High-Rate Capability of LiNi0.9Mn0.1−xAlxO2 (NMA) (x = 0.01, 0.03, 0.05) as Cathode for Lithium-Ion Batteries
Abstract
:1. Introduction
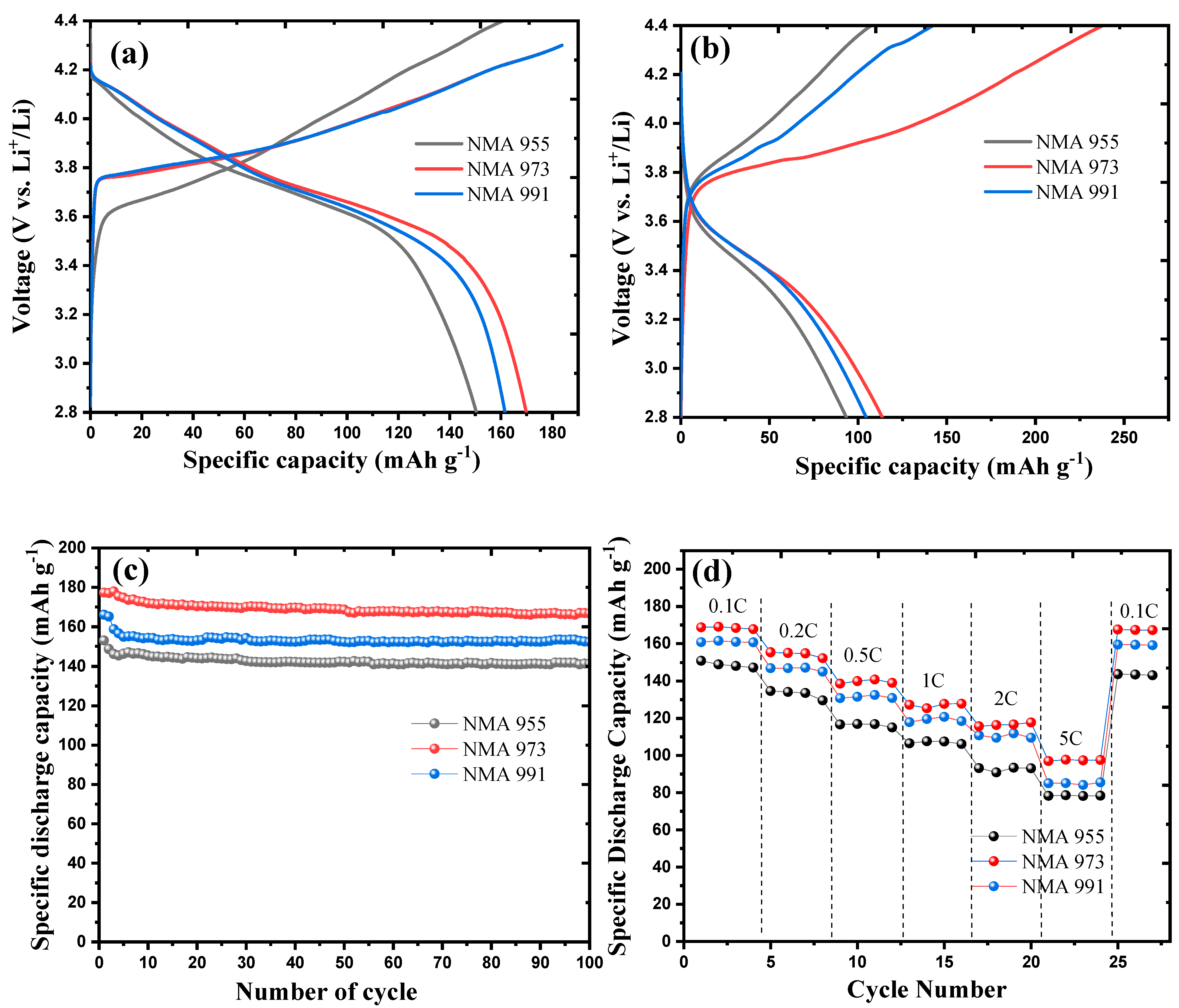
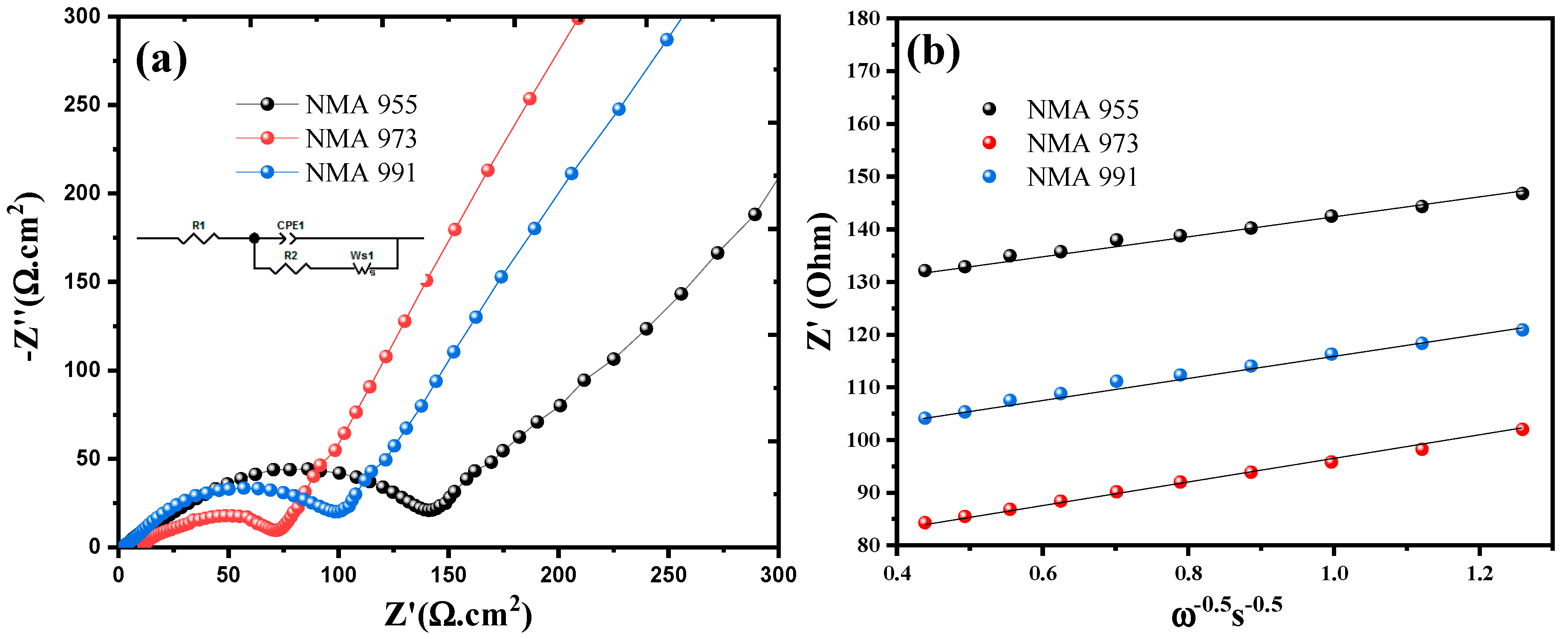
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of NMA
4.2. Material Characterization
4.3. Electrochemical Measurement
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [Green Version]
- Jeevanantham, B.; Shobana, M.K. Enhanced cathode materials for advanced lithium-ion batteries using nickel-rich and lithium/manganese-rich LiNixMnyCozO2. J. Energy Storage 2022, 54, 105353. [Google Scholar] [CrossRef]
- Zhao, H.; Lam, W.-Y.A.; Sheng, L.; Wang, L.; Bai, P.; Yang, Y.; Ren, D.; Xu, H.; He, X. Cobalt-Free Cathode Materials: Families and their Prospects. Adv. Energy Mater. 2022, 12, 2103894. [Google Scholar] [CrossRef]
- Noerochim, L.; Suwarno, S.; Idris, N.H.; Dipojono, H.K. Recent Development of Nickel-Rich and Cobalt-Free Cathode Materials for Lithium-Ion Batteries. Batteries 2021, 7, 84. [Google Scholar] [CrossRef]
- Gourley, S.W.D.; Or, T.; Chen, Z. Breaking Free from Cobalt Reliance in Lithium-Ion Batteries. iScience 2020, 23, 101505. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ye, Y.; Liu, T.; Xiao, Y.; Wang, C.; Wang, F.; Pan, F. Ni/Li Disordering in Layered Transition Metal Oxide: Electrochemical Impact, Origin, and Control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-Free Ni-Rich Single Crystal Positive Electrode Materials for Lithium Ion Batteries: Part I. Two-Step Lithiation Method for Al- or Mg-Doped LiNiO2. J. Electrochem. Soc. 2021, 168, 40531. