Electroforming as a Novel One-Step Manufacturing Method of Structured Aluminum Foil Current Collectors for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
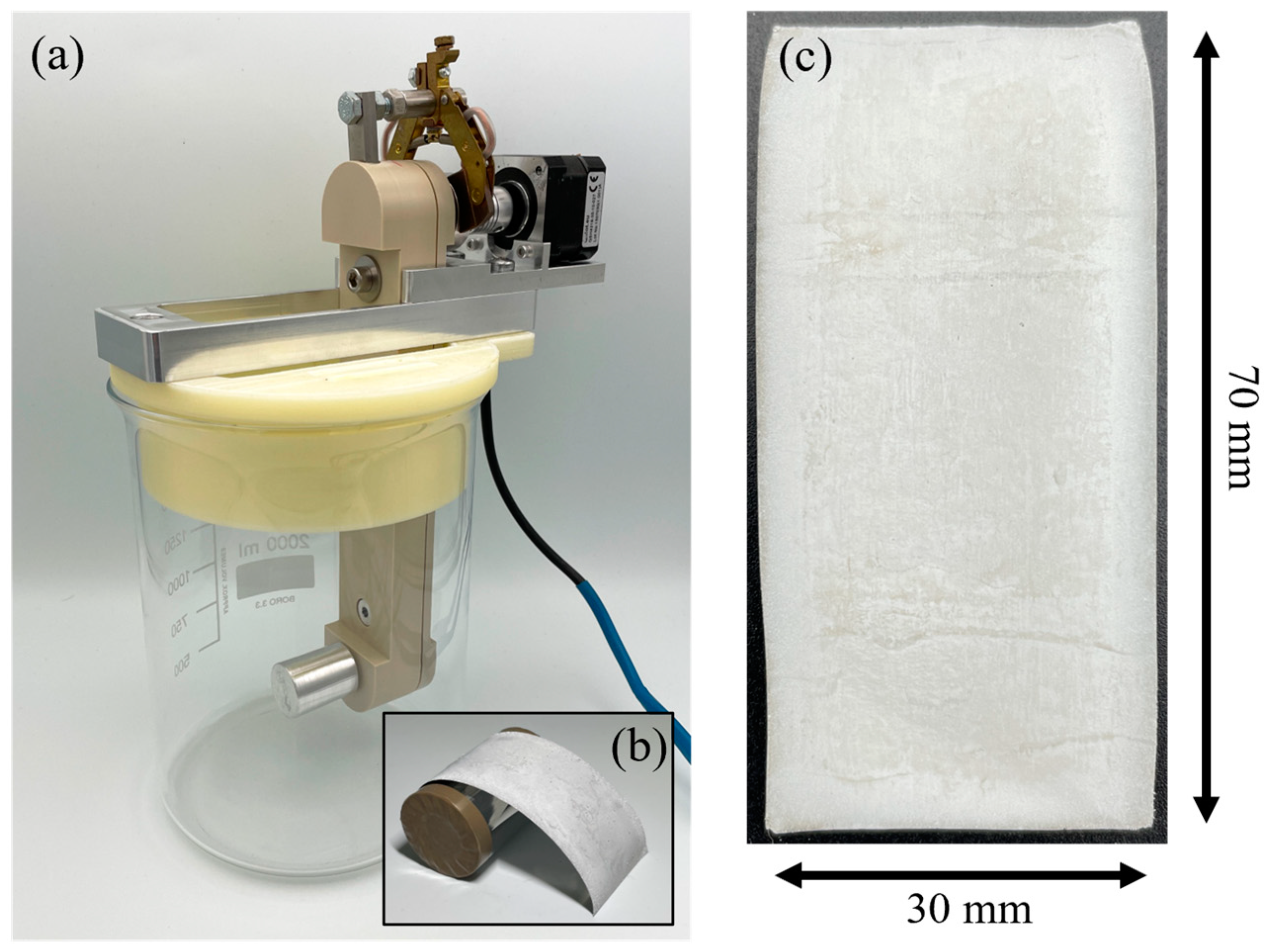
2.1. Materials and Chemicals
2.2. Preparation of the Electrolyte and Electrodes
2.3. Microstructural and Electrochemical Characterization
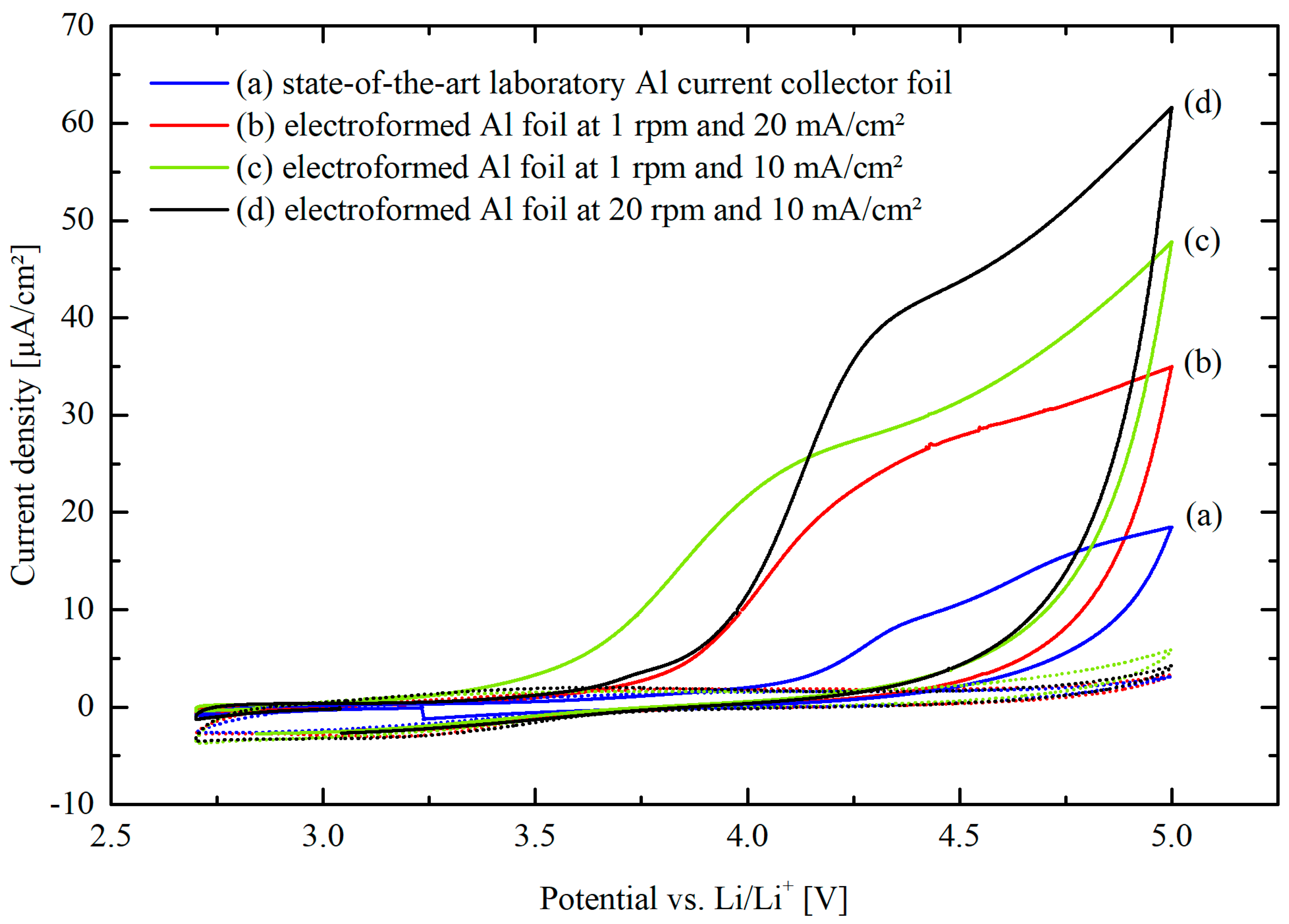
3. Results
3.1. Electroforming of Aluminum Foils
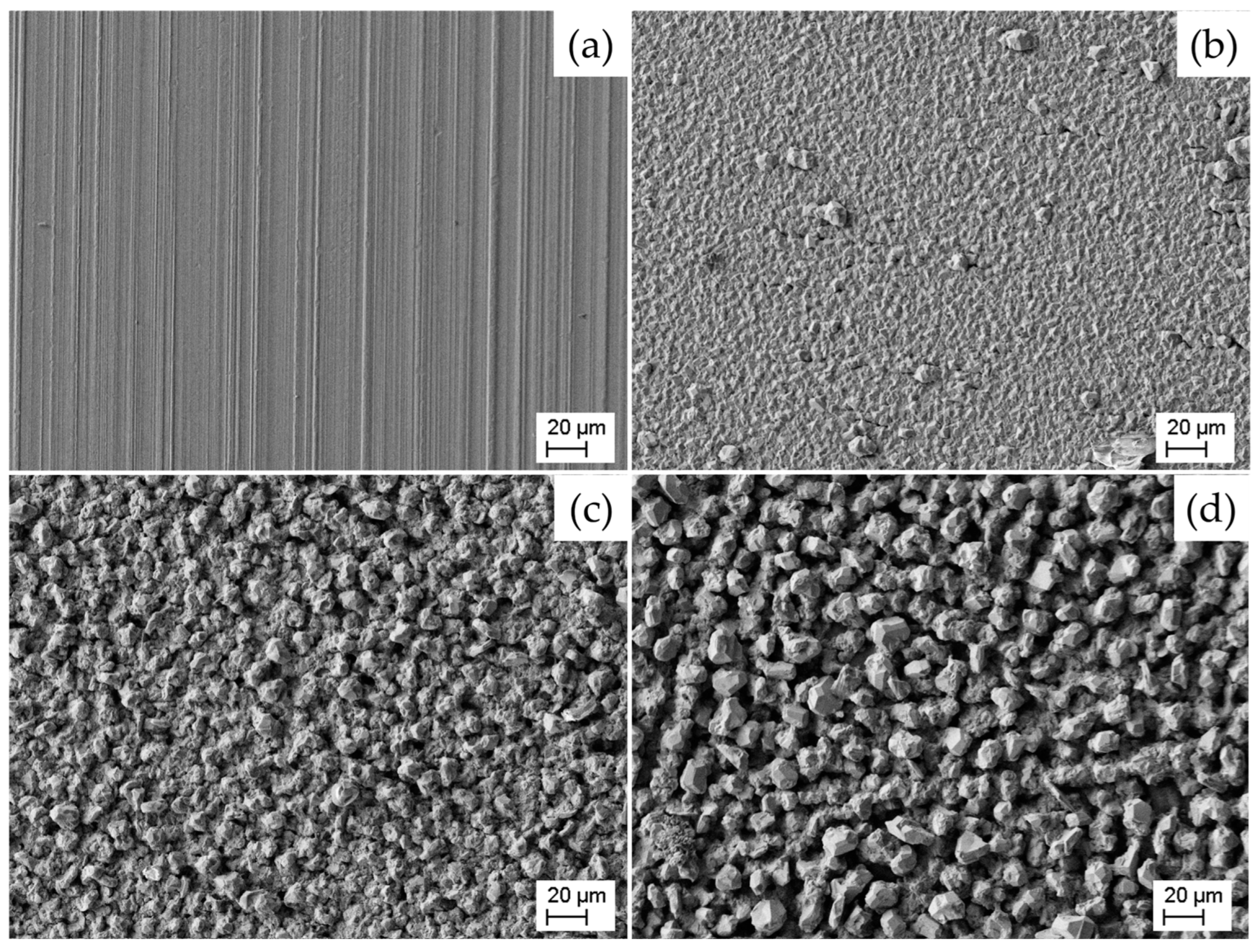
3.2. Microstructural Characterization
3.3. Electrochemical Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javed, M.S.; Zhong, D.; Ma, T.; Song, A.; Ahmed, S. Hybrid pumped hydro and battery storage for renewable energy based power supply system. Appl. Energy 2020, 257, 114026. [Google Scholar] [CrossRef]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering smart grid: A comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, Y.-L.; Cao, P.-F.; Lin, M.-C. Energy storage system: Current studies on batteries and power condition system. Renew. Sustain. Energy Rev. 2018, 82, 3091. [Google Scholar] [CrossRef]
- Hannan, M.A.; Wali, S.B.; Ker, P.J.; Rahman, M.A.; Mansor, M.; Ramachandaramurthy, V.K.; Muttaqi, K.M.; Mahlia, T.M.I.; Dong, Z.Y. Battery energy-storage system: A review of technologies, optimization objectives, constraints, approaches, and outstanding issues. J. Energy Storage 2021, 42, 103023. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, C.-Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.