Tetraethylene Glycol Dimethyl Ether (TEGDME)-Water Hybrid Electrolytes Enable Excellent Cyclability in Aqueous Zn-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
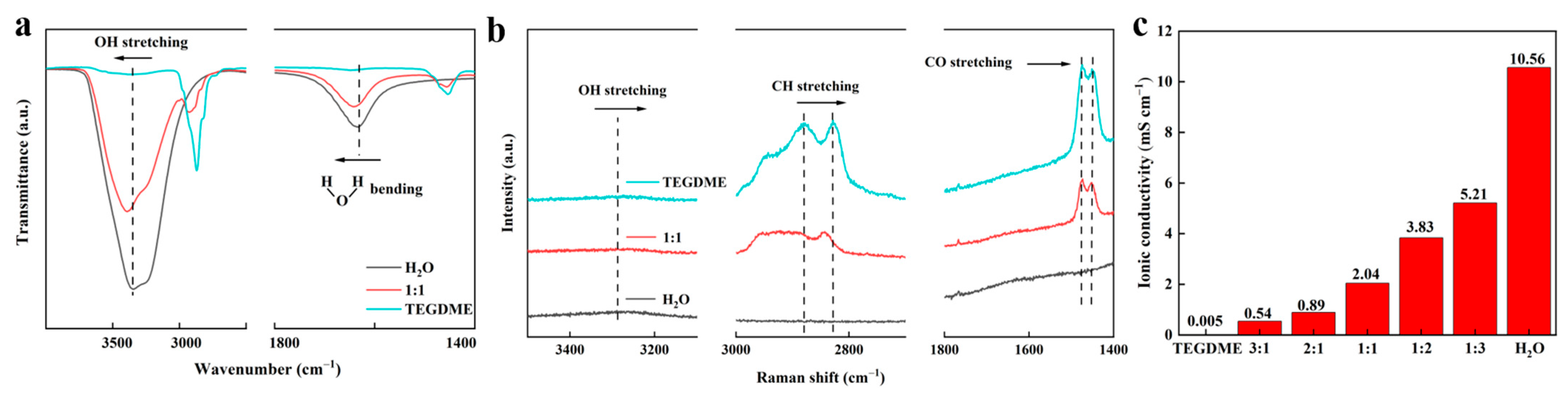
3.1. Properties of 1 M Zn(CF3SO3)2 TEGDME/H2O Hybrid Electrolyte
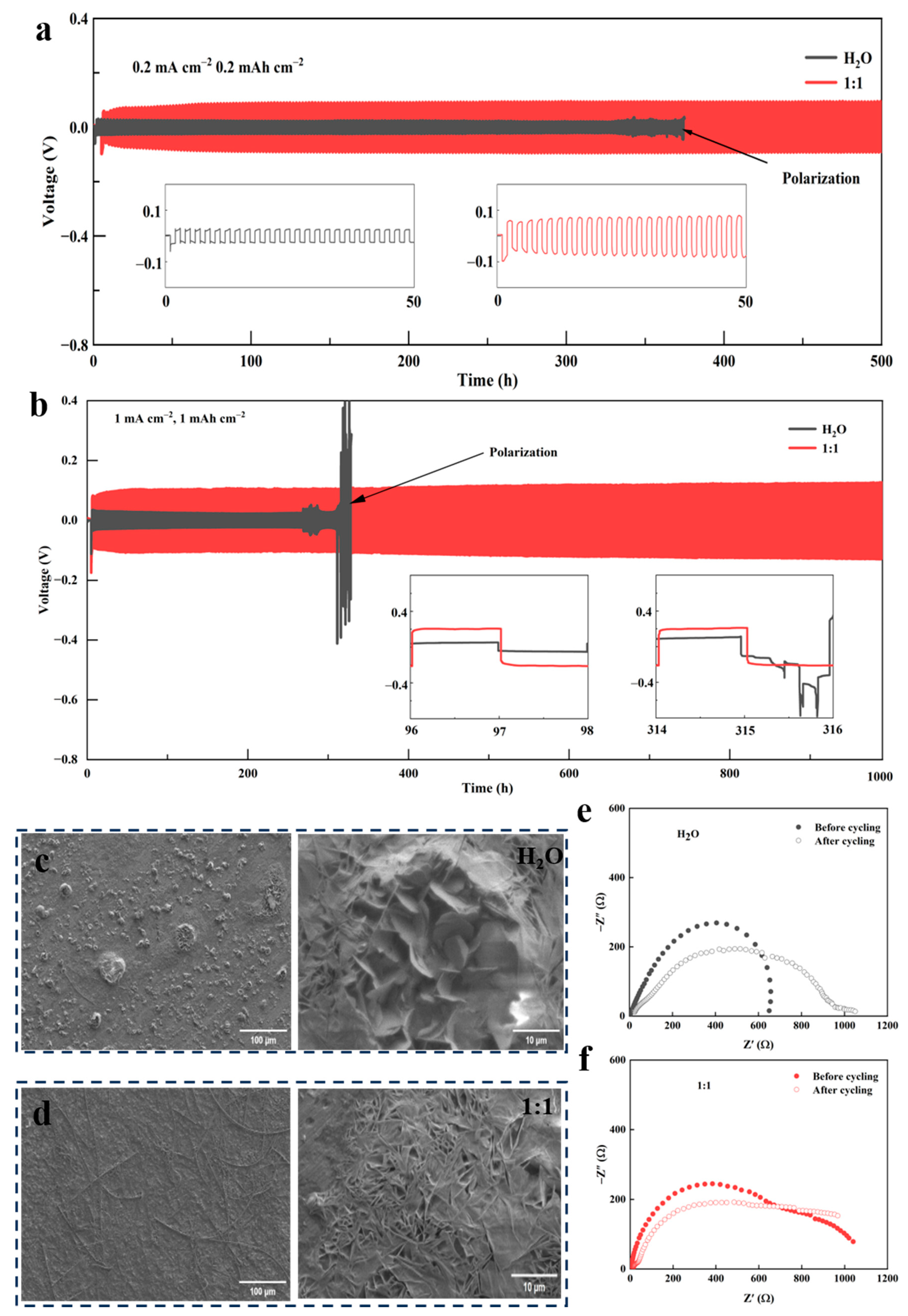
3.2. Electrochemical Properties of Various Hybrid Electrolytes
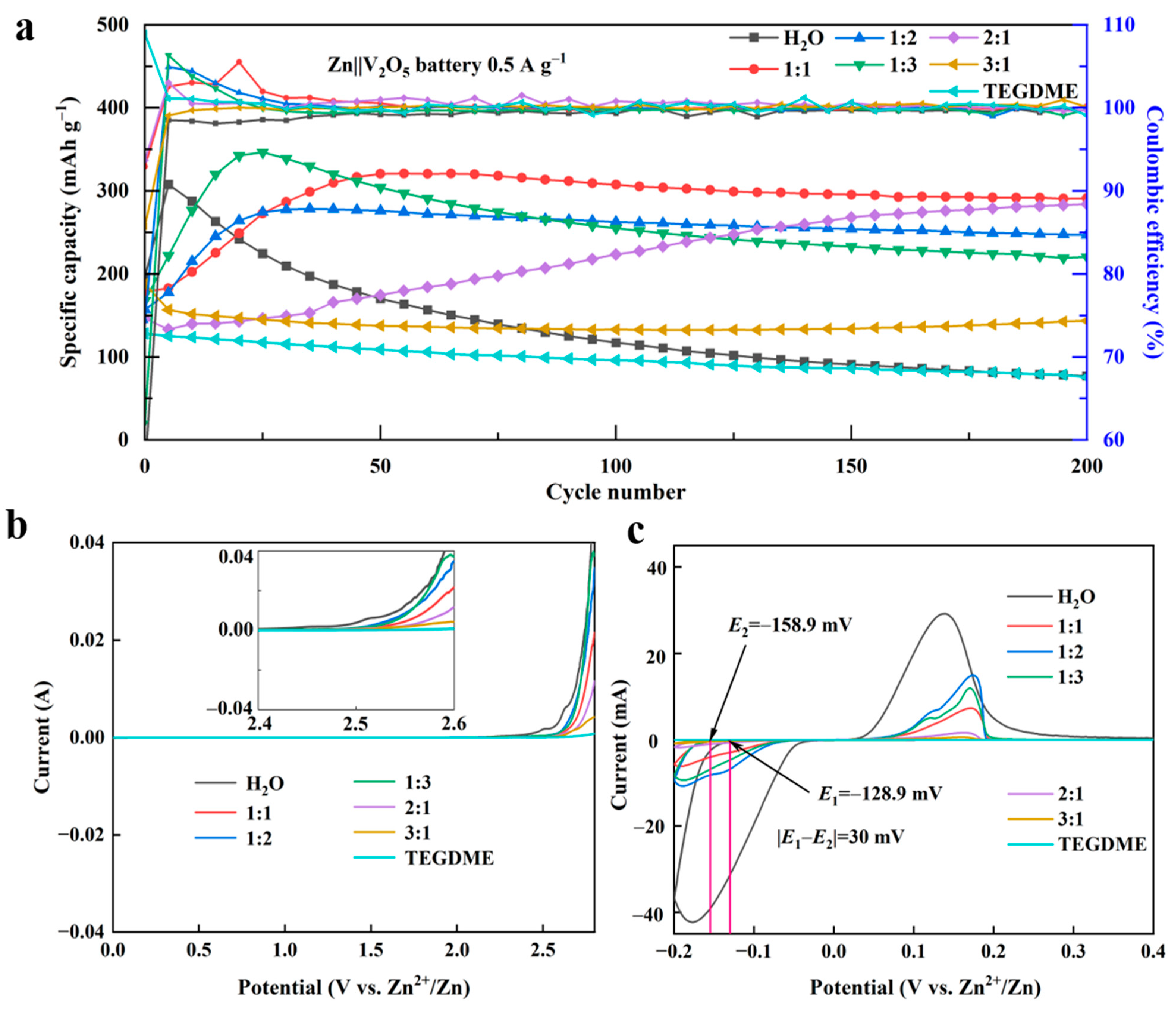
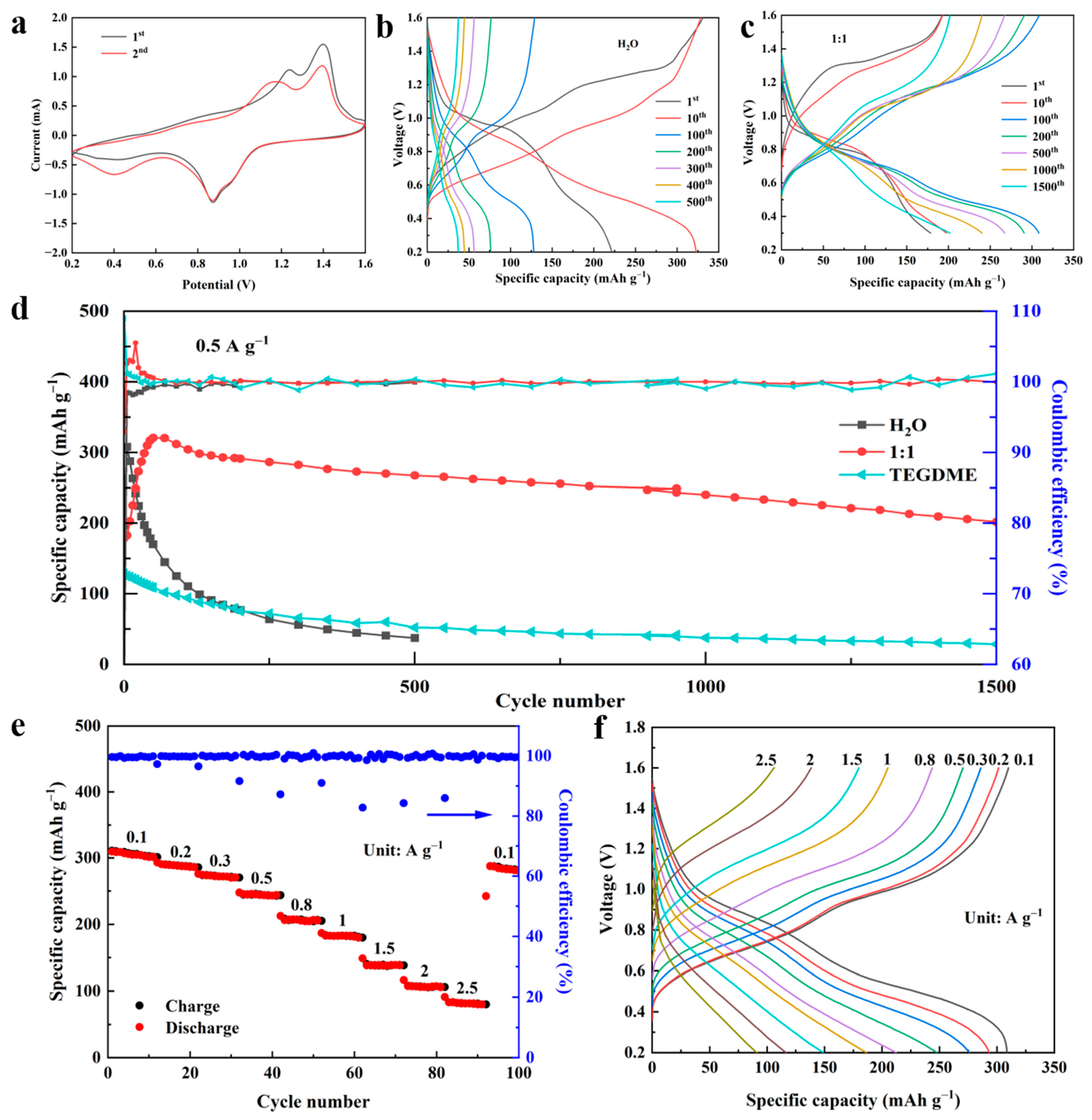
3.3. Characterization of Zn||V2O5 Batteries
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, H.; Li, J.; Yuan, Y.; Wang, J.; Lu, J.; Wang, S. Recent Progress in Biomass-Derived Electrode Materials for High Volumetric Performance Supercapacitors. Adv. Energy Mater. 2018, 8, 1801007. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef]
- Li, T.C.; Fang, D.; Zhang, J.; Pam, M.E.; Leong, Z.Y.; Yu, J.; Li, X.L.; Yan, D.; Yang, H.Y. Recent progress in aqueous zinc-ion batteries: A deep insight into zinc metal anodes. J. Mater. Chem. A 2021, 9, 6013. [Google Scholar] [CrossRef]
- Yao, M.; Yuan, Z.; Li, S.; He, T.; Wang, R.; Yuan, M.; Niu, Z. Scalable Assembly of Flexible Ultrathin All-in-One Zinc-Ion Batteries with Highly Stretchable, Editable, and Customizable Functions. Adv. Mater. 2021, 33, 2008140. [Google Scholar] [CrossRef]
- He, M.; Shu, C.; Hu, A.; Zheng, R.; Li, M.; Ran, Z.; Long, J. Suppressing dendrite growth and side reactions on Zn metal anode via guiding interfacial anion/cation/H2O distribution by artificial multi-functional interface layer. Energy Storage Mater. 2022, 44, 452–460. [Google Scholar] [CrossRef]