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, W.; Fu, P.; Song, Y.; Song, D.; Shi, X.; Zhang, H.; Li, C.; Zhang, L.; Wang, D. Influence of core and shell components on the Ni-rich layered oxides with core–shell and dual-shell structures. Chem. Eng. J. 2020, 400, 125821. [Google Scholar] [CrossRef]
- Wang, R.; He, K.; Liu, J.; Liu, Z.; Lv, X.; Su, J.; Wen, Y. Enhanced structure stability and electrochemical performance of LiNiO2 by Li2SeO4 coating and gradient surface SeO32−/SeO42− doping. Surf. Coat. Technol. 2023, 465, 129587. [Google Scholar] [CrossRef]
- Xu, T.; Du, F.; Wu, L.; Fan, Z.; Shen, L.; Zheng, J. Boosting the electrochemical performance of LiNiO2 by extra low content of Mn-doping and its mechanism. Electrochim. Acta 2022, 417, 140345. [Google Scholar] [CrossRef]
- Wu, J.; Wen, Y.; Zhou, Q.; Wang, J.; Shen, L.; Zheng, J. Simultaneous Bulk Doping and Surface Coating of Sn to Boost the Electrochemical Performance of LiNiO2. ACS Appl. Energy Mater. 2023, 6, 3010–3019. [Google Scholar] [CrossRef]
- Mao, G.; Luo, J.; Zhou, Q.; Xiao, F.; Tang, R.; Li, J.; Zeng, L.; Wang, Y. Improved cycling stability of high nickel cathode material for lithium ion battery through Al- and Ti-based dual modification. Nanoscale 2021, 13, 18741–18753. [Google Scholar] [CrossRef]