-Q.; Yu, D.; Liu, Y.; Titirici, M.-M.; Chueh, Y.-L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y.J. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. Mater. Chem. A 2019, 7, 2942. [Google Scholar]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries—Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Warner, J.T. Lithium-Ion Battery Chemistries. A Primer, 1st ed.; Elsevier: Cambridge, MA, USA, 2019. [Google Scholar]
- Zhang, Y.S.; Courtier, N.E.; Zhang, Z.; Liu, K.; Bailey, J.J.; Boyce, A.M.; Richardson, G.; Shearing, P.R.; Kendrick, E.; Brett, D.J.L. A Review of Lithium-Ion Battery Electrode Drying: Mechanisms and Metrology. Adv. Energy Mater. 2022, 12, 2102233. [Google Scholar] [CrossRef]
- Warner, J.T. The Handbook of Lithium-Ion Battery Pack Design. Chemistry, Components, Types and Terminology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Zhou, S.; Liu, G.; Ding, N.; Shang, L.; Dang, R.; Zhang, J. Improved performances of lithium-ion batteries by graphite-like carbon modified current collectors. Surf. Coat. Technol. 2020, 399, 126150. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Song, X.-F.; Yu, J.-G. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Manag. 2015, 46, 523. [Google Scholar] [CrossRef]
- Lain, M.J.; Brandon, J.; Kendrick, E. Design Strategies for High Power vs. High Energy Lithium Ion Cells. Batteries 2019, 5, 64. [Google Scholar] [CrossRef]