- Yan, H.; Li, S.; Xu, H.; Chen, H.; Yang, S.; Li, B. Triggering Zn2+ Unsaturated Hydration Structure via Hydrated Salt Electrolyte for High Voltage and Cycling Stable Rechargeable Aqueous Zn Battery. Adv. Energy Mater. 2022, 12, 2201599. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ma, L.; Huang, W. Solvation Structures in Aqueous Metal-Ion Batteries. Adv. Energy Mater. 2022, 12, 2202068. [Google Scholar] [CrossRef]
- Dong, C.; Xu, F.; Chen, L.; Chen, Z.; Cao, Y. Design Strategies for High-Voltage Aqueous Batteries. Small Struct. 2021, 2, 2100001. [Google Scholar] [CrossRef]
- Li, B.; Xue, J.; Han, C.; Liu, N.; Ma, K.; Zhang, R.; Wu, X.; Dai, L.; Wang, L.; He, Z. A hafnium oxide-coated dendrite-free zinc anode for rechargeable aqueous zinc-ion batteries. J. Colloid Interf. Sci. 2021, 599, 467–475. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, S.; Lai, Y.; Ding, M.; Cao, Y.; Chen, Z. A stable “rocking-chair” zine-ion battery boosted by low-strain Zn3V4(PO4)6 cathode. Nano Energy 2022, 100, 107520. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 13180–13191. [Google Scholar] [CrossRef]
- Meng, C.; He, W.; Tan, H.; Wu, X.; Liu, H.; Wang, J. A eutectic electrolyte for an ultralong-lived Zn//V2O5 cell: An in situ generated gradient solid electrolyte interphase. Energy Environ. Sci. 2023, 16, 3587–3599. [Google Scholar] [CrossRef]
