Dual-Salts Electrolyte with Fluoroethylene Carbonate Additive for High-Voltage Li-Metal Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Measurements
2.3. Material Characterization
3. Results and Discussion
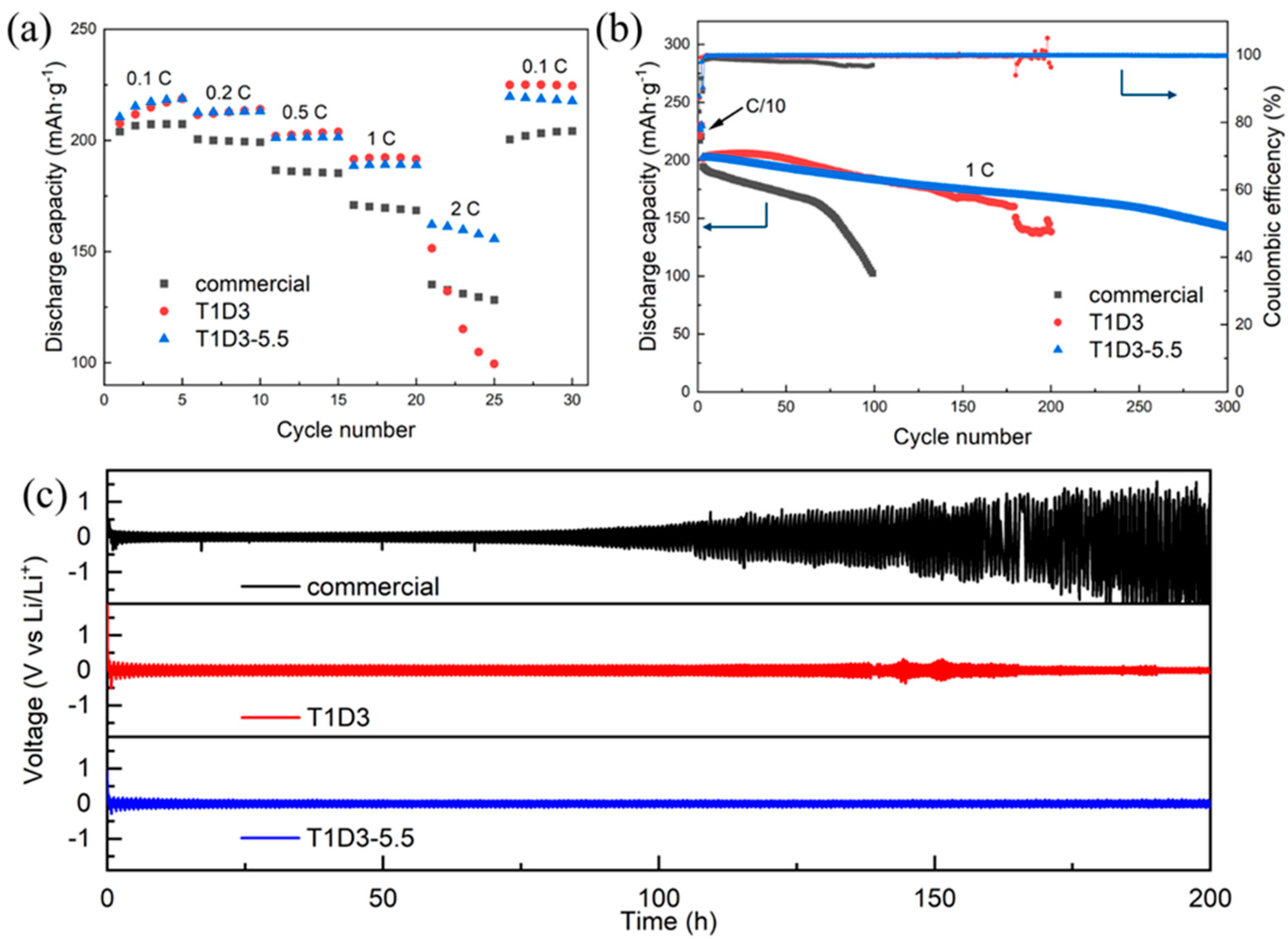
3.1. Electrochemical Performance
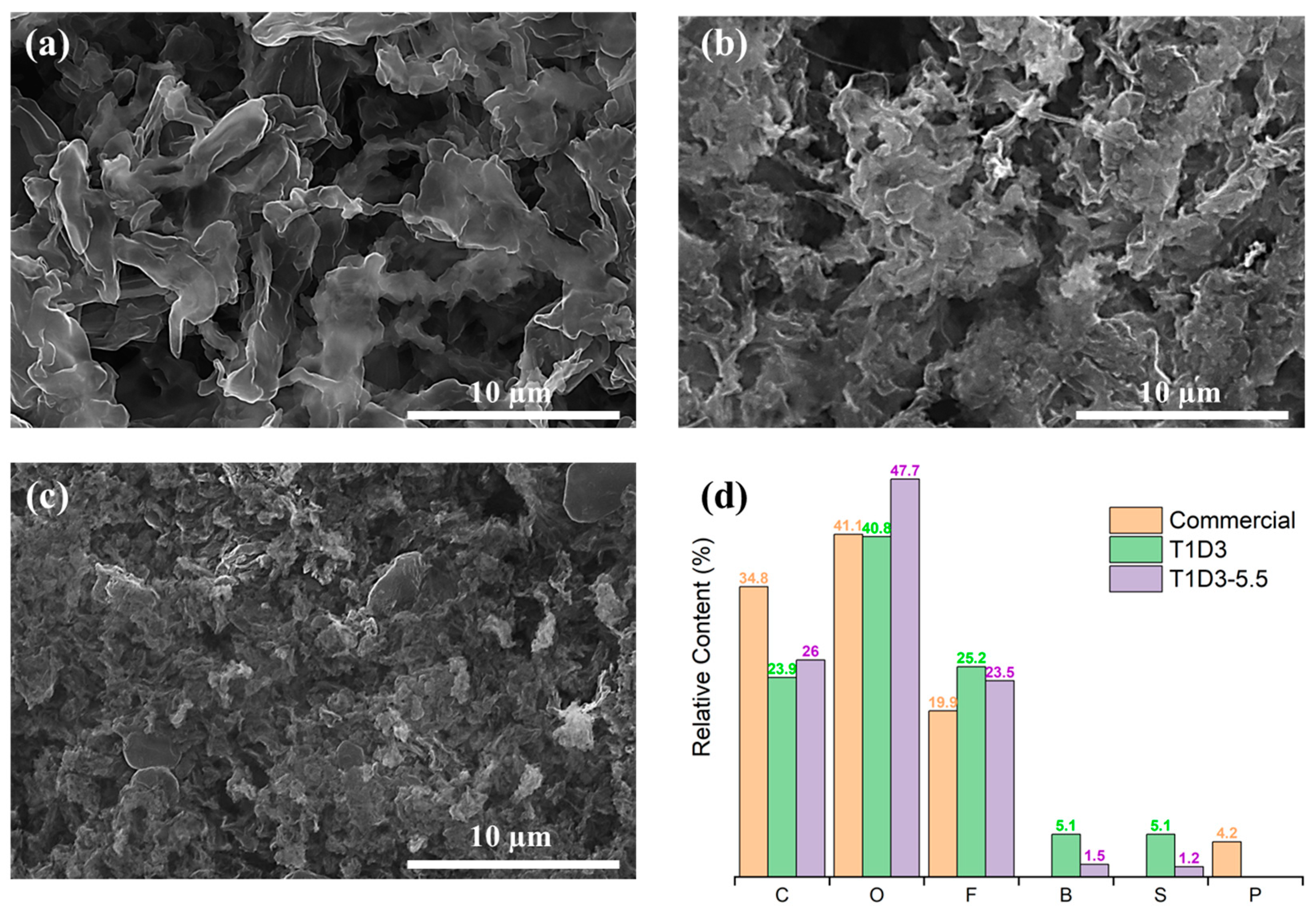
3.2. The Anode/Electrolyte Interface
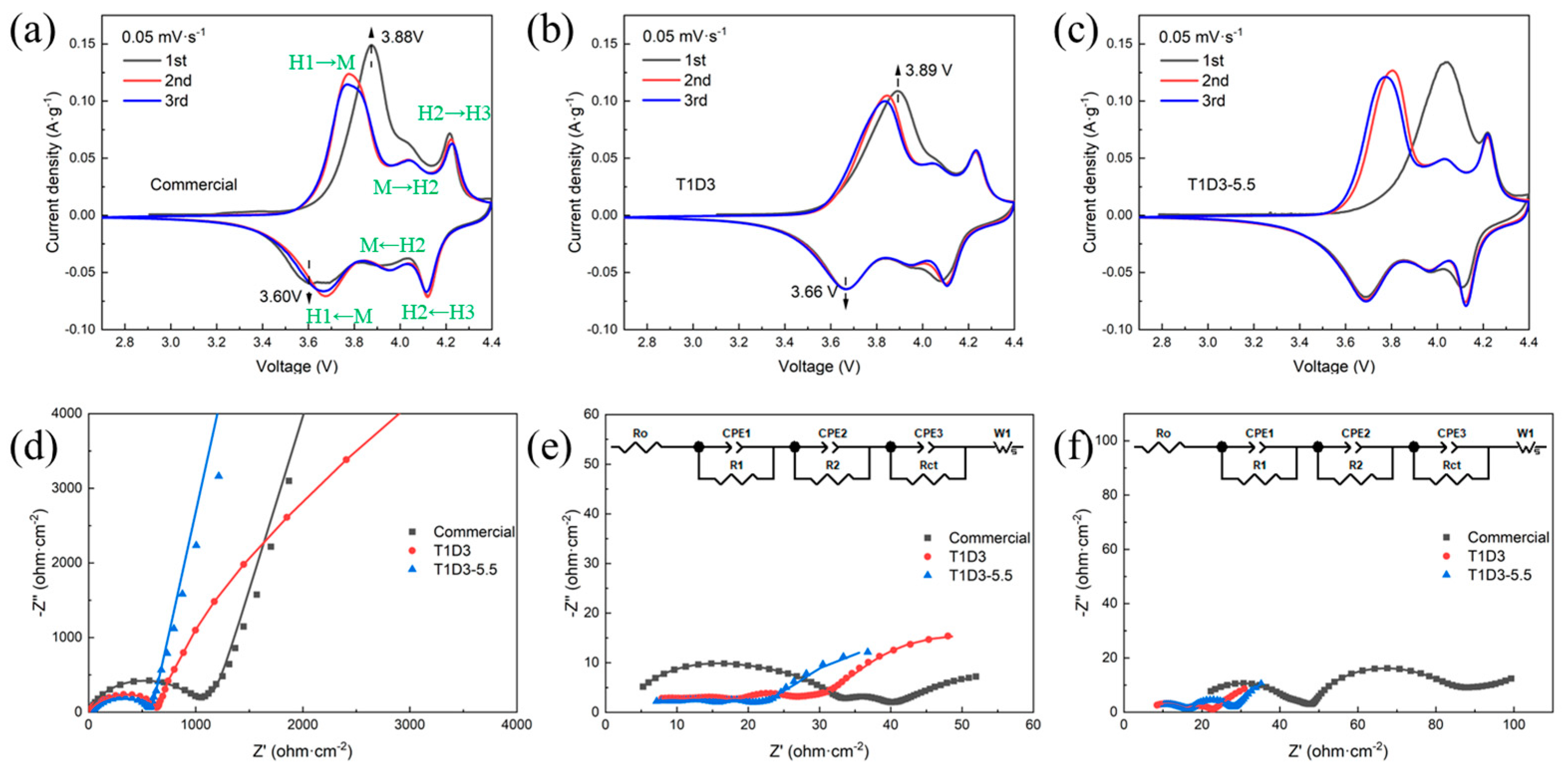
3.3. The Cathode/Electrolyte Interface
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, W.; Chen, N.; Huang, Y.; Li, L.; Wu, F.; Chen, R. Ionic liquid electrolyte with highly concentrated LiTFSI for lithium metal batteries. Electrochim. Acta 2018, 285, 78–85. [Google Scholar] [CrossRef]
- Fang, S.; Shen, L.; Li, S.; Dou, H.; Zhang, X. Self-supported TiN nanorod array/carbon textile as a lithium host that induces dendrite-free lithium plating with high rates and long cycle life. J. Mater. Chem. A 2020, 8, 3293–3299. [Google Scholar] [CrossRef]
- Li, G. Regulating Mass Transport Behavior for High-Performance Lithium Metal Batteries and Fast-Charging Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2002891. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; Meng, Y.S.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Lu, J.; Xu, X.; Fan, W.; Xin, Y.; Wang, W.; Fan, C.; Chen, P.; Zhao, J.; Liu, J.; Hou, Y. Phenyl 4-Fluorobenzene Sulfonate as a Versatile Film-Forming Electrolyte Additive for Wide-Temperature-Range NCM811//Graphite Batteries. ACS Appl. Energy Mater. 2022, 5, 6324–6334. [Google Scholar] [CrossRef]
- Wu, B.; Chen, C.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. All-Solid-State Thin Film Li-Ion Batteries: New Challenges, New Materials, and New Designs. Batteries 2023, 9, 186. [Google Scholar] [CrossRef]
- Dong, X.; Yao, J.; Zhu, W.; Huang, X.; Kuai, X.; Tang, J.; Li, X.; Dai, S.; Shen, L.; Yang, R.; et al. Enhanced high-voltage cycling stability of Ni-rich cathode materials via the self-assembly of Mn-rich shells. J. Mater. Chem. A 2019, 7, 20262–20273. [Google Scholar] [CrossRef]