- Shen, Y.; Yao, X.; Zhang, J.; Wang, S.; Zhang, D.; Yin, D.; Wang, L.; Zhang, Y.; Hu, J.; Cheng, Y.; et al. Sodium doping derived electromagnetic center of lithium layered oxide cathode materials with enhanced lithium storage. Nano Energy 2022, 94, 106900. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Gao, P. Synthesis and characterization of Nickel-rich layered LiNi1−xMnxO2 (x = 0.02, 0.05) cathodes for lithium-ion batteries. Electrochim. Acta 2022, 427, 140891. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Xu, W.; Lu, Y.; Ma, H.; Cheng, F.; Chen, J. Gradient doping Mg and Al to stabilize Ni-rich cathode materials for rechargeable lithium-ion batteries. J. Power Sources 2022, 535, 231445. [Google Scholar] [CrossRef]
- Xin, F.; Zhou, H.; Zong, Y.; Zuba, M.; Chen, Y.; Chernova, N.A.; Bai, J.; Pei, B.; Goel, A.; Rana, J.; et al. What is the Role of Nb in Nickel-Rich Layered Oxide Cathodes for Lithium-Ion Batteries? ACS Energy Lett. 2021, 6, 1377–1382. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Reissig, F.; Frankenstein, L.; Heidbüchel, M.; Winter, M.; Placke, T.; Schmuch, R. Magnesium Substitution in Ni-Rich NMC Layered Cathodes for High-Energy Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103045. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sakaebe, H.; Kageyama, H.; Tatsumi, K.; Arachi, Y.; Kamiyama, T. Changes in the structure and physical properties of the solid solution LiNi1−xMnxO2 with variation in its composition. J. Mater. Chem. 2003, 13, 590–595. [Google Scholar] [CrossRef]
