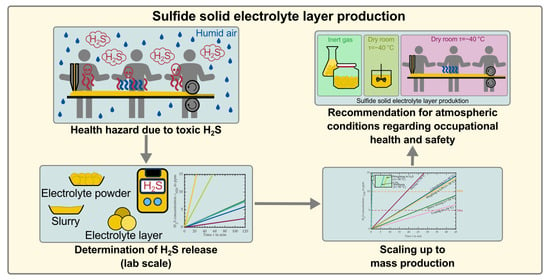
Investigating the Production Atmosphere for Sulfide-Based Electrolyte Layers Regarding Occupational Health and Safety
Abstract
:1. Introduction
1.1. Production Process
1.2. Production Atmosphere
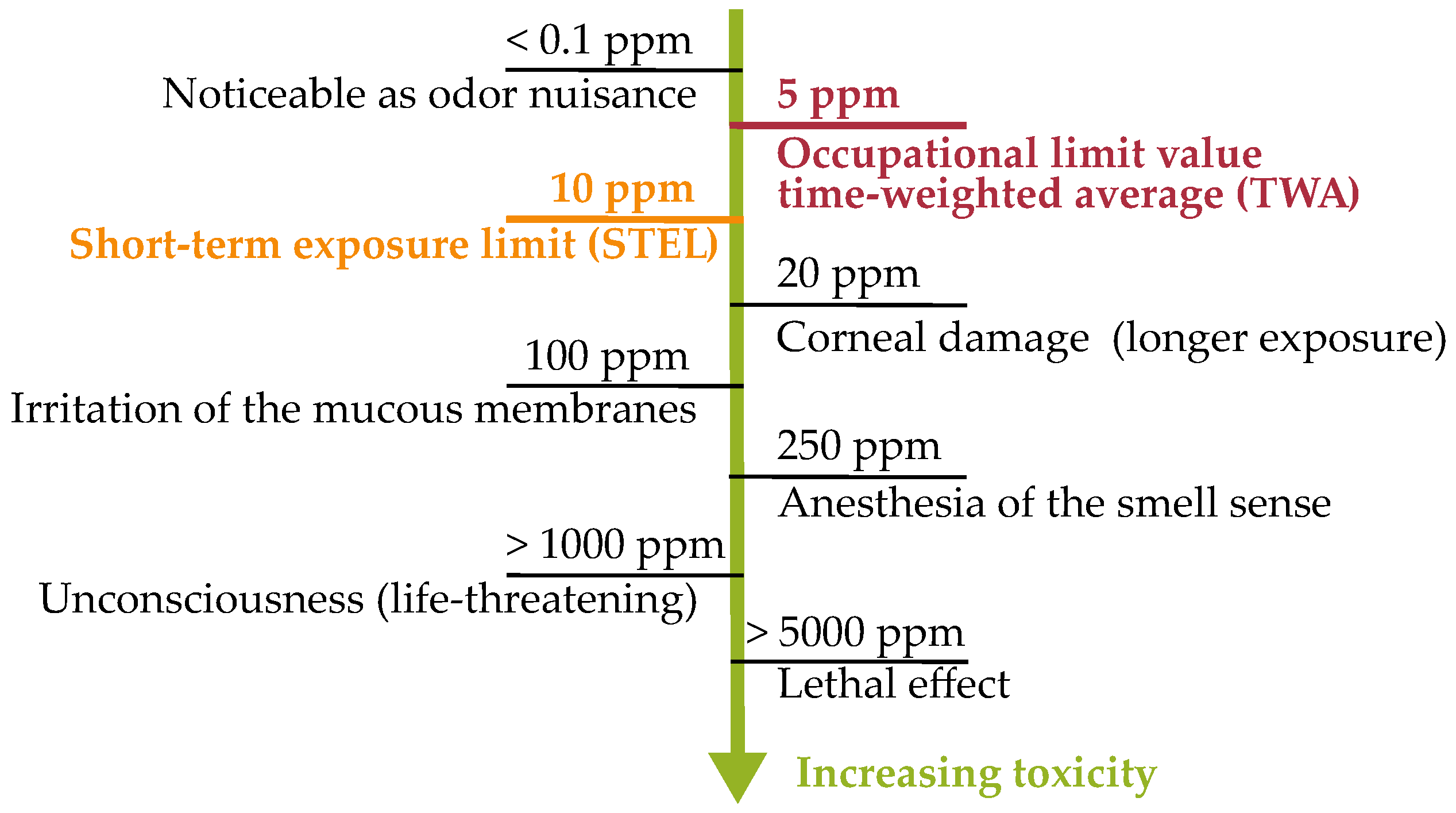
1.3. Hydrogen Sulfide—Toxicology, Health and Safety Regulations and Formation
1.4. Aim of This Study
2. Experimental
2.1. Materials
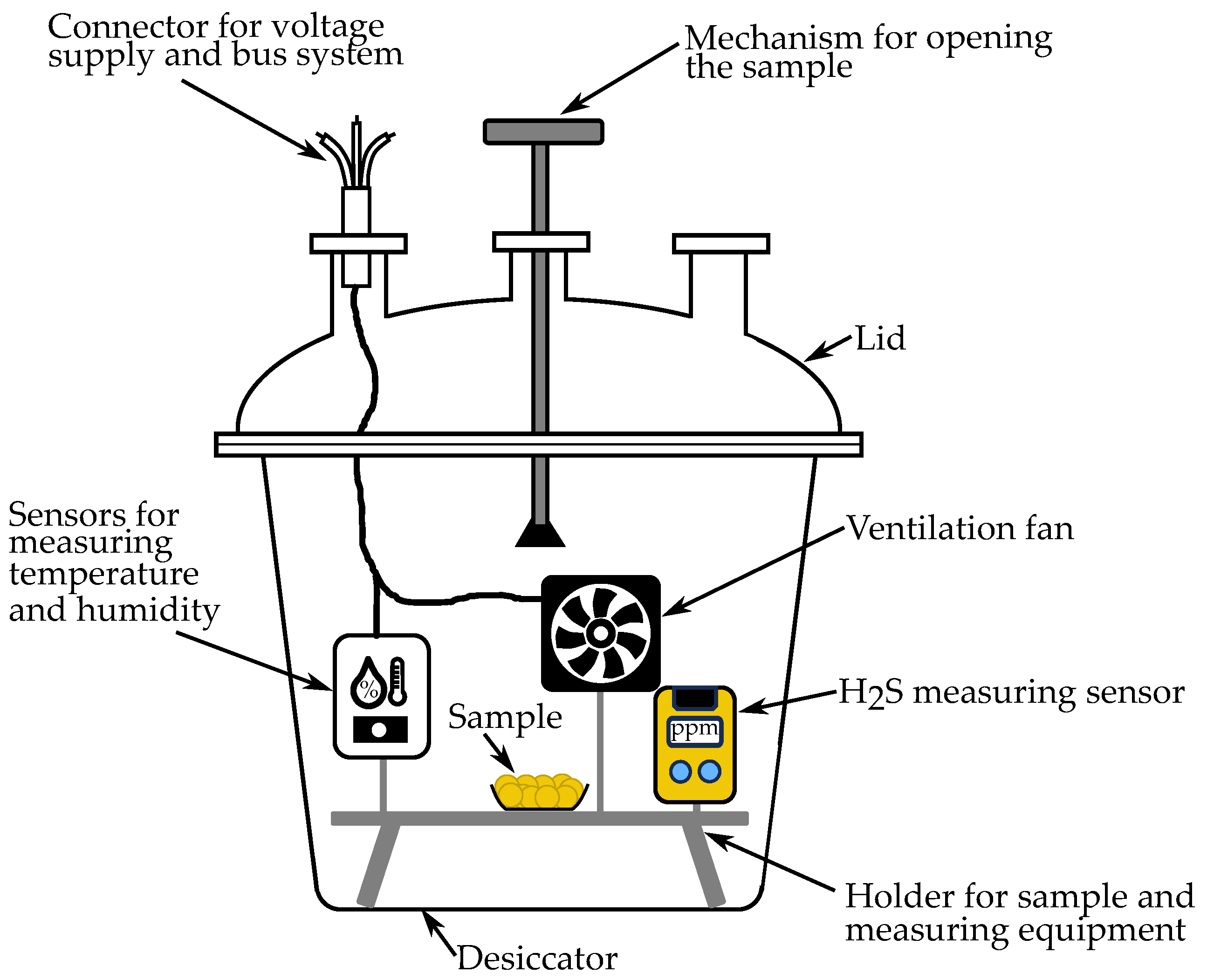
2.2. Experimental Setup
2.3. Upscaling from Laboratory to Mass Production Scale
3. Results and Discussion
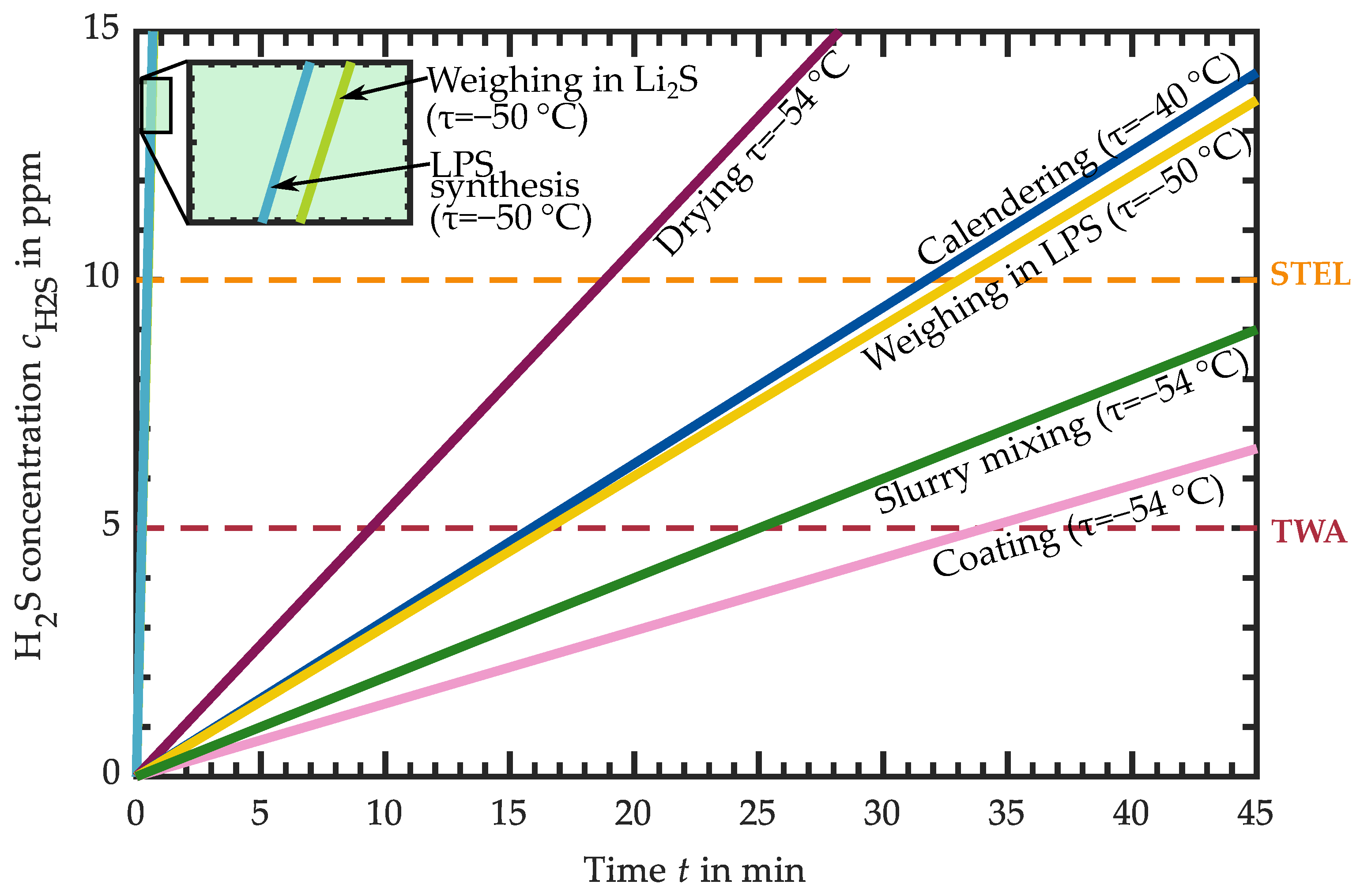
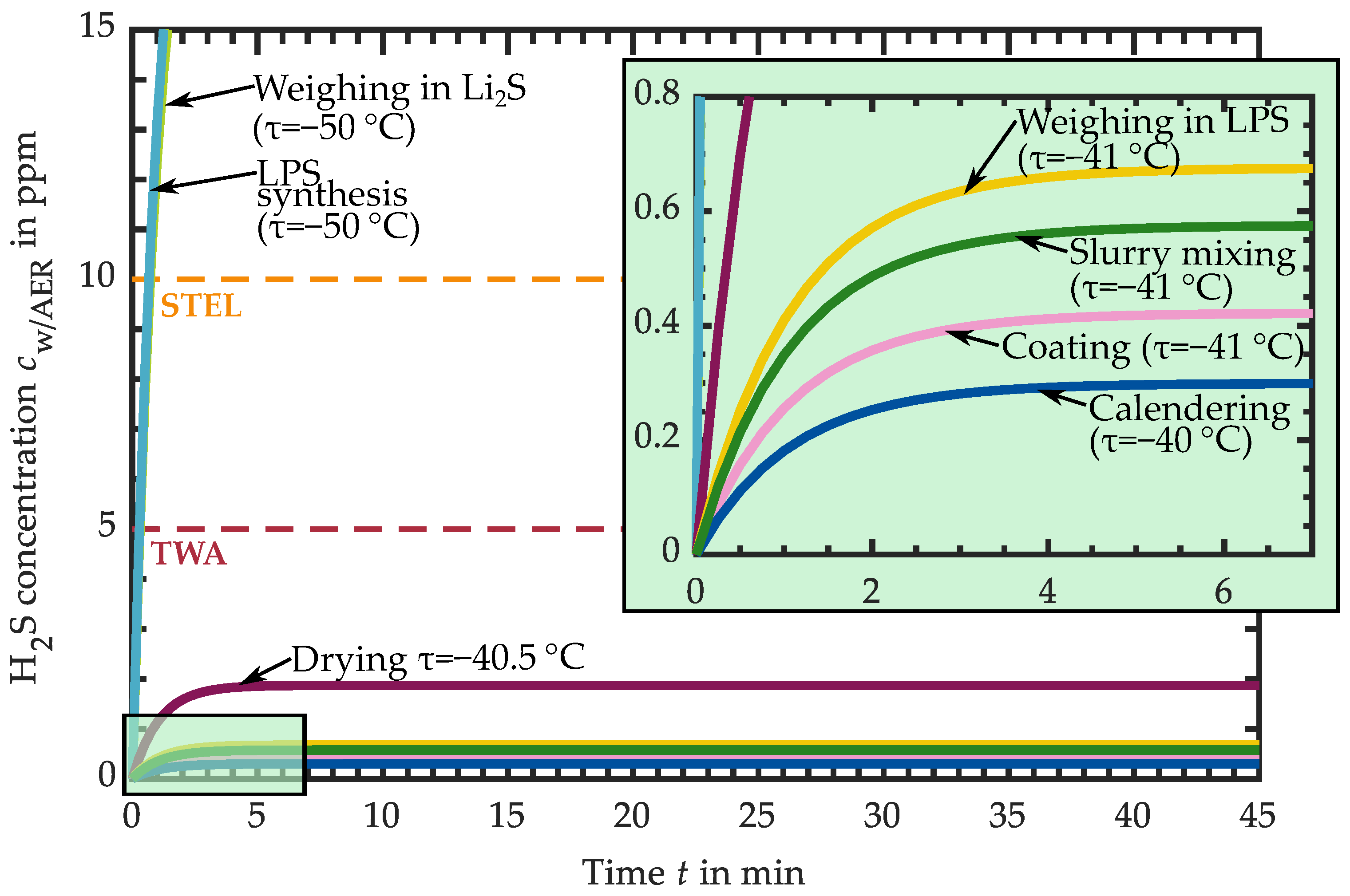
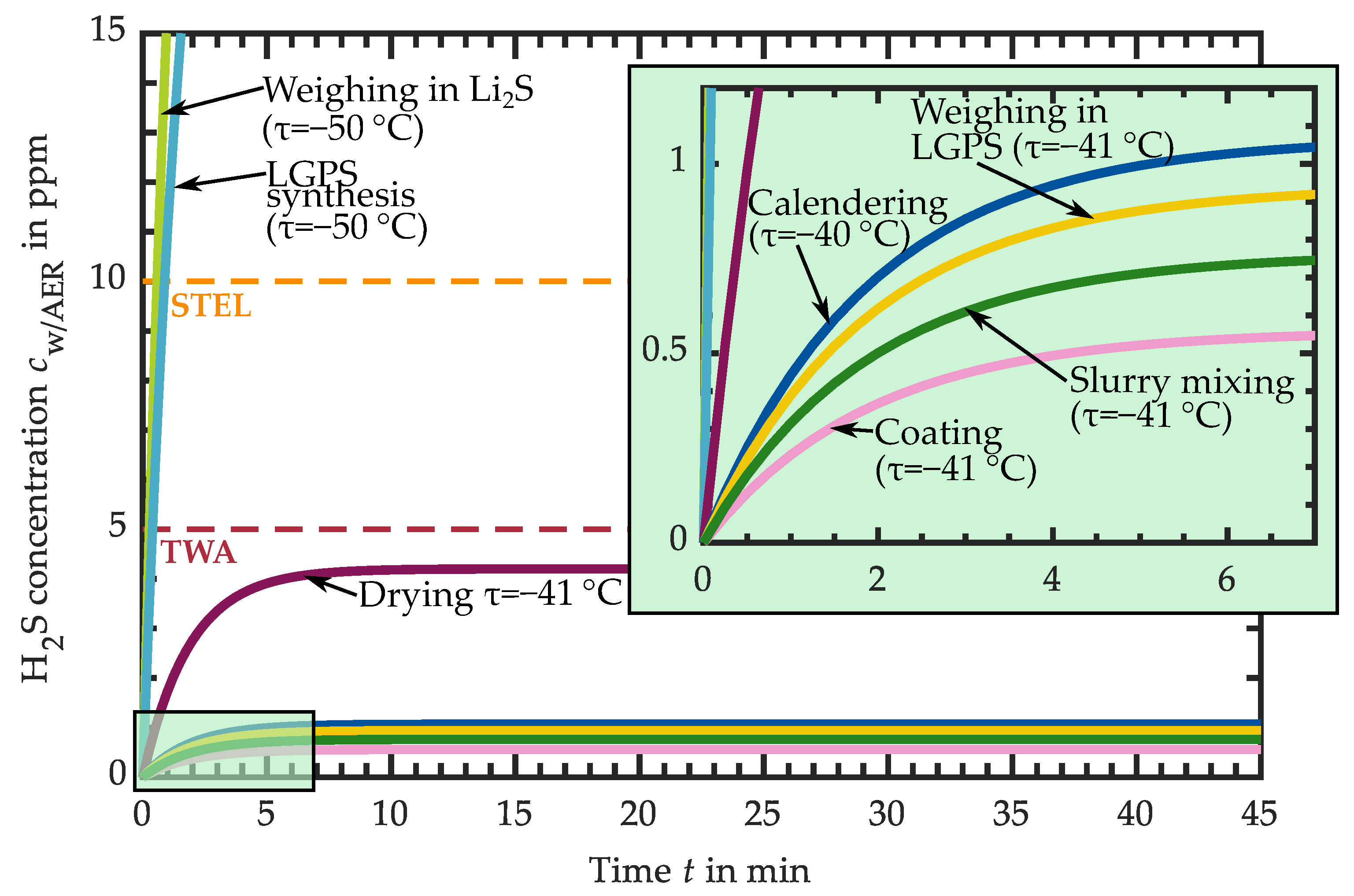
3.1. Hydrogen Sulfide Release of Intermediate Products
3.1.1. H2S Release of Educts for Electrolyte Synthesis
3.1.2. H2S Release of LPS Electrolyte
3.1.3. H2S Release of LPS-Based Slurry
3.1.4. H2S Release of LPS-Based Dried Layers
3.1.5. H2S Release of LPS-Based Calendered Layers
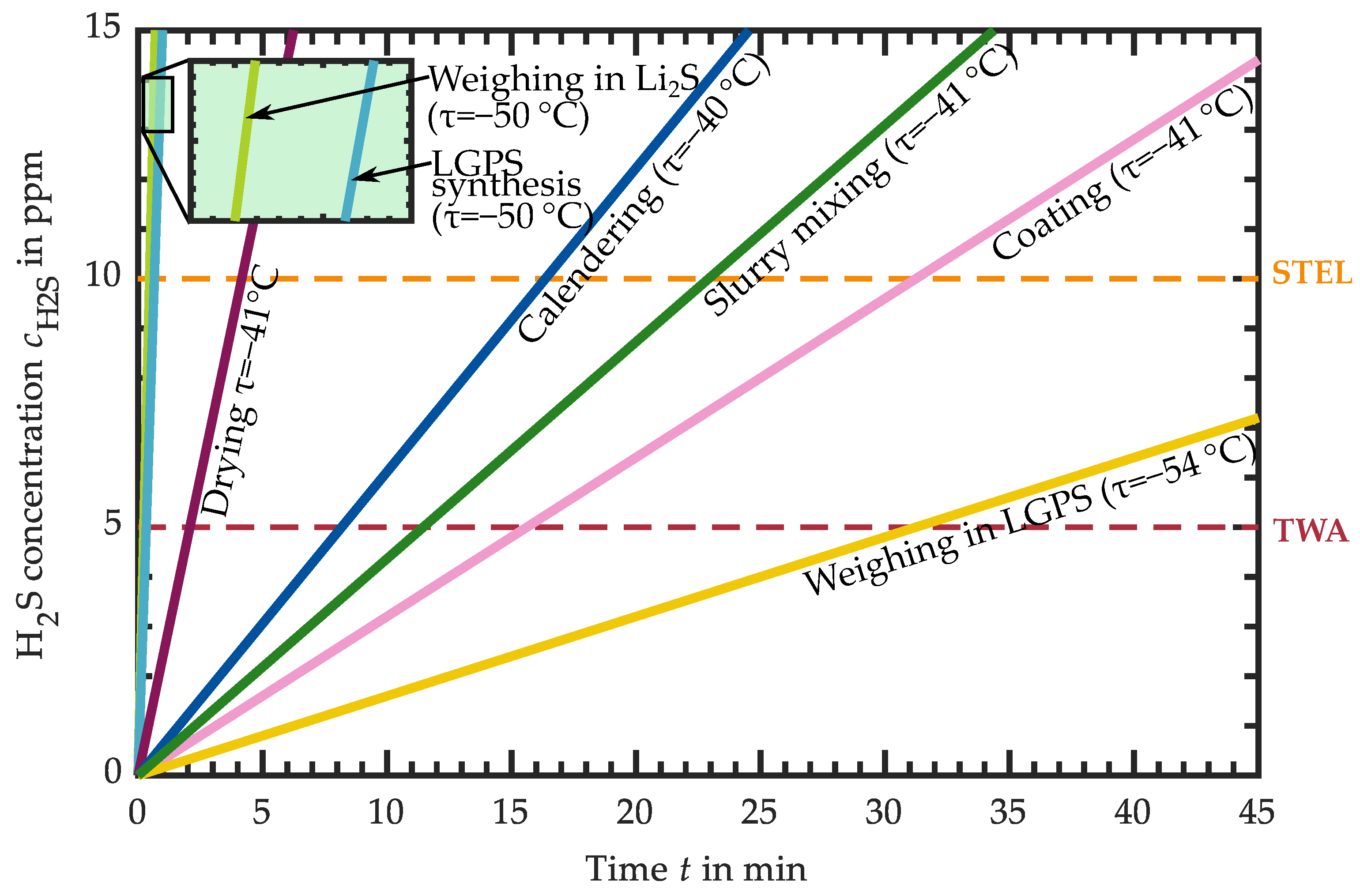
3.1.6. H2S Release of LGPS-Based Intermediates
3.2. Requirements for the Atmospheric Conditions in Industrial Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AER | air exchange rate |
| Ar | argon |
| ASSB | all-solid-state batterie |
| ASSBs | all-solid-state batteries |