- Hu, P.; Yan, M.; Zhu, T.; Wang, X.; Wei, X.; Li, J.; Zhou, L.; Li, Z.; Chen, L.; Mai, L. Zn/V2O5 Aqueous Hybrid-Ion Battery with High Voltage Platform and Long Cycle Life. ACS Appl. Mater. Interfaces 2017, 9, 42717–42722. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zou, Y.; Liu, G.; Wang, Y.; Yin, J.; Ming, J.; Alshareef, H.N. Electrolyte Solvation Structure Design for Sodium Ion Batteries. Adv. Sci. 2022, 9, 2201207. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, X.; Lu, B.; Zhou, J.; Liang, S. Electrolyte Strategies toward Better Zinc-Ion Batteries. ACS Energy Lett. 2021, 6, 1015–1033. [Google Scholar] [CrossRef]
- Wang, C.; Pei, Z.; Meng, Q.; Zhang, C.; Sui, X.; Yuan, Z.; Wang, S.; Chen, Y. Toward Flexible Zinc-Ion Hybrid Capacitors with Superhigh Energy Density and Ultralong Cycling Life: The Pivotal Role of ZnCl2 Salt-Based Electrolytes. Angew. Chem. Int. Ed. 2021, 60, 990–997. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, K.; Ma, Y.; Lu, Y.; Li, L.; Liang, J.; Chou, S.; Chen, J. Chaotropic Anion and Fast-Kinetics Cathode Enabling Low-Temperature Aqueous Zn Batteries. ACS Energy Lett. 2021, 6, 2704–2712. [Google Scholar] [CrossRef]
- Cao, X.; Gao, P.; Ren, X.; Zou, L.; Engelhard, M.H.; Matthews, B.E.; Hu, J.; Niu, C.; Liu, D.; Arey, B.W.; et al. Effects of fluorinated solvents on electrolyte solvation structures and electrode/electrolyte interphases for lithium metal batteries. Proc. Natl. Acad. Sci. USA 2021, 118, e2020357118. [Google Scholar] [CrossRef]
- Shi, J.; Xia, K.; Liu, L.; Liu, C.; Zhang, Q.; Li, L.; Zhou, X.; Liang, J.; Tao, Z. Ultrahigh coulombic efficiency and long-life aqueous Zn anodes enabled by electrolyte additive of acetonitrile. Electrochim. Acta 2020, 358, 136937. [Google Scholar] [CrossRef]
