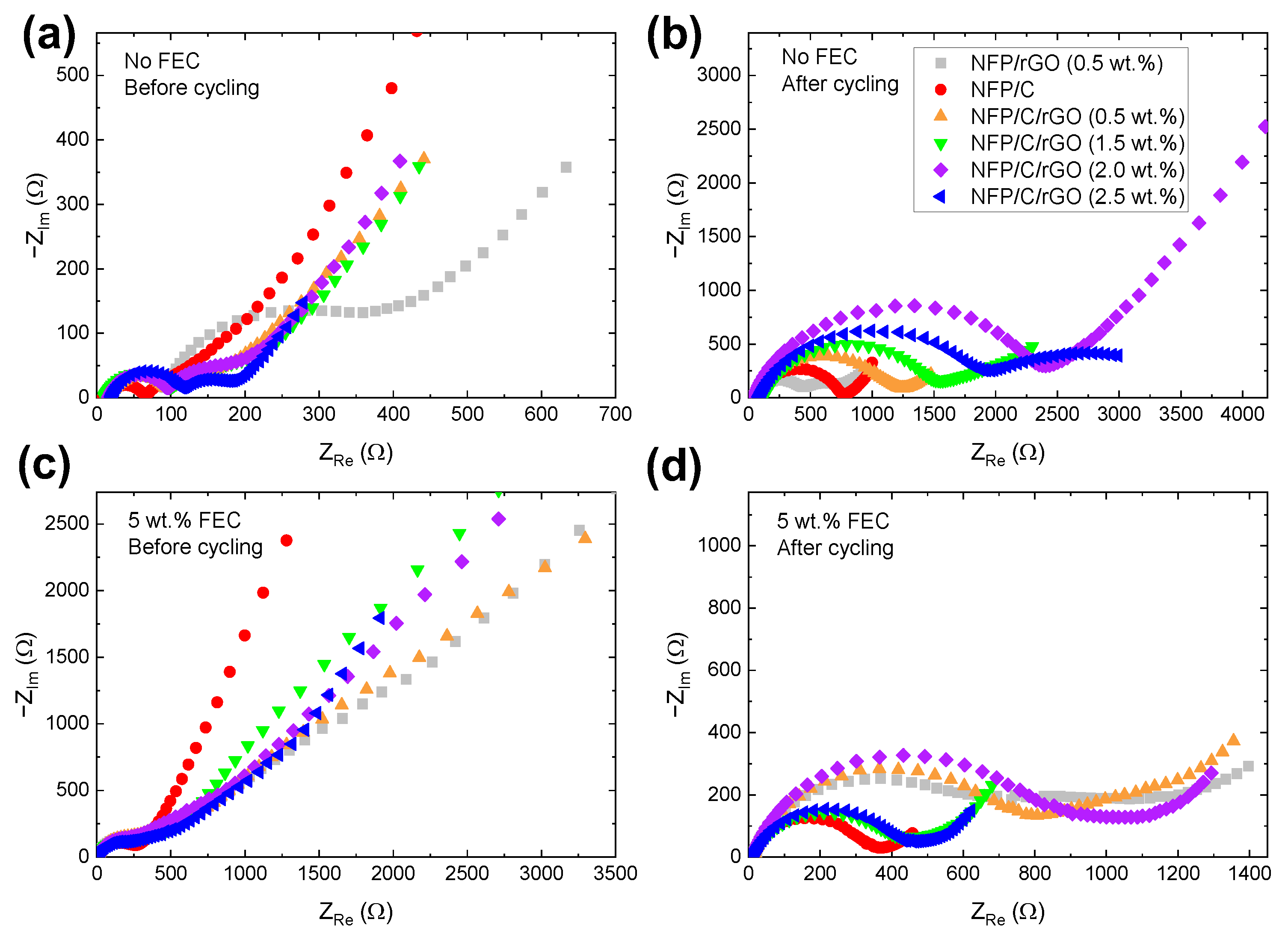
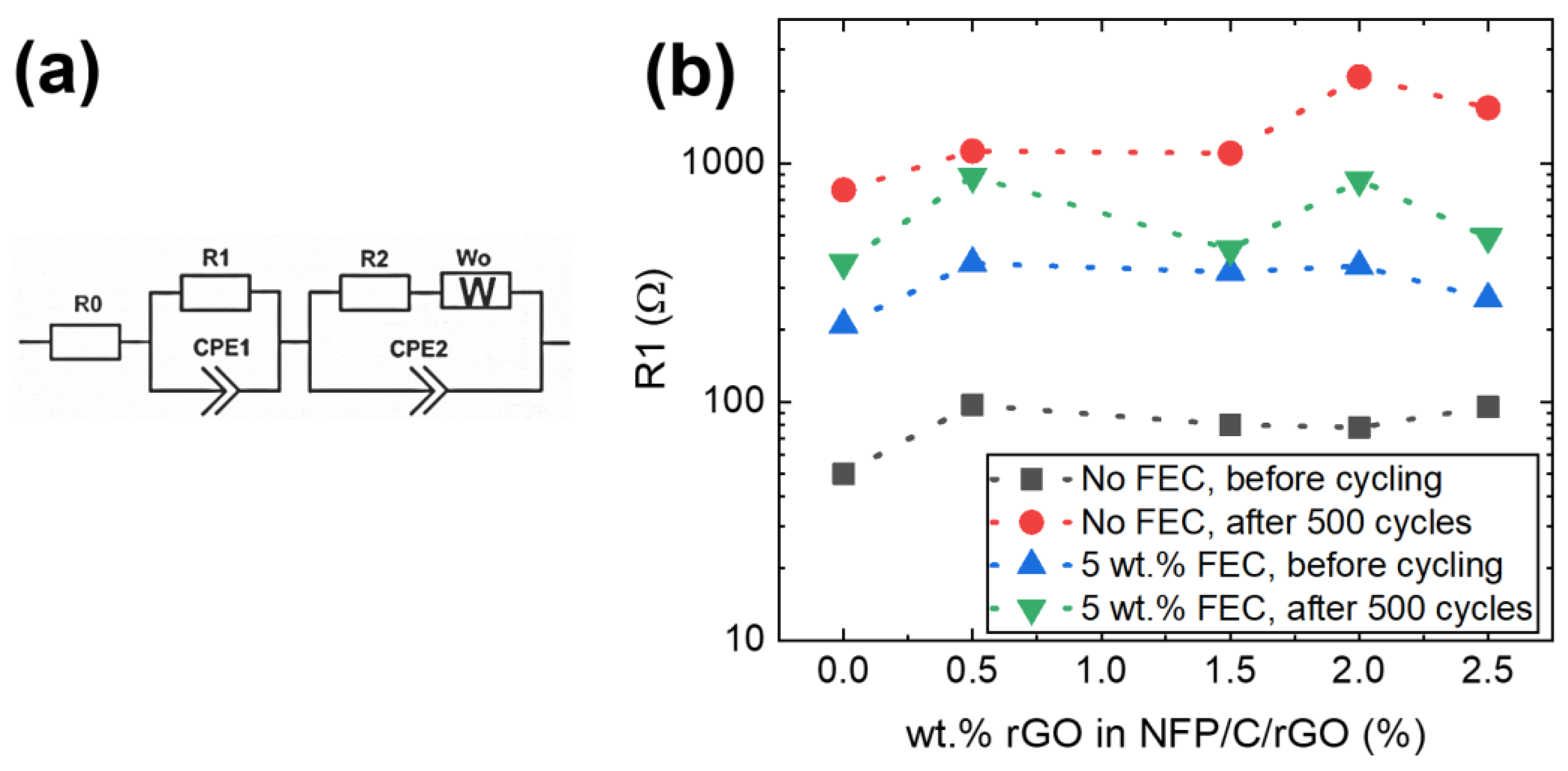
3.3. Electrochemical Impedance Spectroscopy
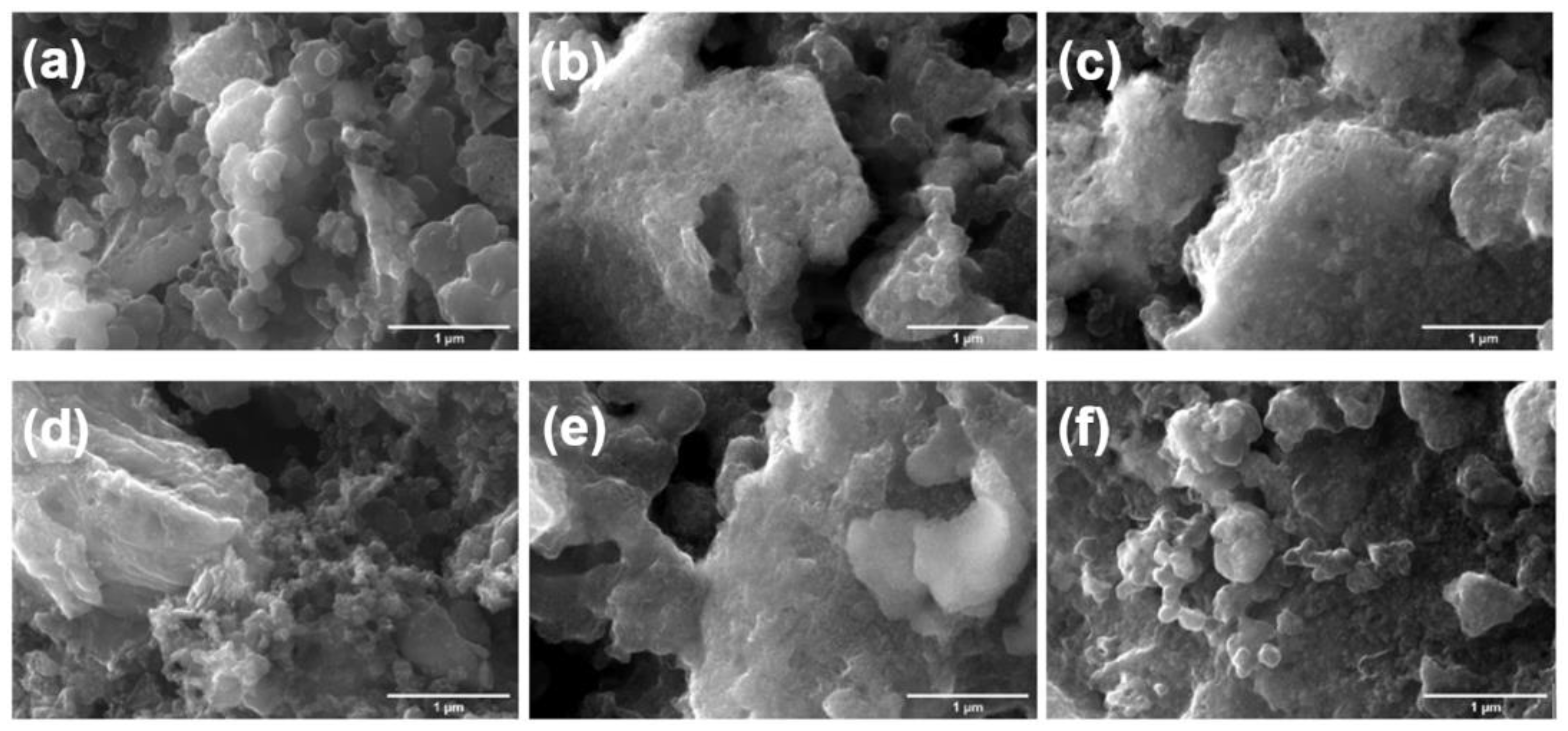
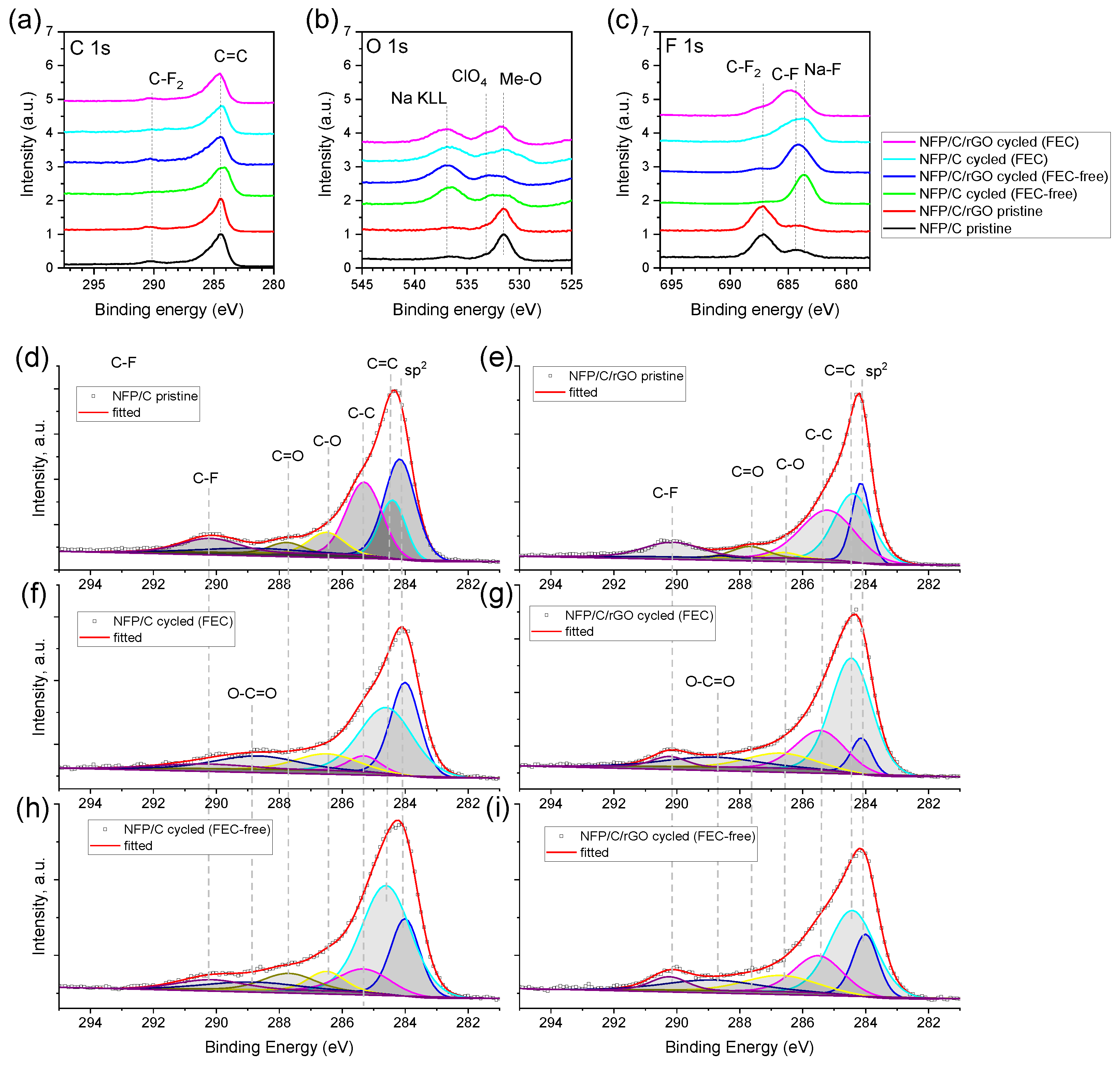
3.4. Post-Mortem Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Ruiz, N.; Armstrong, A.R.; Alptekin, H.; Amores, M.A.; Au, H.; Barker, J.; Boston, R.; Brant, W.R.; Brittain, J.M.; Chen, Y.; et al. 2021 roadmap for sodium-ion batteries. J. Phys. Energy 2021, 3, 031503. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CATL. Unveils Its Latest Breakthrough Technology by Releasing Its First Generation of Sodium-Ion Batteries. Available online: https://www.catl.com/en/news/665.html (accessed on 14 February 2023).