| HNBR | hydrogenated nitrile-butadiene rubber |
| Li+ | lithium ion |
| LGPS | lithium germanium phosphorus sulfide (Li10GeP2S12) |
| LPS | lithium phosphorus sulfide (β−Li3PS4) |
| ppm | parts per million |
| PX | p-xylene |
| RH | relative humidity |
| SE | solid electrolyte |
| SEs | solid electrolytes |
| STEL | short-term exposure limit |
| TWA | time-weighted average |
References
- Wu, M.; Li, M.; Jin, Y.; Chang, X.; Zhao, X.; Gu, Z.; Liu, G.; Yao, X. In situ formed LiF-Li3N interface layer enables ultra-stable sulfide electrolyte-based all-solid-state lithium batteries. J. Energy Chem. 2023, 79, 272–278. [Google Scholar] [CrossRef]
- Zuo, T.T.; Walther, F.; Ahmed, S.; Rueß, R.; Hertle, J.; Mogwitz, B.; Volz, K.; Janek, J. Formation of an Artificial Cathode–Electrolyte Interphase to Suppress Interfacial Degradation of Ni-Rich Cathode Active Material with Sulfide Electrolytes for Solid-State Batteries. ACS Energy Lett. 2023, 8, 1322–1329. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, K.; Zhao, W.; Hu, Z.; Li, S.; Zhong, Y.; Yang, R.; Wang, X.; Wang, J.; Wu, C.; et al. Interfacial Challenges and Strategies toward Practical Sulfide-Based Solid-State Lithium Batteries. Energy Mater. Adv. 2023, 4, 22. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Han, F.; Yao, X.; Wang, C. Lithium/Sulfide All-Solid-State Batteries Using Sulfide Electrolytes. Adv. Mater. 