- Kim, H.-G.; Myung, S.-T.; Lee, J.K.; Sun, Y.-K. Effects of manganese and cobalt on the electrochemical and thermal properties of layered Li[Ni0.52Co0.16+xMn0.32−x]O2 cathode materials. J. Power Sources 2011, 196, 6710–6715. [Google Scholar] [CrossRef]
- You, Y.; Celio, H.; Li, J.; Dolocan, A.; Manthiram, A. Modified High-Nickel Cathodes with Stable Surface Chemistry Against Ambient Air for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 6480–6485. [Google Scholar] [CrossRef]
- Nayak, P.K.; Grinblat, J.; Levi, M.; Levi, E.; Kim, S.; Choi, J.W.; Aurbach, D. Al Doping for Mitigating the Capacity Fading and Voltage Decay of Layered Li and Mn-Rich Cathodes for Li-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502398. [Google Scholar] [CrossRef]
- Brow, R.; Donakowski, A.; Mesnier, A.; Pereira, D.J.; Steirer, K.X.; Santhanagopalan, S.; Manthiram, A. Mechanical Pulverization of Co-Free Nickel-Rich Cathodes for Improved High-Voltage Cycling of Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 6996–7005. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.; Manthiram, A. High-Nickel NMA: A Cobalt-Free Alternative to NMC and NCA Cathodes for Lithium-Ion Batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Li, W.; Manthiram, A. Delineating the Roles of Mn, Al, and Co by Comparing Three Layered Oxide Cathodes with the Same Nickel Content of 70% for Lithium-Ion Batteries. Chem. Mater. 2022, 34, 629–642. [Google Scholar] [CrossRef]