- Zhang, P.; CnEVPost. BYD May Launch New Model with Sodium-Ion Batteries in Q2 2023, Report Says. Available online: https://cnevpost.com/2022/11/30/byd-model-with-sodium-ion-batteries-q2-2023-report/ (accessed on 14 February 2023).
- Hasa, I.; Tapia-Ruiz, N.; Galceran, M. Editorial: Sodium-ion batteries: From materials discovery and understanding to cell development. Front. Energy Res. 2022, 10, 1076764. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, T.; Li, W.; Li, T.; Zhang, L.; Zhang, X.; Wang, Z. Engineering of sodium-ion batteries: Opportunities and challenges. Engineering 2022. [Google Scholar] [CrossRef]
- Honma, T.; Togashi, T.; Ito, N.; Komatsu, T. Fabrication of Na2FeP2O7 Glass-Ceramics for Sodium Ion Battery. J. Ceram. Soc. Jpn. 2012, 120, 344–346. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Shakoor, R.A.; Park, C.; Lim, S.Y.; Kim, J.-S.; Jo, Y.N.; Cho, W.; Miyasaka, K.; Kahraman, R.; Jung, Y.; et al. Na2FeP2O7 as a Promising Iron-Based Pyrophosphate Cathode for Sodium Rechargeable Batteries: A Combined Experimental and Theoretical Study. Adv. Funct. Mater. 2013, 23, 1147–1155. [Google Scholar] [CrossRef]
- Kucinskis, G.; Nesterova, I.; Sarakovskis, A.; Bikse, L.; Hodakovska, J.; Bajars, G. Electrochemical performance of Na2FeP2O7/C cathode for sodium-ion batteries in electrolyte with fluoroethylene carbonate additive. J. Alloys Compd. 2022, 895, 162656. [Google Scholar] [CrossRef]
- Makhlooghiazad, F.; Sharma, M.; Zhang, Z.; Howlett, P.C.; Forsyth, M.; Nazar, L.F. Stable High-Temperature Cycling of Na Metal Batteries on Na3V2(PO4)3 and Na2FeP2O7 Cathodes in NaFSI-Rich Organic Ionic Plastic Crystal Electrolytes. J. Phys. Chem. Lett. 2020, 11, 2092–2100. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Kumakura, S.; Tahara, Y.; Kubota, K.; Chihara, K.; Komaba, S. Sodium and Manganese Stoichiometry of P2-Type Na2/3MnO2. Angew. Chem. Int. Ed. 2016, 55, 12760–12763. [Google Scholar] [CrossRef]
- Pfeiffer, L.F.; Jobst, N.; Gauckler, C.; Linden, M.; Marinaro, M.; Passerini, S.; Wohlfahrt-Mehrens, M.; Axmann, P. Layered P2-NaxMn3/4Ni1/4O2 Cathode Materials For Sodium-Ion Batteries: Synthesis, Electrochemistry and Influence of Ambient Storage. Front. Energy Res. 2022, 10, 910842. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, D.-S.; Kim, J.-C.; Hong, S.-H.; Kim, D.-W. An approach to flexible Na-ion batteries with exceptional rate capability and long lifespan using Na2FeP2O7 nanoparticles on porous carbon cloth. J. Mater. Chem. A 2017, 5, 5502–5510. [Google Scholar] [CrossRef]
- Kosova, N.V.; Rezepova, D.O.; Petrov, S.A.; Slobodyuk, A.B. Electrochemical and Chemical Na+/Li+ Ion Exchange in Na-Based Cathode Materials: Na1.56Fe1.22P2O7 and Na3V2(PO4)2F3. J. Electrochem. Soc. 2016, 164, A6192. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Matsumoto, K.; Nohira, T.; Ding, C.; Yamamoto, T.; Hagiwara, R. Charge–discharge behavior of a Na2FeP2O7 positive electrode in an ionic liquid electrolyte between 253 and 363 K. Electrochim. Acta 2014, 133, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2FeP2O7: A Safe Cathode for Rechargeable Sodium-ion Batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Bo, S.-H.; Wang, Y.; Chen, H.; Twu, N.; Wu, D.; Ceder, G. High-Performance P2-Type Na2/3(Mn1/2Fe1/4Co1/4)O2 Cathode Material with Superior Rate Capability for Na-Ion Batteries. Adv. Energy Mater. 2015, 5, 1500944. [Google Scholar] [CrossRef]
- Mortemard de Boisse, B.; Carlier, D.; Guignard, M.; Bourgeois, L.; Delmas, C. P2-NaxMn1/2Fe1/2O2 Phase Used as Positive Electrode in Na Batteries: Structural Changes Induced by the Electrochemical (De)intercalation Process. Inorg. Chem. 2014, 53, 11197–11205. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Wang, X.; Shen, X.; Kong, Q.; Yu, R.; Lu, G.; Wang, Z.; Chen, L. Iron migration and oxygen oxidation during sodium extraction from NaFeO2. Nano Energy 2018, 47, 519–526. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Winter, M.; Placke, T.; Ramirez-Rico, J. Correlation of Structure and Performance of Hard Carbons as Anodes for Sodium Ion Batteries. Chem. Mater. 2019, 31, 7288–7299. [Google Scholar] [CrossRef]