2021, 33, 2000751. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Jiang, Z.; Liu, Y.; Li, J.; Gu, C.; Wang, X.; Xia, X.; Tu, J. Recent progress of sulfide electrolytes for all-solid-state lithium batteries. Energy Mater. 2022, 2, 200005. [Google Scholar] [CrossRef]
- Chen, S.; Xie, D.; Liu, G.; Mwizerwa, J.P.; Zhang, Q.; Zhao, Y.; Xu, X.; Yao, X. Sulfide solid electrolytes for all-solid-state lithium batteries: Structure, conductivity, stability and application. Energy Storage Mater. 2018, 14, 58–74. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef]
- Singer, C.; Schnell, J.; Reinhart, G. Scalable Processing Routes for the Production of All-Solid-State Batteries—Modeling Interdependencies of Product and Process. Energy Technol. 2021, 9, 2000665. [Google Scholar] [CrossRef]
- Ates, T.; Keller, M.; Kulisch, J.; Adermann, T.; Passerini, S. Development of an all-solid-state lithium battery by slurry-coating procedures using a sulfidic electrolyte. Energy Storage Mater. 2019, 17, 204–210. [Google Scholar] [CrossRef]
- Schnell, J.; Günther, T.; Knoche, T.; Vieider, C.; Köhler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-solid-state lithium-ion and lithium metal batteries—Paving the way to large-scale