- Nian, Q.; Wang, J.; Liu, S.; Sun, T.; Zheng, S.; Zhang, Y.; Tao, Z.; Chen, J. Aqueous Batteries Operated at −50 degrees C. Angew. Chem. Int. Ed. 2019, 58, 16994–16999. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, K.; Huo, W.; Wang, Y.; Yao, G.; Gu, X.; Cheng, H.; Mai, L.; Hu, C.; Wang, X. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy 2019, 69, 275–281. [Google Scholar] [CrossRef]
- Chang, N.; Li, T.; Li, R.; Wang, S.; Yin, Y.; Zhang, H.; Li, X. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices. Energy Environ. Sci. 2020, 13, 3527. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, T.; Li, X.; Cui, H.; Liang, G.; Yang, Q.; Chen, Z.; Chen, A.; Guo, Y.; Fan, J.; et al. Small-Dipole-Molecule-Containing Electrolytes for High-Voltage Aqueous Rechargeable Batteries. Adv. Mater. 2022, 34, 2106180. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Su, B.; Sun, M.; Gu, S.; Lu, Z.; Zhang, K.; Yu, D.Y.W.; Huang, B.; Wang, P.; Lee, C.S.; et al. Dilute Aqueous-Aprotic Hybrid Electrolyte Enabling a Wide Electrochemical Window through Solvation Structure Engineering. Adv. Mater. 2021, 33, 2102390. [Google Scholar] [CrossRef]
- Li, C.; Wu, W.; Shi, H.Y.; Qin, Z.; Yang, D.; Yang, X.; Song, Y.; Guo, D.; Liu, X.X.; Sun, X. The energy storage behavior of a phosphate-based cathode material in rechargeable zinc batteries. Chem. Commun. 2021, 57, 6253. [Google Scholar] [CrossRef]