- Essehli, R.; Parejiya, A.; Muralidharan, N.; Jafta, C.J.; Amin, R.; Dixit, M.B.; Bai, Y.; Liu, J.; Belharouak, I. Hydrothermal synthesis of Co-free NMA cathodes for high performance Li-ion batteries. J. Power Sources 2022, 545, 231938. [Google Scholar] [CrossRef]
- Castro-García, S.; Castro-Couceiro, A.; Señarís-Rodríguez, M.A.; Soulette, F.; Julien, C. Influence of aluminum doping on the properties of LiCoO2 and LiNi0.5Co0.5O2 oxides. Solid State Ion. 2003, 156, 15–26. [Google Scholar] [CrossRef]
- Li, W.; Reimers, J.N.; Dahn, J.R. In situ x-ray diffraction and electrochemical studies of Li1−xNiO2. Solid State Ion. 1993, 67, 123–130. [Google Scholar] [CrossRef]
- Bie, Y.; Yang, J.; Wang, J.; Zhou, J.; Nuli, Y. Li2O2 as a cathode additive for the initial anode irreversibility compensation in lithium-ion batteries. Chem. Commun. 2017, 53, 8324–8327. [Google Scholar] [CrossRef]
- Kim, T.; Ono, L.K.; Fleck, N.; Raga, S.R.; Qi, Y. Transition metal speciation as a degradation mechanism with the formation of a solid-electrolyte interphase (SEI) in Ni-rich transition metal oxide cathodes. J. Mater. Chem. A 2018, 6, 14449–14463. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.S.; Xu, K.; Jow, T.R. Understanding Formation of Solid Electrolyte Interface Film on LiMn2O4 Electrode. J. Electrochem. Soc. 2002, 149, A1521. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Wang, X.; Wu, Q.; Ju, B.; Liu, L.; Yang, X.; Wang, Y.; Bai, Y.; Yang, S. Ammonia Assisted Hydrothermal Synthesis of Monodisperse LiFePO4/C Microspheres as Cathode Material for Lithium Ion Batteries. J. Electrochem. Soc. 2011, 158, A1448–A1454. [Google Scholar] [CrossRef]
- Qian, D.; Gu, Y.; Chen, Y.; Liu, H.; Wang, J.; Zhou, H. Ultra-high specific capacity of Cr3+-doped Li4Ti5O12 at 1.55 V as anode material for lithium-ion batteries. Mater. Lett. 2019, 238, 102–106. [Google Scholar] [CrossRef]
- Noerochim, L.; Caesarendra, W.; Habib, A.; Widyastuti; Suwarno; Ni’mah, Y.L.; Subhan, A.; Prihandoko, B.; Kosasih, B. Role of TiO2 Phase Composition Tuned by LiOH on The Electrochemical Performance of Dual-Phase Li4Ti5O12-TiO2 Microrod as an Anode for Lithium-Ion Battery. Energies 2020, 13, 5251. [Google Scholar] [CrossRef]
- Karunawan, J.; Floweri, O.; Santosa, S.P.; Sumboja, A.; Iskandar, F. Stable layered-layered-spinel structure of the Li1.2Ni0.13Co0.13Mn0.54O2 cathode synthesized by ball-milling assisted solid-state method. J. Electroanal. Chem. 2022, 907, 116050. [Google Scholar] [CrossRef]