production. J. Power Sources 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Lu, P.; Wu, D.; Chen, L.; Li, H.; Wu, F. Air Stability of Solid-State Sulfide Batteries and Electrolytes. Electrochem. Energy Rev. 2022, 5, 3. [Google Scholar] [CrossRef]
- Singer, C.; Töpper, H.C.; Kutsch, T.; Schuster, R.; Koerver, R.; Daub, R. Hydrolysis of Argyrodite Sulfide-Based Separator Sheets for Industrial All-Solid-State Battery Production. ACS Appl. Mater. Interfaces 2022, 14, 24245–24254. [Google Scholar] [CrossRef] [PubMed]
- Teragawa, S.; Aso, K.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Liquid-phase synthesis of a Li3PS4 solid electrolyte using N-methylformamide for all-solid-state lithium batteries. J. Mater. Chem. A 2014, 2, 5095. [Google Scholar] [CrossRef]
- Ito, A.; Kimura, T.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Liquid-phase synthesis of Li3PS4 solid electrolyte using ethylenediamine. J. Sol-Gel Sci. Technol. 2022, 101, 2–7. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Kanno, R. Li10GeP2S12—Type Superionic Conductors: Synthesis, Structure, and Ionic Transportation. Adv. Energy Mater. 2020, 10, 2002153. [Google Scholar] [CrossRef]
- Duchardt, M.; Diels, M.; Roling, B.; Dehnen, S. Flow-Oriented Synthesis of Li2S and Li3PS4·3THF: Opening Up a Completely Solvent-Based Solid Electrolyte Value Chain. ACS Appl. Energy Mater. 2020, 3, 6937–6945. [Google Scholar] [CrossRef]