- Shi, H.Y.; Song, Y.; Qin, Z.; Li, C.; Guo, D.; Liu, X.X.; Sun, X. Inhibiting VOPO4·xH2O Decomposition and Dissolution in Rechargeable Aqueous Zinc Batteries to Promote Voltage and Capacity Stabilities. Angew. Chem. Int. Ed. 2019, 58, 16057–16061. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhao, G.; Hong, H.; Tang, Z.; Xu, X.; Li, H.; Zhi, C.; Han, C. Ether-Water Hybrid Electrolyte Contributing to Excellent Mg Ion Storage in Layered Sodium Vanadate. ACS Nano 2022, 16, 6093–6102. [Google Scholar] [CrossRef]
- Li, M.; Hicks, R.P.; Chen, Z.; Luo, C.; Guo, J.; Wang, C.; Xu, Y. Electrolytes in Organic Batteries. Chem. Rev. 2023, 123, 1712–1773. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Rao, M.M.; Venugopal, N.; Kim, K.-B. Hydrothermal synthesis of SnO2–V2O5 mixed oxide and electrochemical screening of carbon nano-tubes (CNT), V2O5, V2O5–CNT, and SnO2–V2O5–CNT electrodes for supercapacitor applications. J. Power Sources 2007, 166, 578–583. [Google Scholar] [CrossRef]
- Wei, T.T.; Peng, Y.Q.; Mo, L.E.; Chen, S.H.; Ghadari, R.; Li, Z.Q.; Hu, L.H. Modulated bonding interaction in propanediol electrolytes toward stable aqueous zinc-ion batteries. Sci. China Mater. 2022, 65, 1156–1164. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Ji, H.; Liu, J.; Qian, T.; Yan, C. Single-Atom Iron as Lithiophilic Site To Minimize Lithium Nucleation Overpotential for Stable Lithium Metal Full Battery. ACS Appl. Mater. Interfaces 2019, 11, 32008–32014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938. [Google Scholar] [CrossRef]