- Song, J.-M.; Zou, Y.-S.; Kuo, C.-C.; Lin, S.-C. Orientation dependence of the electrochemical corrosion properties of electrodeposited Cu foils. Corros. Sci. 2013, 74, 223. [Google Scholar] [CrossRef]
- Cao, Q.-D.; Fang, L.; Lv, J.-M.; Zhang, X.-P.; Thuy Dat, N. Effects of pulse reverse electroforming parameters on the thickness uniformity of electroformed copper foil. Trans. IMF 2018, 96, 108. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E.J. A review of current collectors for lithium-ion batteries. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Circuit Foil. Available online: https://www.circuitfoil.com (accessed on 19 July 2023).
- Parkinson, R. Nickel Plating and Electroforming: Essential Industries for Today and the Future; Technical Series N° 10 088; Nickel Development Institute: Toronto, ON, Canada, 2001; pp. 1–25. [Google Scholar]
- Heim, F.; Kreher, T.; Birke, K.P. The Influence of Micro-Structured Anode Current Collectors in Combination with Highly Concentrated Electrolyte on the Coulombic Efficiency of In-Situ Deposited Li-Metal Electrodes with Different Counter Electrodes. Batteries 2020, 6, 20. [Google Scholar] [CrossRef]
- Feng, H.; Chen, Y.; Wang, Y. Electrochemical Performance of a Lithium Ion Battery with Different Nanoporous Current Collectors. Batteries 2019, 5, 21. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, W.; Yao, B.; Zheng, F.; Smirnova, A.; Gu, Z. Mediating Lithium Plating/Stripping by Constructing 3D Au@Cu Pentagonal Pyramid Array. Batteries 2023, 9, 279. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Q.; Wang, Y.; Hou, G.; Zhang, J.; Tang, Y. Pulsed Current Constructs 3DM Cu/ZnO Current Collector Composite Anode for Free-Dendritic Lithium Metal Batteries. Batteries 2023, 9, 188. [Google Scholar] [CrossRef]
- Sörgel, Ş.; Kesten, O.; Wengel, A.; Sörgel, T. Nickel/sulfur composite electroplated nickel foams for the use as 3D cathode in lithium/sulfur batteries – A proof of concept. Energy Storage Mater. 2018, 10, 223–232. [Google Scholar] [CrossRef]
- Arnet, R.; El Mofid, W.; Sörgel, T. Combining 3D Printing and Electrochemical Deposition for Manufacturing Tailor-Made 3D Nickel Foams with Highly Competitive Porosity and Specific Surface Area Density. Metals 2023, 13, 857. [Google Scholar] [CrossRef]
- El Mofid, W.; Sörgel, T. Sulfur Loading as a Manufacturing Key Factor of Additive-Free Cathodes for Lithium-Sulfur Batteries Prepared by Composite Electroforming. Energies 2023, 16, 1134. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Tu, X.; Zhang, J.; Zhang, M.; Cai, Y.; Lang, H.; Tian, G.; Wang, Y. Influence of Operating Conditions on Deposition Rate and Smoothness of Electrolytic Aluminum Foil Using Chloroaluminate Ionic Liquids. RSC Adv. 2017, 7, 14790. [Google Scholar] [CrossRef]
- Ui, K.; Kobayashi, S.; Sasaki, K.; Takeguchi, T.; Tsuda, T.; Ueda, M.; Nunomura, J.; Honkawa, Y.; Kojima, Y. Influence of Operating Conditions on Deposition Rate and Smoothness of Electrolytic Aluminum Foil Using Chloroaluminate Ionic Liquids. J. Electrochem. Soc. 2021, 168, 56510. [Google Scholar] [CrossRef]
