Fibrillar Collagen Type I Participates in the Survival and Aggregation of Primary Hepatocytes Cultured on Soft Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hepatocytes Isolation and Culture
2.3. Liver Decellularization and Pepsin Digestion
2.4. Polyacrylamide (PAA) Hydrogels (HGs)
2.5. SDS Polyacrylamide Gels Electrophoresis (SDS-PAGE) and Protein Staining
2.6. Immunoblotting
2.7. Immunofluorescence and TUNEL Assay
2.8. Periodic Acid-Schiff (PAS) Staining
2.9. Quantification and Statistics
3. Results
3.1. Stiffness Promotes Cell Aggregation and Triggers Spreading in Primary Hepatocytes, Whereas Collagen Type I Promotes Cell Adhesion and Aggregation in Soft Conditions
3.2. Actin Cytoskeleton Remodeling Is Inhibited as the Nuclear Area Is Confined in Parallel in Primary Hepatocytes Cultured on Soft PAA HGs
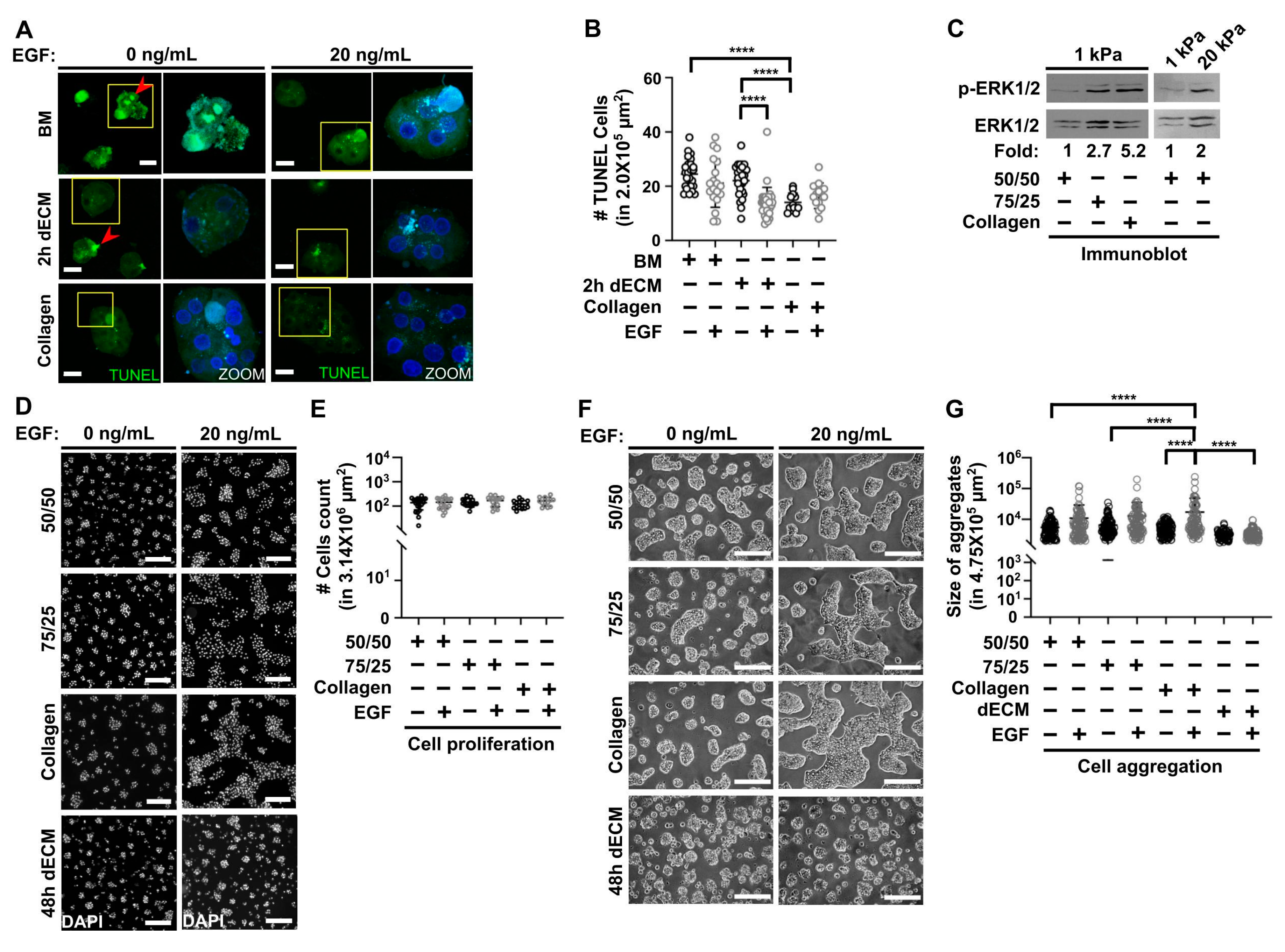
3.3. Collagen Type I Increases Viability in Primary Hepatocytes Cultured on Soft PAA HGs and Participates with Epidermal Growth Factor (EGF) in Regulating Cell Aggregation
3.4. Primary Hepatocytes on Soft PAA HGs Maintain their Actin Cytoskeleton Proximal to the Plasma Membrane, Canaliculi-Like Structures, and Synthesis of Glycogen, Whereas They Lose Albumin Protein Expression during Long Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colnot, S.; Perret, C. Liver Zonation. In Molecular Pathology of Liver Diseases; Monga, S.P.S., Ed.; Molecular Pathology Library; Springer: Boston, MA, USA, 2011; Volume 5, pp. 7–16. ISBN 9781441971067. [Google Scholar]
- Kuntz, E.; Kuntz, H.-D. Hepatology, Textbook and Atlas, 3rd ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2008; ISBN 9783540768395. [Google Scholar]
- Jones, A.L.; Mills, E.S. Ultrastructural concepts of drug metabolism. I. The hepatocyte: Structure and function. Am. J. Drug Alcohol Abuse 1974, 1, 111–135. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, L.; De Bleser, P.; Vanderkerken, K.; Geerts, B.; Wisse, E. Liver cell heterogeneity: Functions of non-parenchymal cells. Enzyme 1992, 46, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Liver regeneration. J. Cell. Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Morales-Navarrete, H.; Segovia-Miranda, F.; Klukowski, P.; Meyer, K.; Nonaka, H.; Marsico, G.; Chernykh, M.; Kalaidzidis, A.; Zerial, M.; Kalaidzidis, Y. A versatile pipeline for the multi-scale digital reconstruction and quantitative analysis of 3D tissue architecture. Elife 2015, 4. [Google Scholar]
- Treyer, A.; Müsch, A. Hepatocyte polarity. Compr. Physiol. 2013, 3, 243–287. [Google Scholar] [PubMed] [Green Version]
- Gissen, P.; Arias, I.M. Structural and functional hepatocyte polarity and liver disease. J. Hepatol. 2015, 63, 1023–1037. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.; Morales-Navarrete, H.; Seifert, S.; Wilsch-Braeuninger, M.; Dahmen, U.; Tanaka, E.M.; Brusch, L.; Kalaidzidis, Y.; Zerial, M. Bile canaliculi remodeling activates YAP via the actin cytoskeleton during liver regeneration. Mol. Syst. Biol. 2020, 16, e8985. [Google Scholar] [CrossRef]