- Adam, L.; Guesdon, A.; Raveau, B. A new lithium manganese phosphate with an original tunnel structure in the A2MP2O7 family. J. Solid State Chem. 2008, 181, 3110–3115. [Google Scholar] [CrossRef]
- Barpanda, P.; Nishimura, S.; Yamada, A. High-Voltage Pyrophosphate Cathodes. Adv. Energy Mater. 2012, 2, 841–859. [Google Scholar] [CrossRef]
- Dugas, R.; Ponrouch, A.; Gachot, G.; David, R.; Palacin, M.R.; Tarascon, J.M. Na Reactivity toward Carbonate-Based Electrolytes: The Effect of FEC as Additive. J. Electrochem. Soc. 2016, 163, A2333. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Zhang, X.; Chung, M.; Less, G.; Sastry, A. A Review of Conduction Phenomena in Li-Ion Batteries. J. Power Source 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Hein, S.; Danner, T.; Westhoff, D.; Prifling, B.; Scurtu, R.; Kremer, L.; Hoffmann, A.; Hilger, A.; Osenberg, M.; Manke, I.; et al. Influence of Conductive Additives and Binder on the Impedance of Lithium-Ion Battery Electrodes: Effect of Morphology. J. Electrochem. Soc. 2020, 167, 013546. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Pai, C.-T.; Chen, Y.-F.; Chen, I.-L.; Chen, W.-Y. Preparation of lithium iron phosphate cathode materials with different carbon contents using glucose additive for Li-ion batteries. J. Taiwan Inst. Chem. Eng. 2014, 45, 1501–1508. [Google Scholar] [CrossRef]
- Yang, S.-L.; Ma, R.-G.; Hu, M.-J.; Xi, L.-J.; Lu, Z.-G.; Chung, C.Y. Solvothermal synthesis of nano-LiMnPO4 from Li3PO4 rod-like precursor: Reaction mechanism and electrochemical properties. J. Mater. Chem. 2012, 22, 25402–25408. [Google Scholar]
- Gaberscek, M.; Dominko, R.; Jamnik, J. Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes. Electrochem. Commun. 2007, 9, 2778–2783. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, X.; Liu, D.; Wang, S.; Zhang, L.; Zhao, R.; Kang, F.; Li, B. Understanding the Conductive Carbon Additive on Electrode/Electrolyte Interface Formation in Lithium-Ion Batteries via in situ Scanning Electrochemical Microscopy. Front. Chem. 2020, 8, 114. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Source 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Bikova, K.; Kaprans, K.; Kleperis, J. Microstructural Influence on Electrochemical Properties of LiFePO4/C/Reduced Graphene Oxide Composite Cathode. Russ. J. Electrochem. 2019, 55, 517–523. [Google Scholar] [CrossRef]
- Arifin, M.; Iskandar, F.; Aimon, A.; Munir, M.M.; Nuryadin, B. Synthesis of LiFePO4/Li2SiO3/reduced Graphene Oxide (rGO) Composite via Hydrothermal Method. J. Phys. Conf. Ser. 2016, 739, 012087. [Google Scholar] [CrossRef]
- Jiang, G.; Hu, Z.; Xiong, J.; Zhu, X.; Yuan, S. Enhanced performance of LiFePO4 originating from the synergistic effect of graphene modification and carbon coating. J. Alloys Compd. 2018, 767, 528–537. [Google Scholar] [CrossRef]
- Miao, R.; He, J.; Sahoo, S.; Luo, Z.; Zhong, W.; Chen, S.-Y.; Guild, C.; Jafari, T.; Dutta, B.; Cetegen, S.; et al. Reduced Graphene Oxide Supported Nickel-Manganese-Cobalt Spinel Ternary Oxide Nanocomposites and Their Chemical-Converted Sulfide Nanocomposites as Efficient Electrocatalysts for Alkaline Water Splitting. ACS Catal. 2016, 7, 819–832. [Google Scholar] [CrossRef]
- Chen, X.; Du, K.; Lai, Y.; Shang, G.; Li, H.; Xiao, Z.; Li, J.; Zhang, Z. In-situ carbon-coated Na2FeP2O7 anchored in three-dimensional reduced graphene oxide framework as a durable and high-rate sodium-ion battery cathode. J. Power Source 2017, 357, 164–172. [Google Scholar] [CrossRef]
- Ben, J.; Jia, Y.; Wu, T.; Liu, X.; Li, X. Sodium birnessite@graphene hierarchical structures for ultrafast sodium ion storage. J. Electroanal. Chem. 2022, 906, 116007. [Google Scholar] [CrossRef]
- Kumar, P.R.; Jung, Y.H.; Ahad, S.A.; Kim, D.K. A high rate and stable electrode consisting of a Na3V2O2X(PO4)2F3−2X–rGO composite with a cellulose binder for sodium-ion batteries. RSC Adv. 2017, 7, 21820–21826. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhao, Y.; Xia, L.; Yang, Z.; Wei, J.; Zhou, Z. Well-dispersed Na3V2(PO4)2F3@rGO with improved kinetics for high-power sodium-ion batteries. J. Mater. Chem. A 2020, 8, 12391–12397. [Google Scholar] [CrossRef]