- Ui, K.; Kobayashi, S.; Takeguchi, T.; Tsuda, T.; Ueda, M.; Nunomura, J.; Honkawa, Y.; Oya, Y.; Kojima, Y. Influence of Pulse Electrolytic Conditions on Properties of Electrolytic Aluminum Foil Using Chloroaluminate Ionic Liquids. Meet. Abstr. 2021, MA2021-02, 723. [Google Scholar] [CrossRef]
- Ui, K.; Kobayashi, S.; Takeguchi, T.; Tsuda, T.; Ueda, M.; Nunomura, J.; Honkawa, Y.; Kojima, Y. Investigation on Operating Conditions Influencing the Aluminum Electrolysis Using Chloroaluminate Ionic Liquids. Meet. Abstr. 2020, MA2020-02, 3002. [Google Scholar] [CrossRef]
- Ui, K.; Kobayashi, S.; Kono, M.; Takeguchi, T.; Tsuda, T.; Ueda, M.; Nunomura, J.; Honkawa, Y.; Oya, Y.; Kojima, Y. (Digital Presentation) Influence of Pulse Electrolytic Conditions on Deposition Morphology of Electrolytic Aluminum Foil Using Chloroaluminate Ionic Liquids. ECS Trans. 2022, 109, 105. [Google Scholar] [CrossRef]
- Ui, K.; Kobayashi, S.; Mandai, T.; Takeguchi, T.; Tsuda, T.; Ueda, M.; Nunomura, J.; Honkawa, Y.; Kojima, Y. Influence of Electrolytic Condition on Surface Smoothness of Electrolytic Aluminum Foil from AlCl3-EMIC Melt. Meet. Abstr. 2019, MA2019-02, 961. [Google Scholar] [CrossRef]
- Ui, K.; Kobayashi, S.; Takeguchi, T.; Tsuda, T.; Ueda, M.; Nunomura, J.; Honkawa, Y.; Kojima, Y. Investigation on Operating Conditions Influencing the Aluminum Electrolysis Using Chloroaluminate Ionic Liquids. ECS Trans. 2020, 98, 223. [Google Scholar] [CrossRef]