- Bissell, D.M.; Arenson, D.M.; Maher, J.J.; Roll, F.J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J. Clin. Investig. 1987, 79, 801–812. [Google Scholar] [CrossRef] [Green Version]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef] [Green Version]
- Wisse, E.; Braet, F.; Luo, D.; De Zanger, R.; Jans, D.; Crabbé, E.; Vermoesen, A. Structure and function of sinusoidal lining cells in the liver. Toxicol. Pathol. 1996, 24, 100–111. [Google Scholar] [CrossRef]
- Baratta, J.L.; Ngo, A.; Lopez, B.; Kasabwalla, N.; Longmuir, K.J.; Robertson, R.T. Cellular organization of normal mouse liver: A histological, quantitative immunocytochemical, and fine structural analysis. Histochem. Cell Biol. 2009, 131, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaas, M.; Kangur, T.; Viil, J.; Mäemets-Allas, K.; Minajeva, A.; Vadi, K.; Antsov, M.; Lapidus, N.; Järvekülg, M.; Jaks, V. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 2016, 6, 27398. [Google Scholar] [CrossRef] [PubMed]
- Aycock, R.S.; Seyer, J.M. Collagens of Normal and Cirrhotic Human Liver. Connect. Tissue Res. 1989, 23, 19–31. [Google Scholar] [CrossRef]
- Hahn, E.; Wick, G.; Pencev, D.; Timpl, R. Distribution of basement membrane proteins in normal and fibrotic human liver: Collagen type IV, laminin, and fibronectin. Gut 1980, 21, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.; Yamamoto, K.; Nagano, T.; Okamoto, R.; Ibuki, N.; Tagashira, M.; Tsuji, T. Immunohistochemical study on phenotypical changes of hepatocytes in liver disease with reference to extracellular matrix composition. Liver Int. 1999, 19, 32–38. [Google Scholar] [CrossRef]
- Mak, K.M.; Mei, R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat. Rec. 2017, 300, 1371–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenta, P.S.; Harrison, D. Expression and potential role of the extracellular matrix in hepatic ontogenesis: A review. Microsc. Res. Tech. 1997, 39, 372–386. [Google Scholar] [CrossRef]
- Cameron, K.; Tan, R.; Schmidt-Heck, W.; Campos, G.; Lyall, M.J.; Wang, Y.; Lucendo-Villarin, B.; Szkolnicka, D.; Bates, N.; Kimber, S.J.; et al. Recombinant Laminins Drive the Differentiation and Self-Organization of hESC-Derived Hepatocytes. Stem Cell Rep. 2015, 5, 1250–1262. [Google Scholar] [CrossRef] [Green Version]
- Reid, L.M.; Fiorino, A.S.; Sigal, S.H.; Brill, S.; Holst, P.A. Extracellular matrix gradients in the space of Disse: Relevance to liver biology. Hepatology 1992, 15, 1198–1203. [Google Scholar] [CrossRef]
- Ushiki, T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Godoy, P.; Hengstler, J.G.; Ilkavets, I.; Meyer, C.; Bachmann, A.; Müller, A.; Tuschl, G.; Mueller, S.O.; Dooley, S. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology 2009, 49, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Dzieran, J.; Liu, Y.; Schindler, F.; Munker, S.; Müller, A.; Coulouarn, C.; Dooley, S. Distinct dedifferentiation processes affect caveolin-1 expression in hepatocytes. Cell Commun. Signal. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, V.; Berglund, E.J.; Chen, D.X.; Kidambi, S. Substrate stiffness regulates primary hepatocyte functions. RSC Adv. 2015, 5, 80956–80966. [Google Scholar] [CrossRef]