- Xiaoman Wang, H.-L.Z. Effect of Calcining Temperatures on the Electrochemical Performances of LiNi0.5Co0.2Mn0.3O2 Cathode Material for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2021, 16, 151011. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Celio, H.; Smith, P.; Dolocan, A.; Chi, M.; Manthiram, A. Mn versus Al in Layered Oxide Cathodes in Lithium-Ion Batteries: A Comprehensive Evaluation on Long-Term Cyclability. Adv. Energy Mater. 2018, 8, 1703154. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Li, X.; Zhu, Y.; Gao, P. Synthesis and characterization of Co-free NMA cathodes for fast charging lithium-ion batteries. J. Alloys Compd. 2023, 955, 170226. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An overview of modification strategies to improve LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode performance for automotive lithium-ion batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Pang, S.; Wang, Y.; Chen, T.; Shen, X.; Xi, X.; Liao, D. The effect of AlF3 modification on the physicochemical and electrochemical properties of Li-rich layered oxide. Ceram. Int. 2016, 42, 5397–5402. [Google Scholar] [CrossRef]
- Minnici, K.; Kwon, Y.H.; Huie, M.M.; de Simon, M.V.; Zhang, B.; Bock, D.C.; Wang, J.; Wang, J.; Takeuchi, K.J.; Takeuchi, E.S.; et al. High capacity Li-ion battery anodes: Impact of crystallite size, surface chemistry and PEG-coating. Electrochim. Acta 2018, 260, 235–245. [Google Scholar] [CrossRef]
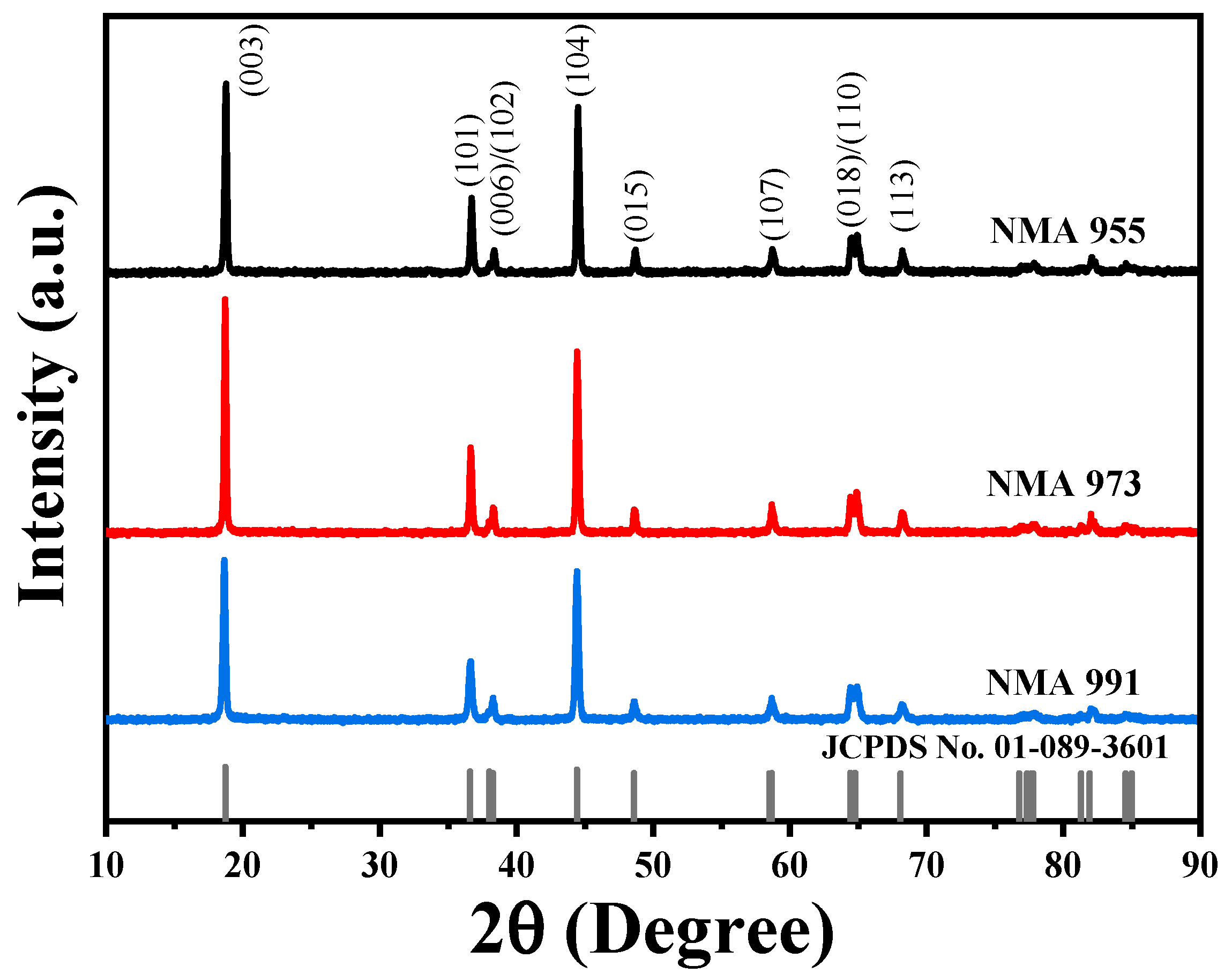

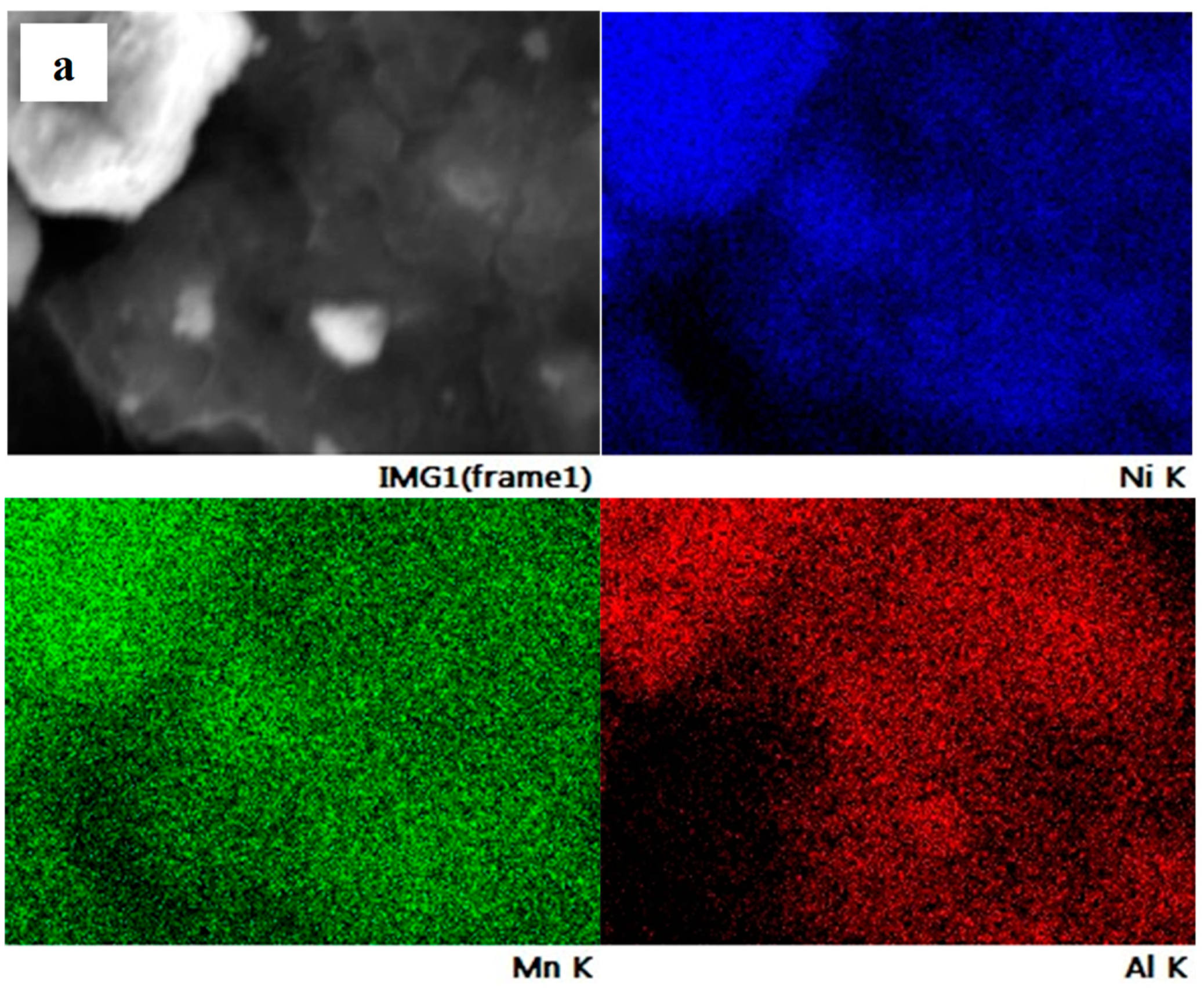

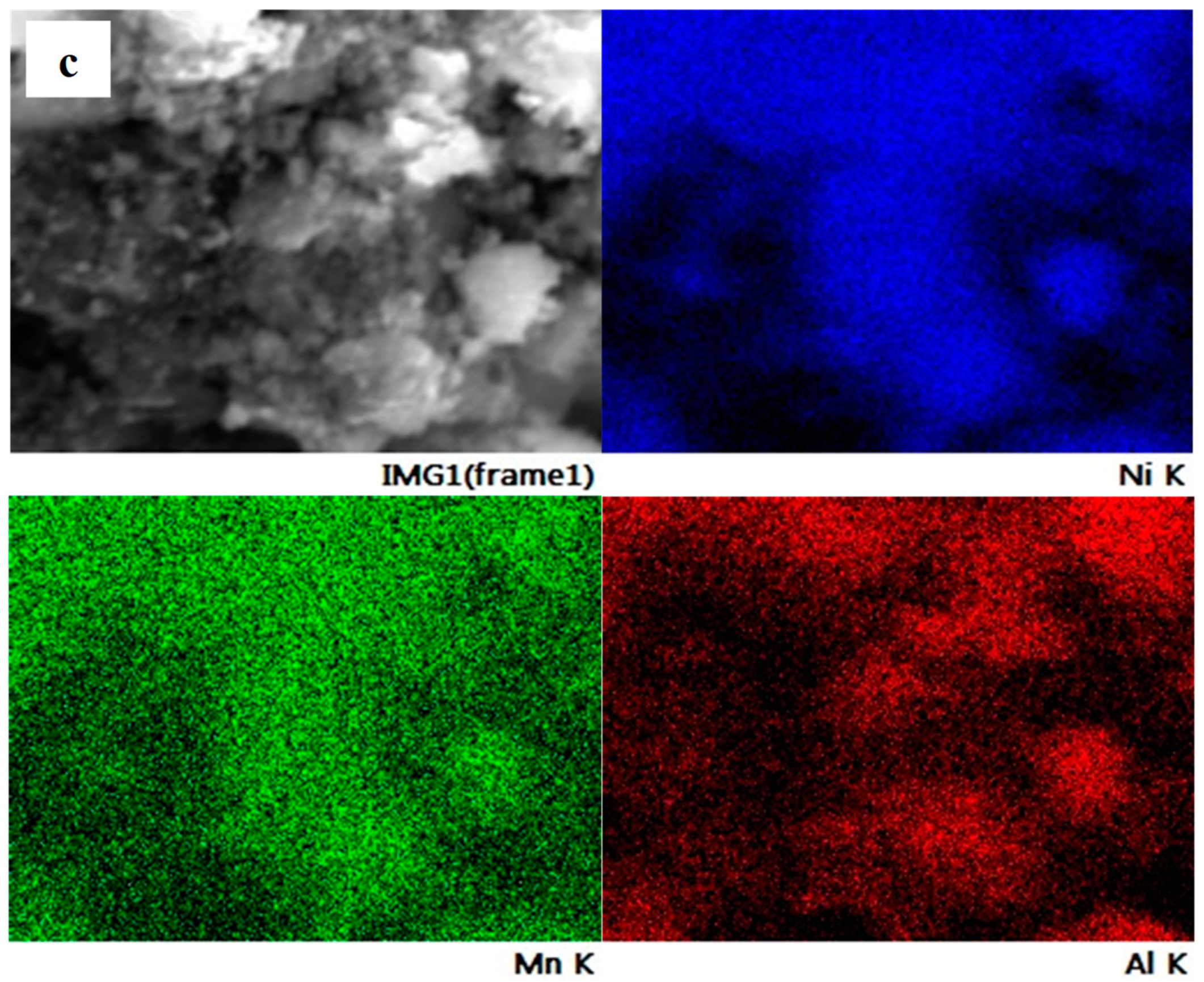
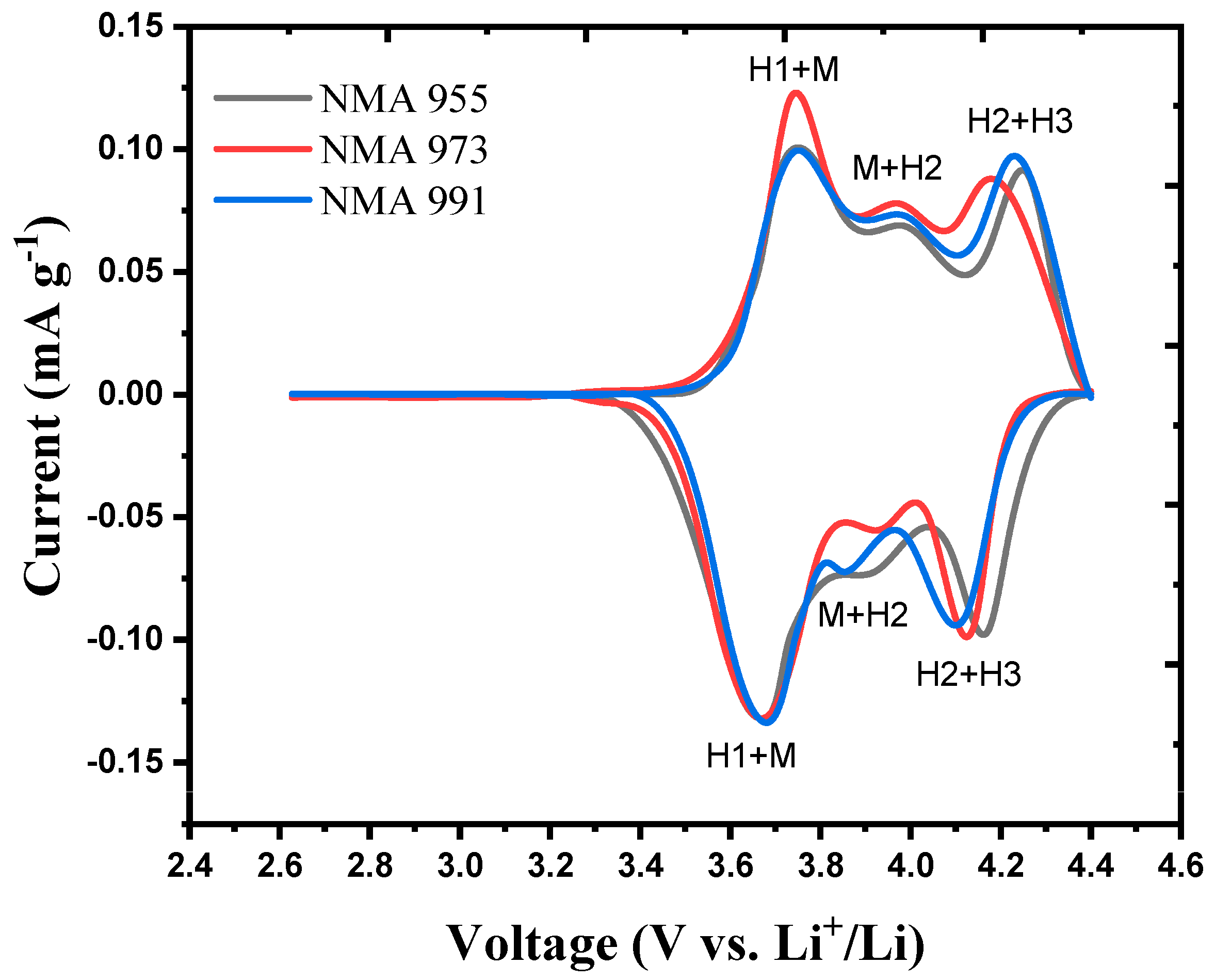