- Li, F.; Yu, L.; Hu, Q.; Guo, S.; Mei, Y.; Liu, Q.; He, Y.; Hu, X. Fabricating low-temperature-tolerant and durable Zn-ion capacitors via modulation of co-solvent molecular interaction and cation solvation. Sci. China Mater. 2021, 64, 1609–1620. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, N.; Li, Y.; Chen, S.; Lai, J.; Huang, Y.; Qu, W.; Wu, F.; Chen, R. An “Ether-In-Water” Electrolyte Boosts Stable Interfacial Chemistry for Aqueous Lithium-Ion Batteries. Adv. Mater. 2020, 32, 2004017. [Google Scholar] [CrossRef] [PubMed]
- Tot, A.; Zhang, L.; Berg, E.J.; Svensson, P.H.; Kloo, L. Water-in-salt electrolytes made saltier by Gemini ionic liquids for highly efficient Li-ion batteries. Sci. Rep. 2023, 13, 2154. [Google Scholar] [CrossRef]
- Hendricks, C.; Williard, N.; Mathew, S.; Pecht, M. A failure modes, mechanisms, and effects analysis (FMMEA) of lithium-ion batteries. J. Power Sources 2015, 297, 113–120. [Google Scholar] [CrossRef]
- Meng, C.; He, W.; Kong, Z.; Liang, Z.; Zhao, H.; Lei, Y.; Wu, Y.; Hao, X. Multifunctional water-organic hybrid electrolyte for rechargeable zinc ions batteries. Chem. Eng. J. 2022, 450, 138265. [Google Scholar] [CrossRef]
- He, H.; Tong, H.; Song, X.; Song, X.; Liu, J. Highly stable Zn metal anodes enabled by atomic layer deposited Al2O3 coating for aqueous zinc-ion batteries. J. Mater. Chem. A 2020, 8, 7836. [Google Scholar] [CrossRef]