- Ruan, S.; Schuh, C.A. Towards electroformed nanostructured aluminum alloys with high strength and ductility. J. Mater. Res. 2012, 27, 1638. [Google Scholar] [CrossRef]
- Xia, L.; Jiang, Y.; Pan, Y.; Li, S.; Wang, J.; He, Y.; Xia, Y.; Liu, Z.; Chen, G.Z. Lithium Bis(fluorosulfony)imide-Lithium Hexafluorophosphate Binary-Salt Electrolytes for Lithium-Ion Batteries: Aluminum Corrosion Behaviors and Electrochemical Properties. ChemistrySelect 2018, 3, 1954. [Google Scholar] [CrossRef]
- Myung, S.-T.; Sasaki, Y.; Sakurada, S.; Sun, Y.-K.; Yashiro, H. Electrochemical behavior of current collectors for lithium batteries in non-aqueous alkyl carbonate solution and surface analysis by ToF-SIMS. Electrochim. Acta 2009, 55, 288. [Google Scholar] [CrossRef]
- Kanamura, K.; Okagawa, T.; Takehara, Z. Electrochemical oxidation of propylene carbonate (containing various salts) on aluminium electrodes. J. Power Sources 1995, 57, 119. [Google Scholar] [CrossRef]
- Zhang, X.; Devine, T.M. Identity of Passive Film Formed on Aluminum in Li-Ion Battery Electrolytes with LiPF6. J. Electrochem. Soc. 2006, 153, B344. [Google Scholar] [CrossRef]
- Streipert, B.; Röser, S.; Kasnatscheew, J.; Janßen, P.; Cao, X.; Wagner, R.; Cekic-Laskovic, I.; Winter, M.J. Influence of LiPF6 on the Aluminum Current Collector Dissolution in High Voltage Lithium Ion Batteries after Long-Term Charge/Discharge Experiments. Electrochem. Soc. 2017, 164, 1474. [Google Scholar] [CrossRef]