- Su, F.; He, Y.-B.; Li, B.; Chen, X.-C.; You, C.-H.; Wei, W.; Lv, W.; Yang, Q.-H.; Kang, F. Could graphene construct an effective conducting network in a high-power lithium ion battery? Nano Energy 2012, 1, 429–439. [Google Scholar] [CrossRef]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190–193. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Wang, J.; Ma, L.; Ma, S.; Zhang, Y.; Wang, E.; Bi, Y.; Wang, D.; McKee, W.C.; et al. Unlocking the energy capabilities of micron-sized LiFePO4. Nat. Commun. 2015, 6, 7898. [Google Scholar] [CrossRef] [Green Version]
- Griffin, E.; Mogg, L.; Hao, G.-P.; Kalon, G.; Bacaksiz, C.; Lopez-Polin, G.; Zhou, T.Y.; Guarochico, V.; Cai, J.; Neumann, C.; et al. Proton and Li-Ion Permeation through Graphene with Eight-Atom-Ring Defects. ACS Nano 2020, 14, 7280–7286. [Google Scholar] [CrossRef]
- Mogg, L.; Zhang, S.; Hao, G.-P.; Gopinadhan, K.; Barry, D.; Liu, B.L.; Cheng, H.M.; Geim, A.K.; Lozada-Hidalgo, M. Perfect proton selectivity in ion transport through two-dimensional crystals. Nat. Commun. 2019, 10, 4243. [Google Scholar] [CrossRef] [Green Version]
- Farah, S.; Farkas, A.; Madarász, J.; László, K. Comparison of thermally and chemically reduced graphene oxides by thermal analysis and Raman spectroscopy. J. Therm. Anal. Calorim. 2020, 142, 331–337. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.S.; Yoon, G.; Park, M.; Cho, S.; Lim, H.-D.; Kim, H.; Park, Y.; Kim, B.H.; Kang, K.; Jin, H.-J. Restoration of thermally reduced graphene oxide by atomic-level selenium doping. NPG Asia Mater. 2016, 8, e338. [Google Scholar] [CrossRef] [Green Version]
- Biswas, C.; Lee, Y.H. Graphene vs Carbon Nanotubes in Electronic Devices: Graphene Versus Carbon Nanotubes in Electronic Devices (Adv. Funct. Mater. 20/2011). Adv. Funct. Mater. 2011, 21, 3798. [Google Scholar] [CrossRef]
- Kaprans, K.; Mateuss, J.; Dorondo, A.; Bajars, G.; Kucinskis, G.; Lesnicenoks, P.; Kleperis, J. Electrophoretically deposited α-Fe2O3 and TiO2 composite anchored on rGO with excellent cycle performance as anode for lithium ion batteries. Solid State Ion. 2018, 319, 1–6. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Wang, X.; Zhang, B.; Tong, H.; Yu, W.; Zhang, J. Comparative Investigation of Na2FeP2O7 Sodium Insertion Material Synthesized by Using Different Sodium Sources. ACS Sustain. Chem. Eng. 2018, 6, 4966–4972. [Google Scholar] [CrossRef]
- Sengupta, I.; Chakraborty, S.; Talukdar, M.; Pal, S.K.; Chakraborty, S. Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 2018, 33, 4113–4122. [Google Scholar] [CrossRef]
- Slobodian, O.M.; Lytvyn, P.M.; Nikolenko, A.S.; Naseka, V.M.; Khyzhun, O.Y.; Vasin, A.V.; Sevostianov, S.V.; Nazarov, A.N. Low-Temperature Reduction of Graphene Oxide: Electrical Conductance and Scanning Kelvin Probe Force Microscopy. Nanoscale Res. Lett. 2018, 13, 139. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, H.; Robinson, J.T.; Sanchez, H.; Diankov, G.; Dai, H. Simultaneous Nitrogen Doping and Reduction of Graphene Oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.-E.; Sa, Y.J.; Park, S.; Lee, J.; Shin, K.-H.; Joo, S.H.; Ko, H. An ice-templated, pH-tunable self-assembly route to hierarchically porous graphene nanoscroll networks. Nanoscale 2014, 6, 9734–9741. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Matsumoto, K.; Nohira, T.; Hagiwara, R.; Orikasa, Y.; Uchimoto, Y. Pyrophosphate Na2FeP2O7 as a low-cost and high-performance positive electrode material for sodium secondary batteries utilizing an inorganic ionic liquid. J. Power Source 2014, 246, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Shakoor, R.A.; Park, C.S.; Raja, A.A.; Shin, J.; Kahraman, R. A mixed iron–manganese based pyrophosphate cathode, Na2Fe0.5Mn0.5P2O7, for rechargeable sodium ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 3929–3935. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, J.; Alfaruqi, M.H.; Park, W.; Park, S.; Kim, S.; Jo, J.; Kim, J.; Song, J.; Yang, J.; et al. Pyro-synthesis of Na2FeP2O7 Nano-plates as Cathode for Sodium-ion Batteries with Long Cycle Stability. J. Korean Ceram. Soc. 2016, 53, 406–410. [Google Scholar] [CrossRef] [Green Version]