- Tatara, R.; Karayaylali, P.; Yu, Y.; Zhang, Y.; Giordano, L.; Maglia, F.; Jung, R.; Schmidt, J.P.; Lund, I.; Shao-Horn, Y. The Effect of Electrode-Electrolyte Interface on the Electrochemical Impedance Spectra for Positive Electrode in Li-Ion Battery. J. Electrochem. Soc. 2019, 166, A5090–A5098. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Z.; Shen, D.; Chen, L.; Song, T.; Kang, T.; Tong, Z.; Tang, Y.; Wang, H.; Lee, C.S. Electrolyte engineering enables stable Zn-Ion deposition for long-cycling life aqueous Zn-ion batteries. Energy Storage Mater. 2022, 45, 1084–1091. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Kalambate, P.K.; Zhong, Y.; Huang, Z.; Xie, M.; Shen, Y.; Huang, Y. V2O5 nanopaper as a cathode material with high capacity and long cycle life for rechargeable aqueous zinc-ion battery. Nano Energy 2019, 60, 752–759. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shangguan, M.; Wang, K.; Zhao, Y.; Xia, L. Tetraethylene Glycol Dimethyl Ether (TEGDME)-Water Hybrid Electrolytes Enable Excellent Cyclability in Aqueous Zn-Ion Batteries. Batteries 2023, 9, 462. https://doi.org/10.3390/batteries9090462
Shangguan M, Wang K, Zhao Y, Xia L. Tetraethylene Glycol Dimethyl Ether (TEGDME)-Water Hybrid Electrolytes Enable Excellent Cyclability in Aqueous Zn-Ion Batteries. Batteries. 2023; 9(9):462. https://doi.org/10.3390/batteries9090462
Chicago/Turabian StyleShangguan, Mingliang, Kehuang Wang, Yibo Zhao, and Lan Xia. 2023. "Tetraethylene Glycol Dimethyl Ether (TEGDME)-Water Hybrid Electrolytes Enable Excellent Cyclability in Aqueous Zn-Ion Batteries" Batteries 9, no. 9: 462. https://doi.org/10.3390/batteries9090462