- Miura, A.; Rosero-Navarro, N.C.; Sakuda, A.; Tadanaga, K.; Phuc, N.H.H.; Matsuda, A.; Machida, N.; Hayashi, A.; Tatsumisago, M. Liquid-phase syntheses of sulfide electrolytes for all-solid-state lithium battery. Nat. Rev. Chem. 2019, 3, 189–198. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Singer, C.; Töpper, H.C.; Günter, F.J.; Reinhart, G. Plant Technology for the Industrial Coating Process for Sulfide-Based All-Solid-State Batteries. Procedia CIRP 2021, 104, 56–61. [Google Scholar] [CrossRef]
- von Hann, J.F. Handbuch der Klimatologie: Bände 1-2; Salzwasser: Paderborn, Germany, 2012. [Google Scholar]
- Bencloski, J.W. Air temperature and relative humidity: A simulation. J. Geogr. 1982, 81, 64–65. [Google Scholar] [CrossRef]
- Lawrence, M.G. The Relationship between Relative Humidity and the Dewpoint Temperature in Moist Air: A Simple Conversion and Applications. Bull. Am. Meteorol. Soc. 2005, 86, 225–234. [Google Scholar] [CrossRef]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- DIN EN ISO 14644; Reinräume und Zugehörige Reinraumbereiche. Deutsche Norm, Beuth Verlag GmbH: Berlin, Germany, 2016.