- Egashira, M.; Takahashi, H.; Okada, S.; Yamaki, J.-I. Measurement of the electrochemical oxidation of organic electrolytes used in lithium batteries by microelectrode. J. Power Sources 2001, 92, 267. [Google Scholar] [CrossRef]
- Fritsch, M.; Standke, G.; Heubner, C.; Langklotz, U.; Michaelis, A.J. 3D-cathode design with foam-like aluminum current collector for high energy density lithium-ion batteries. Energy Storage 2018, 16, 125. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, T.; Gunji, T.; Wu, J.; Matsumoto, F. Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries. Electrochem 2020, 1, 124–159. [Google Scholar] [CrossRef]
- Plieth, W. Der Galvanische Prozess. Grundlagen der Metallabscheidung und Strukturbildung, 1st ed.; Leuze Verlag: Bad Saulgau, Germany, 2018. [Google Scholar]
- Paunovic, M.; Schlesinger, M. Fundamentals of Electrochemical Deposition, 1st ed.; Wiley: New York, NY, USA; Weinheim, Germany, 1998. [Google Scholar]
- Plieth, W. Electrochemistry for Materials Science, 1st ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2008. [Google Scholar]
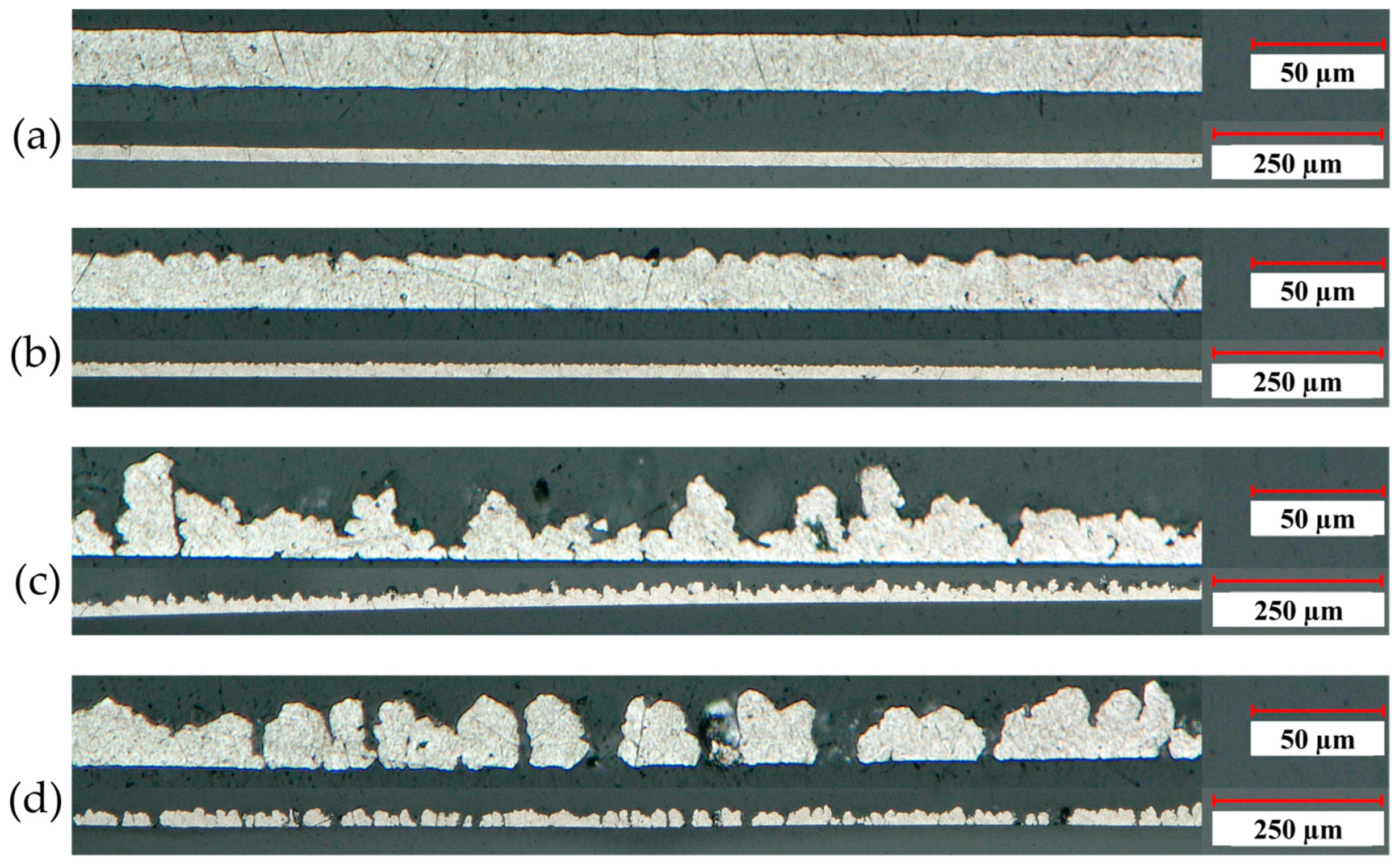
 ) show the position of the measured profile lines from Figure 4.
) show the position of the measured profile lines from Figure 4.
 ) show the position of the measured profile lines from Figure 4.
) show the position of the measured profile lines from Figure 4.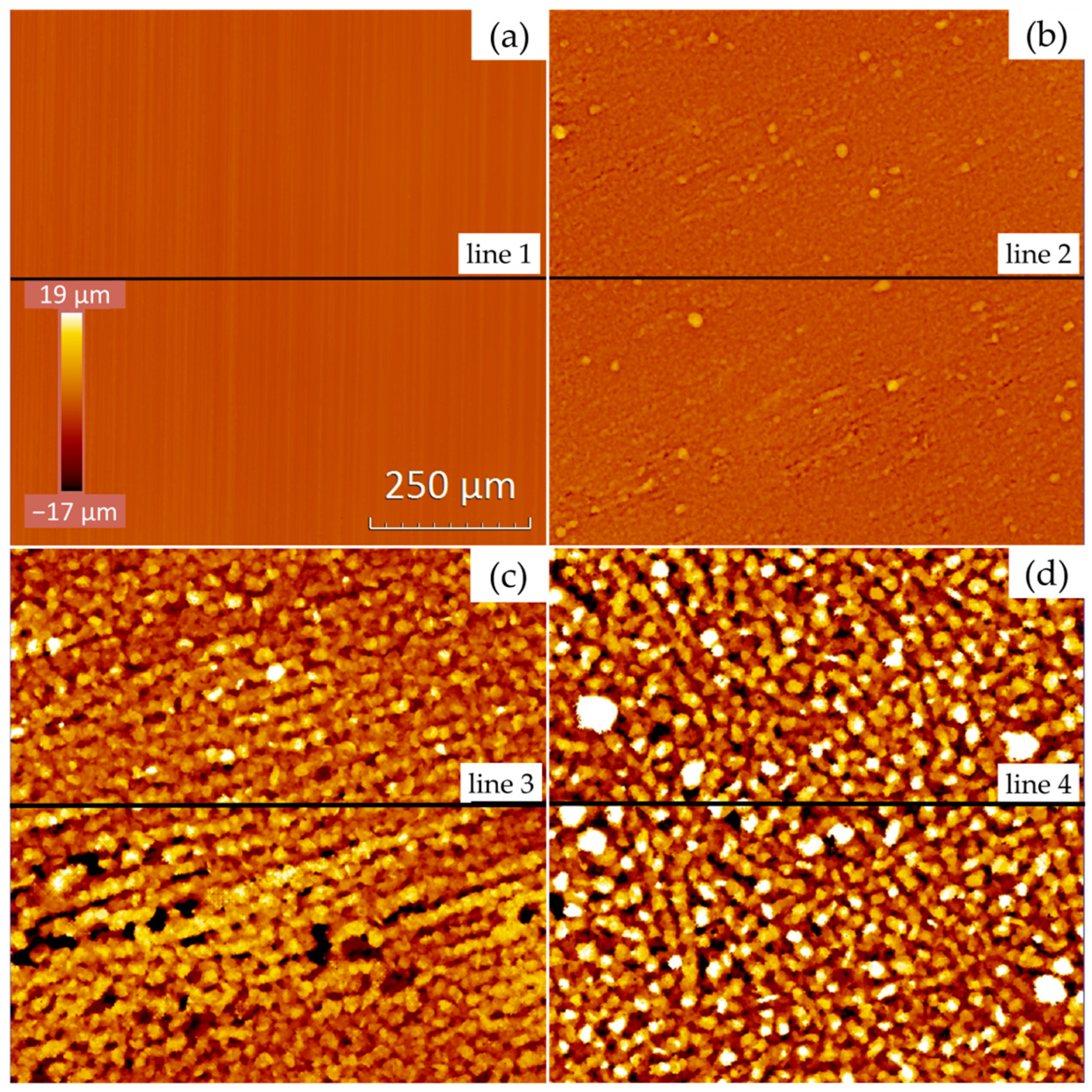