- Pham, T.D.; Bin, F.A.; Kim, J.; Oh, H.M.; Lee, K.K. Practical High-Voltage Lithium Metal Batteries Enabled by Tuning the Solvation Structure in Weakly Solvating Electrolyte. Small 2022, 18, e2107492. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; An, X.; Wu, Z.; Yue, X.; Wang, J.; Hao, X.; Abudula, A.; Guan, G. Fluoropyridine family: Bifunction as electrolyte solvent and additive to achieve dendrites-free lithium metal batteries. J. Mater. Sci. Technol. 2021, 74, 119–127. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, K.-J.; Yoon, C.; Sun, Y.-K. Capacity Fading of Ni-Rich Li[NixCoyMn1−x−y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Li, W.; Kim, U.H.; Dolocan, A.; Sun, Y.K.; Manthiram, A. Formation and Inhibition of Metallic Lithium Microstructures in Lithium Batteries Driven by Chemical Crossover. ACS Nano 2017, 11, 5853–5863. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Xu, G.-L.; Liu, Q.; Lau, K.K.S.; Liu, Y.; Liu, X.; Gao, H.; Zhou, X.; Zhang, M.; Ren, Y.; Li, J.; et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy 2019, 4, 484–494. [Google Scholar] [CrossRef]
- Xue, W.; Huang, M.; Li, Y.; Zhu, Y.G.; Gao, R.; Xiao, X.; Zhang, W.; Li, S.; Xu, G.; Yu, Y.; et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 2021, 6, 495–505. [Google Scholar] [CrossRef]
- Tikekar, M.; Choudhury, S.; Tu, Z.; Archer, L. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, M.; Zhou, T.; Raijmakers, L.; Vezhlev, E.; Wu, B.; Schülli, T.U.; Danilov, D.L.; Wei, Y.; Eichel, R.-A.; et al. Interface Aspects in All-Solid-State Li-Based Batteries Reviewed. Adv. Energy Mater. 2021, 11, 2003939. [Google Scholar] [CrossRef]
- Zhang, C.; Lyu, R.; Lv, W.; Li, H.; Jiang, W.; Li, J.; Gu, S.; Zhou, G.; Huang, Z.; Zhang, Y.; et al. A Lightweight 3D Cu Nanowire Network with Phosphidation Gradient as Current Collector for High-Density Nucleation and Stable Deposition of Lithium. Adv. Mater. 2019, 31, 1904991. [Google Scholar] [CrossRef]