- Gail, L.; Gommel, U. (Eds.) Reinraumtechnik, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ahmed, S.; Nelson, P.A.; Dees, D.W. Study of a dry room in a battery manufacturing plant using a process model. J. Power Sources 2016, 326, 490–497. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Schnell, J.; Knörzer, H.; Imbsweiler, A.J.; Reinhart, G. Solid versus Liquid—A Bottom–Up Calculation Model to Analyze the Manufacturing Cost of Future High–Energy Batteries. Energy Technol. 2020, 8, 1901237. [Google Scholar] [CrossRef]
- Cubitt, A.; Henderson, C.; Staveley, L.; Fonseca, I.; Ferreira, A.; Lobo, L. Some thermodynamic properties of liquid hydrogen sulphide and deuterium sulphide. J. Chem. Thermodyn. 1987, 19, 703–710. [Google Scholar] [CrossRef]
- GESTIS-Substance Database: Hydrogen Sulfide. 2020. Available online: https://gestis.dguv.de/data?name=001130 (accessed on 30 August 2023).
- Goubern, M.; Andriamihaja, M.; Nübel, T.; Blachier, F.; Bouillaud, F. Sulfide, the first inorganic substrate for human cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 1699–1706. [Google Scholar] [CrossRef]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef]
- Sicher Atmen—Auch Bei Extremen H2S-Konzentrationen. Available online: https://www.draeger.com/library/content/fachartikel-h2s-hanscray-999-de.pdf (accessed on 30 August 2023).