| Lattice Parameter | JCPDS No. 01-089-3601 | NMA 991 | NMA 973 | NMA 955 |
|---|---|---|---|---|
| a = b (Å) | 2.88 | 2.88 | 2.87 | 2.87 |
| c (Å) | 14.19 | 14.16 | 14.22 | 14.26 |
| Cathodes | c/a | I003/I104 |
|---|---|---|
| NMA 991 | 4.92 | 1.15 |
| NMA 973 | 4.95 | 1.36 |
| NMA 955 | 4.97 | 1.11 |
| Cathode | Rs (Ω cm−2) | Rct (Ω cm−2) | Li Diffusion (cm−2 s−1) |
|---|---|---|---|
| NMA 991 | 3.89 | 101.39 | 3.74 × 10−11 |
| NMA 973 | 2.51 | 71.58 | 4.27 × 10−10 |
| NMA 955 | 4.43 | 141.02 | 3.12 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noerochim, L.; Gunawan, E.A.; Pintowantoro, S.; Nurdiansah, H.; Adam, A.D.; Idris, N.H. High-Rate Capability of LiNi0.9Mn0.1−xAlxO2 (NMA) (x = 0.01, 0.03, 0.05) as Cathode for Lithium-Ion Batteries. Batteries 2023, 9, 420. https://doi.org/10.3390/batteries9080420
Noerochim L, Gunawan EA, Pintowantoro S, Nurdiansah H, Adam AD, Idris NH. High-Rate Capability of LiNi0.9Mn0.1−xAlxO2 (NMA) (x = 0.01, 0.03, 0.05) as Cathode for Lithium-Ion Batteries. Batteries. 2023; 9(8):420. https://doi.org/10.3390/batteries9080420
Chicago/Turabian StyleNoerochim, Lukman, Elsanti Anggraini Gunawan, Sungging Pintowantoro, Haniffudin Nurdiansah, Ariiq Dzurriat Adam, and Nurul Hayati Idris. 2023. "High-Rate Capability of LiNi0.9Mn0.1−xAlxO2 (NMA) (x = 0.01, 0.03, 0.05) as Cathode for Lithium-Ion Batteries" Batteries 9, no. 8: 420. https://doi.org/10.3390/batteries9080420