| Substrate Rotation Speed [rpm] | Current Density [mA/cm2] |
|---|---|
| 20 | 15 |
| 20 | 10 |
| 20 | 5 |
| 10 | 15 |
| 10 | 10 |
| 10 | 5 |
| 1 | 15 |
| 1 | 10 |
| 1 | 5 |
| 1 | 20 |
| Image | Spv [µm] | Sq [µm] | Sa [µm] |
|---|---|---|---|
| (a) | 6.8 | 0.35 | 0.279 |
| (b) | 29.1 | 1.38 | 0.984 |
| (c) | 58.9 | 7.55 | 6.014 |
| (d) | 96.6 | 10.92 | 8.783 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherzl, P.; Kaupp, M.; El Mofid, W.; Sörgel, T. Electroforming as a Novel One-Step Manufacturing Method of Structured Aluminum Foil Current Collectors for Lithium-Ion Batteries. Batteries 2023, 9, 422. https://doi.org/10.3390/batteries9080422
Scherzl P, Kaupp M, El Mofid W, Sörgel T. Electroforming as a Novel One-Step Manufacturing Method of Structured Aluminum Foil Current Collectors for Lithium-Ion Batteries. Batteries. 2023; 9(8):422. https://doi.org/10.3390/batteries9080422
Chicago/Turabian StyleScherzl, Phillip, Michael Kaupp, Wassima El Mofid, and Timo Sörgel. 2023. "Electroforming as a Novel One-Step Manufacturing Method of Structured Aluminum Foil Current Collectors for Lithium-Ion Batteries" Batteries 9, no. 8: 422. https://doi.org/10.3390/batteries9080422
APA StyleScherzl, P., Kaupp, M., El Mofid, W., & Sörgel, T. (2023). Electroforming as a Novel One-Step Manufacturing Method of Structured Aluminum Foil Current Collectors for Lithium-Ion Batteries. Batteries, 9(8), 422. https://doi.org/10.3390/batteries9080422