- Nikodimos, Y.; Huang, C.J.; Taklu, B.W.; Su, W.N.; Hwang, B.J. Chemical stability of sulfide solid-state electrolytes: Stability toward humid air and compatibility with solvents and binders. Energy Environ. Sci. 2022, 15, 991–1033. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Gu, X. Failure mechanism of solid-state electrolyte Li10GeP2S12 in a moist atmosphere: A first-principles study. Mater. Adv. 2022, 3, 3143–3150. [Google Scholar] [CrossRef]
- Muramatsu, H.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. Structural change of Li2S–P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ionics 2011, 182, 116–119. [Google Scholar] [CrossRef]
- Picot, A.; Grenouillet, P. Safety in the Chemistry and Biochemistry Laboratory, 1st ed.; VCH Publishers: New York, NY, USA, 1995. [Google Scholar]
- Rochow, E.G.; Abel, E.W. The Chemistry of Germanium, Tin and Lead; Elsevier Science: Burlington, VT, USA, 2014. [Google Scholar]
- Horton, J.H.; Peat, K.L.; Lambert, R.M. Surface photo-oxidation and Ag deposition on amorphous GeS2. J. Phys. Condens. Matter 1993, 5, 9037–9048. [Google Scholar] [CrossRef]
- Xue, X.; Liu, G.; Zhao, X.; Xie, W.; He, H.; Yao, X. Superior Air–Stable Li4P0 · 9 Sb0 · 1 S4I Solid Electrolyte for All–Solid–State Lithium Batteries. Energy Technol. 2023, 11, 2201320. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, J.; Lee, H.J.; Kim, M.; Han, D.; Kim, H.; Yuk, J.M.; Nam, K.W.; Chung, K.Y.; Jung, H.G.; et al. Lithium Argyrodite Sulfide Electrolytes with High Ionic Conductivity and Air Stability for All-Solid-State Li-Ion Batteries. ACS Energy Lett. 2022, 7, 171–179. [Google Scholar] [CrossRef]
- Yersak, T.A.; Zhang, Y.; Hao, F.; Cai, M. Moisture Stability of Sulfide Solid-State Electrolytes. Front. Energy Res. 2022, 10, 882508. [Google Scholar] [CrossRef]
- Kimura, T.; Nakano, T.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Hydration and Dehydration Behavior of Li4SnS4 for Applications as a Moisture-Resistant All-Solid-State Battery Electrolyte. J. Phys. Chem. C 2023, 127, 1303–1309. [Google Scholar] [CrossRef]
- Hayashi, A.; Muramatsu, H.; Ohtomo, T.; Hama, S.; Tatsumisago, M. Improvement of chemical stability of Li3PS4 glass electrolytes by adding MxOy (M = Fe, Zn, and Bi) nanoparticles. J. Mater. Chem. A 2013, 1, 6320. [Google Scholar] [CrossRef]
- Chen, Y.T.; Marple, M.A.T.; Tan, D.H.S.; Ham, S.Y.; Sayahpour, B.; Li, W.K.; Yang, H.; Lee, J.B.; Hah, H.J.; Wu, E.A.; et al. Investigating dry room compatibility of sulfide solid-state electrolytes for scalable manufacturing. J. Mater. Chem. A 2022, 10, 7155–7164. [Google Scholar] [CrossRef]
- Lu, P.; Liu, L.; Wang, S.; Xu, J.; Peng, J.; Yan, W.; Wang, Q.; Li, H.; Chen, L.; Wu, F. Superior All-Solid-State Batteries Enabled by a Gas-Phase-Synthesized Sulfide Electrolyte with Ultrahigh Moisture Stability and Ionic Conductivity. Adv. Mater. 2021, 33, 2100921. [Google Scholar] [CrossRef]