- Kosova, N.V.; Rezepova, D.O.; Podgornova, O.A.; Slobodyuk, A.B.; Petrov, S.A.; Avdeev, M. A comparative study of structure, air sensitivity and electrochemistry of sodium iron pyrophosphates Na2−xFe1+x/2P2O7 (x = 0; 0.44). Electrochim. Acta 2017, 235, 42–55. [Google Scholar] [CrossRef]
- Gond, R.; Meena, S.S.; Pralong, V.; Barpanda, P. Structural and electrochemical investigation of binary Na2Fe1-xZnxP2O7 (0 ≤ x ≤ 1) pyrophosphate cathodes for sodium-ion batteries. J. Solid State Chem. 2019, 277, 329–336. [Google Scholar] [CrossRef]
- Vogt, L.O.; El Kazzi, M.; Jämstorp Berg, E.; Pérez Villar, S.; Novák, P.; Villevieille, C. Understanding the Interaction of the Carbonates and Binder in Na-Ion Batteries: A Combined Bulk and Surface Study. Chem. Mater. 2015, 27, 1210–1216. [Google Scholar] [CrossRef]
- Dahbi, M.; Nakano, T.; Yabuuchi, N.; Ishikawa, T.; Kubota, K.; Fukunishi, M.; Shibahara, S.; Son, J.-Y.; Cui, Y.-T.; Oji, H.; et al. Sodium carboxymethyl cellulose as a potential binder for hard-carbon negative electrodes in sodium-ion batteries. Electrochem. Commun. 2014, 44, 66–69. [Google Scholar] [CrossRef]
- Kucinskis, G.; Kruze, B.; Korde, P.; Sarakovskis, A.; Viksna, A.; Hodakovska, J.; Bajars, G. Enhanced Electrochemical Properties of Na0.67MnO2 Cathode for Na-Ion Batteries Prepared with Novel Tetrabutylammonium Alginate Binder. Batteries 2022, 8, 6. [Google Scholar] [CrossRef]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na Insertion and Solid Electrolyte Interphase for Hard-Carbon Electrodes and Application to Na-Ion Batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar]
- Webb, S.; Baggetto, L.; Bridges, C.; Veith, G. The electrochemical reactions of pure indium with Li and Na: Anomalous electrolyte decomposition, benefits of FEC additive, phase transitions and electrode performance. J. Power Source 2014, 248, 1105–1117. [Google Scholar]
- Jeong, S.-K.; Inaba, M.; Mogi, R.; Iriyama, Y.; Abe, T.; Ogumi, Z. Surface Film Formation on a Graphite Negative Electrode in Lithium-Ion Batteries: Atomic Force Microscopy Study on the Effects of Film-Forming Additives in Propylene Carbonate Solutions. Langmuir 2001, 17, 8281–8286. [Google Scholar] [CrossRef]
- Kallel, A.Y.; Petrychenko, V.; Kanoun, O. State-of-Health of Li-Ion Battery Estimation Based on the Efficiency of the Charge Transfer Extracted from Impedance Spectra. Appl. Sci. 2022, 12, 885. [Google Scholar] [CrossRef]
- Atebamba, J.-M.; Moskon, J.; Pejovnik, S.; Gaberscek, M. On the Interpretation of Measured Impedance Spectra of Insertion Cathodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2010, 157, A1218. [Google Scholar] [CrossRef]
- Gaberscek, M.; Moskon, J.; Erjavec, B.; Dominko, R.; Jamnik, J. The Importance of Interphase Contacts in Li Ion Electrodes: The Meaning of the High-Frequency Impedance Arc. Electrochem. Solid-State Lett. 2008, 11, A170. [Google Scholar] [CrossRef]
- Pritzl, D.; Bumberger, A.E.; Wetjen, M.; Landesfeind, J.; Solchenbach, S.; Gasteiger, H.A. Identifying Contact Resistances in High-Voltage Cathodes by Impedance Spectroscopy. J. Electrochem. Soc. 2019, 166, A582. [Google Scholar] [CrossRef] [Green Version]
- Ariyoshi, K.; Tanimoto, M.; Yamada, Y. Impact of particle size of lithium manganese oxide on charge transfer resistance and contact resistance evaluated by electrochemical impedance analysis. Electrochim. Acta 2020, 364, 137292. [Google Scholar] [CrossRef]
- Kavaliukė, V.; Nesterova, I.; Kežionis, A.; Balciunas, S.; Bajars, G.; Salkus, T. Combined conductivity and electrochemical impedance spectroscopy study of Na2FeP2O7 cathode material for sodium ion batteries. Solid State Ion. 2022, 385, 116024. [Google Scholar] [CrossRef]
- Murray, V.; Hall, D.S.; Dahn, J.R. A Guide to Full Coin Cell Making for Academic Researchers. J. Electrochem. Soc. 2019, 166, A329. [Google Scholar] [CrossRef]