- Qin, K.; Holguin, K.; Mohammadiroudbari, M.; Huang, J.; Kim, E.Y.S.; Hall, R.; Luo, C. Strategies in Structure and Electrolyte Design for High-Performance Lithium Metal Batteries. Adv. Funct. Mater. 2021, 31, 2009694. [Google Scholar] [CrossRef]
- Piao, Z.; Gao, R.; Liu, Y.; Zhou, G.; Cheng, H.-M. A Review on Regulating Li Solvation Structures in Carbonate Electrolytes for Lithium. Adv. Mater. 2022, 35, 2206009. [Google Scholar]
- Zhang, J.; Zhang, H.; Deng, L.; Yang, Y. An additive-enabled ether-based electrolyte to realize stable cycling of high-voltage anode-free lithium metal batteries. Energy Storage Mater. 2023, 54, 450–460. [Google Scholar] [CrossRef]
- Jiang, G.; Li, F.; Wang, H.; Wu, M.; Qi, S.; Liu, X.; Yang, S.; Ma, J. Perspective on High-Concentration Electrolytes for Lithium Metal Batteries. Small Struct. 2021, 2, 2000122. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Jiao, S.; Mei, D.; Engelhard, M.H.; Li, Q.; Lee, H.; Niu, C.; Adams, B.D.; Wang, C.; et al. High-Concentration Ether Electrolytes for Stable High-Voltage Lithium Metal Batteries. ACS Energy Lett. 2019, 4, 896–902. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Ji, X.; Deng, T.; Hou, S.; Chen, J.; Zheng, J.; Wang, F.; Jiang, J.; Xu, K.; et al. Highly Fluorinated Interphases Enable High-Voltage Li-Metal Batteries. Chem 2018, 4, 174–185. [Google Scholar] [CrossRef]
- Jiao, S.; Ren, X.; Cao, R.; Engelhard, M.H.; Liu, Y.; Hu, D.; Mei, D.; Zheng, J.; Zhao, W.; Li, Q.; et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 2018, 3, 739–746. [Google Scholar] [CrossRef]
- Zheng, Y.; Soto, F.A.; Ponce, V.; Seminario, J.M.; Cao, X.; Zhang, J.-G.; Balbuena, P.B. Localized high concentration electrolyte behavior near a lithium–metal anode surface. J. Mater. Chem. A 2019, 7, 25047–25055. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Yu, L.; Ren, X.; Engelhard, M.H.; Niu, C.; Lee, H.; Xu, W.; Xiao, J.; Liu, J.; et al. High-Efficiency Lithium Metal Batteries with Fire-Retardant Electrolytes. Joule 2018, 2, 1548–1558. [Google Scholar] [CrossRef]