- Khurram Tufail, M.; Ahmad, N.; Zhou, L.; Faheem, M.; Yang, L.; Chen, R.; Yang, W. Insight on air-induced degradation mechanism of Li7P3S11 to design a chemical-stable solid electrolyte with high Li2S utilization in all-solid-state Li/S batteries. Chem. Eng. J. 2021, 425, 130535. [Google Scholar] [CrossRef]
- Kreher, T.; Heim, F.; Pross-Brakhage, J.; Hemmerling, J.; Birke, K.P. Comparison of Different Current Collector Materials for In Situ Lithium Deposition with Slurry-Based Solid Electrolyte Layers. Batteries 2023, 9, 412. [Google Scholar] [CrossRef]
- Drägerwerk AG & Co. KGaA. Datasheet Dräger Pac® 6500 Eingasmessgerät. 2021. Available online: https://www.draeger.com/Products/Content/pac-6500-pi-9104115-de-dach.pdf (accessed on 30 August 2023).
- Sensirion, A.G. Datasheet SHT45. 2023. Available online: https://sensirion.com/media/documents/33FD6951/64D3B030/Sensirion_Datasheet_SHT4x.pdf (accessed on 30 August 2023).
- Simon, R. Facilities of a lithium-ion battery production plant. In Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 227–235. [Google Scholar] [CrossRef]
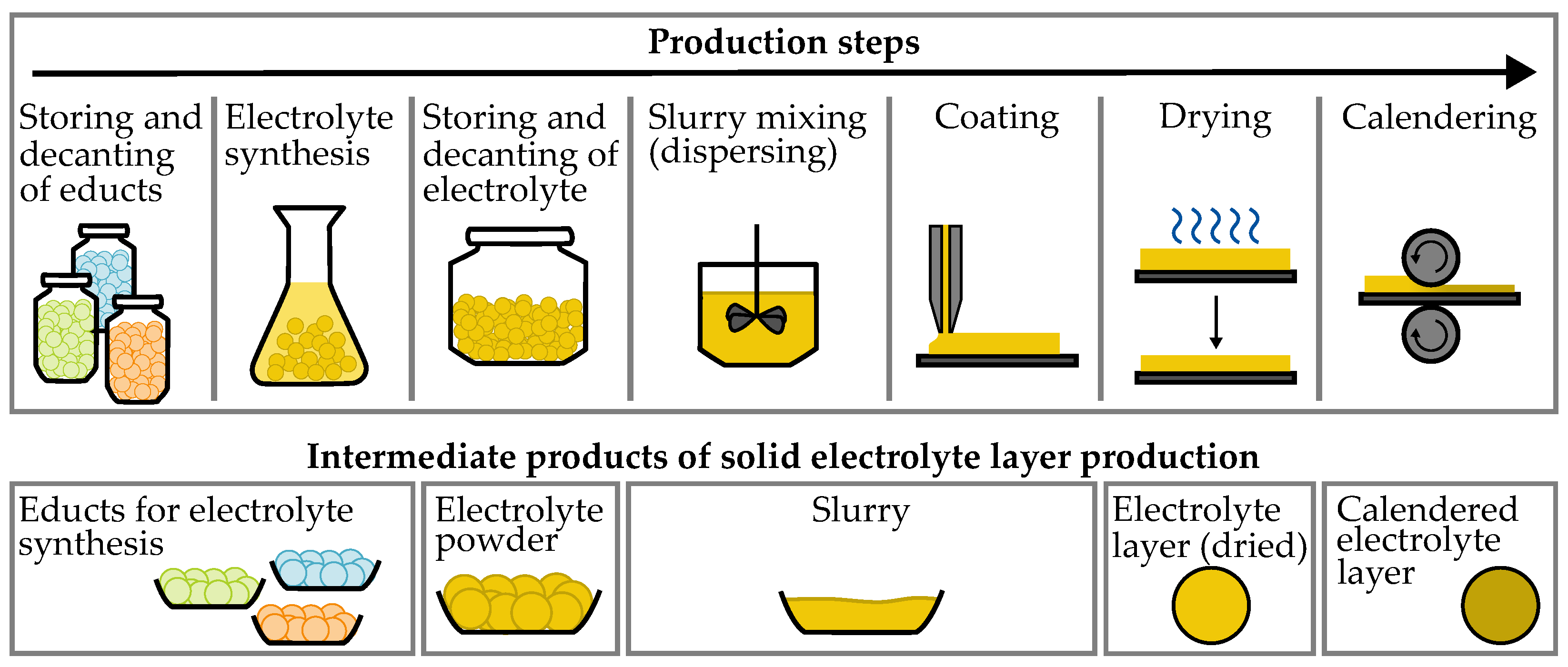
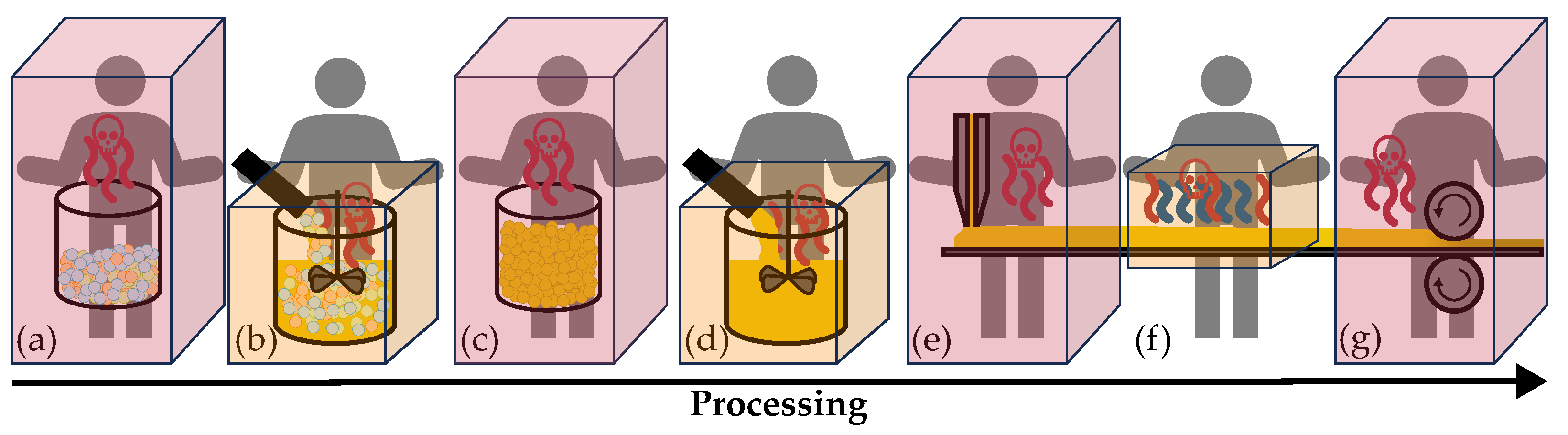





| Production Step | Area of H2S- | Working Area | Air Volume |
|---|---|---|---|
| Releasing Product | |||
| (a) Weighing in and decanting educts for electrolyte synthesis | Storage in a 100 l drum | ||
| (b) Fully automated electrolyte synthesis | Synthesis container | ||
| (c) Weighing in and decanting electrolyte powder | Storage in a 100 l drum | ||
| (d) Fully automated slurry mixing | Mixer | ||
| (e) Coating on carrier substrate | |||
| (f) Fully automated drying | |||
| (g) Calendering and quality control | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreher, T.; Jäger, P.; Heim, F.; Birke, K.P. Investigating the Production Atmosphere for Sulfide-Based Electrolyte Layers Regarding Occupational Health and Safety. Batteries 2023, 9, 472. https://doi.org/10.3390/batteries9090472
Kreher T, Jäger P, Heim F, Birke KP. Investigating the Production Atmosphere for Sulfide-Based Electrolyte Layers Regarding Occupational Health and Safety. Batteries. 2023; 9(9):472. https://doi.org/10.3390/batteries9090472
Chicago/Turabian StyleKreher, Tina, Patrick Jäger, Fabian Heim, and Kai Peter Birke. 2023. "Investigating the Production Atmosphere for Sulfide-Based Electrolyte Layers Regarding Occupational Health and Safety" Batteries 9, no. 9: 472. https://doi.org/10.3390/batteries9090472
APA StyleKreher, T., Jäger, P., Heim, F., & Birke, K. P. (2023). Investigating the Production Atmosphere for Sulfide-Based Electrolyte Layers Regarding Occupational Health and Safety. Batteries, 9(9), 472. https://doi.org/10.3390/batteries9090472