- Koenig, G.M.; Gupta, D.; Kim, Y. Perspective—Expected Variation in Reported Coin Cell Capacities Due to Current Collector Mass Distribution. J. Electrochem. Soc. 2020, 167, 120529. [Google Scholar] [CrossRef]
- Luc, P.-M.; Bauer, S.; Kowal, J. Reproducible Production of Lithium-Ion Coin Cells. Energies 2022, 15, 7949. [Google Scholar] [CrossRef]
- Ogihara, N.; Itou, Y.; Sasaki, T.; Takeuchi, Y. Impedance Spectroscopy Characterization of Porous Electrodes under Different Electrode Thickness Using a Symmetric Cell for High-Performance Lithium-Ion Batteries. J. Phys. Chem. C 2015, 119, 4612–4619. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Liu, Z.; Yang, W.; Liu, J. A highly conductive porous graphene electrode prepared via in situ reduction of graphene oxide using Cu nanoparticles for the fabrication of high performance supercapacitors. RSC Adv. 2015, 5, 54275–54282. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.A.; Zarrabeitia, M.; Castillo-Martínez, E.; Eguía-Barrio, A.; Rojo, T.; Casas-Cabanas, M. Composition and evolution of the solid-electrolyte interphase in Na2Ti3O7 electrodes for Na-ion batteries: XPS and Auger parameter analysis. ACS Appl. Mater. Interfaces 2015, 7, 7801–7808. [Google Scholar] [CrossRef]
- Moreau, P.; Guyomard, D.; Gaubicher, J.; Boucher, F. Structure and Stability of Sodium Intercalated Phases in Olivine FePO4. Chem. Mater. 2010, 22, 4126–4128. [Google Scholar] [CrossRef]
- Staišiūnas, L.; Pilipavičius, J.; Tediashvili, D.; Juodkazytė, J.; Vilčiauskas, L. Engineering of Conformal Electrode Coatings by Atomic Layer Deposition for Aqueous Na-ion Battery Electrodes. J. Electrochem. Soc. 2023, 170, 050533. [Google Scholar] [CrossRef]
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the Design of Cathode Materials for Na-Ion Batteries. ACS Omega 2022, 7, 5605–5614. [Google Scholar] [CrossRef]
- Persson, K.; Sethuraman, V.; Hardwick, L.; Hinuma, Y.; Meng, Y.; Ven, A.; Srinivasan, V.; Kostecki, R.; Ceder, G. Lithium Diffusion in Graphitic Carbon. J. Phys. Chem. Lett. 2011, 1, 1176–1180. [Google Scholar] [CrossRef] [Green Version]
- Yao, F.; Güneş, F.; Ta, H.Q.; Lee, S.M.; Chae, S.J.; Sheem, K.Y.; Cojocaru, C.S.; Xie, S.S.; Lee, Y.H. Diffusion Mechanism of Lithium Ion through Basal Plane of Layered Graphene. J. Am. Chem. Soc. 2012, 134, 8646–8654. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Sun, S.; Zang, Z.; Li, X.; Sun, X.; Cao, F.; Wu, J. Effects of graphene with different sizes as conductive additives on the electrochemical performance of a LiFePO4 cathode. RSC Adv. 2017, 7, 20882–20887. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Luo, G.; Qin, W.; Wu, C.; Jia, C. One-pot synthesis of boron-doped cobalt oxide nanorod coupled with reduced graphene oxide for sodium ion batteries. J. Colloid Interface Sci. 2023, 640, 710–718. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Z.; Song, J.; Manivannnan, A. Chemically Bonded Phosphorus/Graphene Hybrid As a High-Performance Anode for Lithium- and Sodium-Ion Batteries. ECS Meet. Abstr. 2015, MA2015-01, 2. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterova, I.; Britala, L.; Sarakovskis, A.; Kruze, B.; Bajars, G.; Kucinskis, G. The Impact of Graphene in Na2FeP2O7/C/Reduced Graphene Oxide Composite Cathode for Sodium-Ion Batteries. Batteries 2023, 9, 406. https://doi.org/10.3390/batteries9080406
Nesterova I, Britala L, Sarakovskis A, Kruze B, Bajars G, Kucinskis G. The Impact of Graphene in Na2FeP2O7/C/Reduced Graphene Oxide Composite Cathode for Sodium-Ion Batteries. Batteries. 2023; 9(8):406. https://doi.org/10.3390/batteries9080406
Chicago/Turabian StyleNesterova, Inara, Liga Britala, Anatolijs Sarakovskis, Beate Kruze, Gunars Bajars, and Gints Kucinskis. 2023. "The Impact of Graphene in Na2FeP2O7/C/Reduced Graphene Oxide Composite Cathode for Sodium-Ion Batteries" Batteries 9, no. 8: 406. https://doi.org/10.3390/batteries9080406
APA StyleNesterova, I., Britala, L., Sarakovskis, A., Kruze, B., Bajars, G., & Kucinskis, G. (2023). The Impact of Graphene in Na2FeP2O7/C/Reduced Graphene Oxide Composite Cathode for Sodium-Ion Batteries. Batteries, 9(8), 406. https://doi.org/10.3390/batteries9080406