- Fu, J.; Ji, X.; Chen, J.; Chen, L.; Fan, X.; Mu, D.; Wang, C. Lithium Nitrate Regulated Sulfone Electrolytes for Lithium Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 22194–22201. [Google Scholar] [CrossRef]
- Cao, X.; Ren, X.; Zou, L.; Engelhard, M.H.; Huang, W.; Wang, H.; Matthews, B.E.; Lee, H.; Niu, C.; Arey, B.W.; et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 2019, 4, 796–805. [Google Scholar] [CrossRef]
- Yu, L.; Cheng, S.; Lee, H.; Zhang, L.; Engelhard, M.H.; Li, Q.; Jiao, S.; Liu, J.; Xu, W.; Zhang, J.-G. A Localized High-Concentration Electrolyte with Optimized Solvents and Lithium Difluoro(oxalate)borate Additive for Stable Lithium Metal Batteries. ACS Energy Lett. 2018, 3, 2059–2067. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, L.; Cao, X.; Engelhard, M.H.; Liu, W.; Burton, S.D.; Lee, H.; Niu, C.; Matthews, B.E.; Zhu, Z.; et al. Enabling High-Voltage Lithium-Metal Batteries under Practical Conditions. Joule 2019, 3, 1662–1676. [Google Scholar] [CrossRef]
- Huang, H.; Yin, S.-C.; Nazar, L. Approaching theoretical capacity of LiFePO4 at room temperature at high rates. Electrochem. Solid-State Lett. 2001, 4, A170–A172. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B. Cell degradation in commercial LiFePO4 cells with high-power and high-energy designs. J. Power Sources 2014, 258, 408–419. [Google Scholar] [CrossRef]
- Alqahtani, Y.M.; Williams, Q.L. Reduction of Capacity Fading in High-Voltage NMC Batteries with the Addition of Reduced Graphene Oxide. Materials 2022, 15, 2146. [Google Scholar] [CrossRef]
- Tran, M.X.; Smyrek, P.; Park, J.; Pfleging, W.; Lee, J.K. Ultrafast-Laser Micro-Structuring of LiNi0.8Mn0.1Co0.1O2 Cathode for High-Rate Capability of Three-Dimensional Li-ion Batteries. Nanomaterials 2022, 12, 3897. [Google Scholar] [CrossRef]
- Shen, C.-H.; Wang, Q.; Chen, H.-J.; Shi, C.-G.; Zhang, H.-Y.; Huang, L.; Li, J.-T.; Sun, S.-G. In Situ Multitechnical Investigation into Capacity Fading of High-Voltage LiNi0.5Co0.2Mn0.3O2. ACS Appl. Mater. Interfaces 2016, 8, 35323–35335. [Google Scholar] [CrossRef]
- Mao, M.; Huang, B.; Li, Q.; Wnag, C.; He, Y.-B.; Knag, F. In-situ construction of hierarchical cathode electrolyte interphase for high performance LiNi0.8Co0.1Mn0.1O2/Li metal battery. Nano Energy 2020, 78, 105282. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Xu, W.; Xiao, J.; Cao, X.; Liu, J. Lithium Metal Anodes with Nonaqueous Electrolytes. Chem. Rev. 2020, 120, 13312–13348. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Chen, X.; Zhang, J.; Engelhard, M.H.; Zhang, Y.; Johnsin, B.R.; Crum, J.V.; Blake, T.A.; Liu, X.; et al. Effects of Carbonate Solvents and Lithium Salts on Morphology and Coulombic Efficiency of Lithium Electrode. J. Electrochem. Soc. 2013, 160, A1894–A1901. [Google Scholar] [CrossRef]
- Geng, Z.; Lu, J.; Li, Q.; Qiu, J.; Wang, Y.; Peng, J.; Huang, J.; Li, W.; Yu, X.; Li, H. Lithium metal batteries capable of stable operation at elevated temperature. Energy Storage Mater. 2019, 23, 646–652. [Google Scholar] [CrossRef]
- Markevich, E.; Fridman, K.; Sharabi, R.; Elazari, R.; Salitra, G.; Gottlieb, H.E.; Gershinsky, G.; Garsuch, A.; Semrau, G.; Schmidt, M.A.; et al. Amorphous Columnar Silicon Anodes for Advanced High Voltage Lithium Ion Full Cells: Dominant Factors Governing Cycling Performance. J. Electrochem. Soc. 2013, 160, A1824–A1833. [Google Scholar] [CrossRef]
- Hou, T.; Ynag, G.; Rajput, N.N.; Self, J.; Park, S.-W.; Nanda, J.; Persson, K.A. The influence of FEC on the solvation structure and reduction reaction of LiPF6/EC electrolytes and its implication for solid electrolyte interphase formation. Nano Energy 2019, 64, 103881. [Google Scholar] [CrossRef]
- Zhang, J.-N.; Li, Q.; Wang, Y.; Zhang, J.; Yu, X.; Li, H. Dynamic evolution of cathode electrolyte interphase (CEI) on high voltage LiCoO2 cathode and its interaction with Li anode. Energy Storage Mater. 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Park, M.; Park, S.; Choi, N. Unanticipated Mechanism of the Trimethylsilyl Motif in Electrolyte Additives on Nickel-Rich Cathodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 43694–43704. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Qu, K.; Wang, Y.; Shen, K.; Jiang, C.; Yu, B.; Luo, P.; Li, Z.; Chen, M.; et al. Concentrated ternary ether electrolyte allows for stable cycling of a lithium metal battery with commercial mass loading high-nickel NMC and thin anodes. Carbon Energy 2022, 5, e275. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Park, S.-J.; Yoon, C.S.; Sun, Y.-K. Customizing a Li–metal battery that survives practical operating conditions for electric vehicle applications. Energy Environ. Sci. 2019, 12, 2174–2184. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Fluoroethylene Carbonate as an Important Component for the Formation of an Effective Solid Electrolyte Interphase on Anodes and Cathodes for Advanced Li-Ion Batteries. ACS Energy Lett. 2017, 2, 1337–1345. [Google Scholar] [CrossRef]


| After 2 Cycles | After 100 Cycles | |||||
|---|---|---|---|---|---|---|
| Notation | Commercial | T1D3 | T1D3-5.5 | Commercial | T1D3 | T1D3-5.5 |
| Ro (Ω·cm−2) | 2.82 | 3.64 | 4.10 | 16.84 | 5.58 | 5.79 |
| R1 (Ω·cm−2) | 14.70 | 8.76 | 5.92 | 25.46 | 10.58 | 10.15 |
| R2 (Ω·cm−2) | 15.47 | 5.20 | 5.54 | 3.25 | 2.65 | 2.30 |
| RCT (Ω·cm−2) | 4.80 | 9.53 | 4.56 | 28.75 | 3.03 | 8.73 |
| Rtotal (Ω·cm−2) | 37.78 | 27.12 | 20.12 | 74.30 | 21.84 | 26.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.; Wu, B.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Dual-Salts Electrolyte with Fluoroethylene Carbonate Additive for High-Voltage Li-Metal Batteries. Batteries 2023, 9, 477. https://doi.org/10.3390/batteries9090477
Qin Z, Wu B, Danilov DL, Eichel R-A, Notten PHL. Dual-Salts Electrolyte with Fluoroethylene Carbonate Additive for High-Voltage Li-Metal Batteries. Batteries. 2023; 9(9):477. https://doi.org/10.3390/batteries9090477
Chicago/Turabian StyleQin, Zhizhen, Baolin Wu, Dmitri L. Danilov, Rüdiger-A. Eichel, and Peter H. L. Notten. 2023. "Dual-Salts Electrolyte with Fluoroethylene Carbonate Additive for High-Voltage Li-Metal Batteries" Batteries 9, no. 9: 477. https://doi.org/10.3390/batteries9090477