- Desai, S.S.; Tung, J.C.; Zhou, V.X.; Grenert, J.P.; Malato, Y.; Rezvani, M.; Español-Suñer, R.; Willenbring, H.; Weaver, V.M.; Chang, T.T. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology 2016, 64, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Stevens, K.R.; Schwartz, R.E.; Alejandro, B.S.; Huang, J.H.; Bhatia, S.N. Micropatterned Cell–Cell Interactions Enable Functional Encapsulation of Primary Hepatocytes in Hydrogel Microtissues. Tissue Eng. Part A 2014, 20, 2200–2212. [Google Scholar] [CrossRef] [Green Version]
- Zeisberg, M.; Kramer, K.; Sindhi, N.; Sarkar, P.; Upton, M.; Kalluri, R. De-differentiation of primary human hepatocytes depends on the composition of specialized liver basement membrane. Mol. Cell. Biochem. 2006, 283, 181–189. [Google Scholar] [CrossRef]
- Zeigerer, A.; Wuttke, A.; Marsico, G.; Seifert, S.; Kalaidzidis, Y.; Zerial, M. Functional properties of hepatocytes in vitro are correlated with cell polarity maintenance. Exp. Cell Res. 2017, 350, 242–252. [Google Scholar] [CrossRef]
- Moghe, P.V.; Berthiaume, F.; Ezzell, R.M.; Toner, M.; Tompkins, R.G.; Yarmush, M.L. Culture matrix configuration and composition in the maintenance of hepatocyte polarity and function. Biomaterials 1996, 17, 373–385. [Google Scholar] [CrossRef]
- Ballatori, N.; Henson, J.H.; Seward, D.J.; Cai, S.-Y.; Runnegar, M.; Fricker, G.; Miller, D.S.; Boyer, J.L. Retention of structural and functional polarity in cultured skate hepatocytes undergoing in vitro morphogenesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 167–179. [Google Scholar] [CrossRef]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, G.; Su, X.; Jin, C.; Yu, B.; Yu, X.; Lv, Z.; Ma, H.; Zhang, M.; Wei, W.; et al. Maintenance of Primary Hepatocyte Functions In Vitro by Inhibiting Mechanical Tension-Induced YAP Activation. Cell Rep. 2019, 29, 3212–3222.e4. [Google Scholar] [CrossRef] [Green Version]
- Pocaterra, A.; Santinon, G.; Romani, P.; Brian, I.; Dimitracopoulos, A.; Ghisleni, A.; Carnicer-Lombarte, A.; Forcato, M.; Braghetta, P.; Montagner, M.; et al. F-actin dynamics regulates mammalian organ growth and cell fate maintenance. J. Hepatol. 2019, 71, 130–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadriraju, K.; Hansen, L.K. Extracellular matrix-dependent myosin dynamics during G1-S phase cell cycle progression in hepatocytes. Exp. Cell Res. 2004, 300, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Görtzen, J.; Schierwagen, R.; Bierwolf, J.; Klein, S.; Uschner, F.E.; van der Ven, P.F.; Fürst, D.O.; Strassburg, C.P.; Laleman, W.; Pollok, J.-M.; et al. Interplay of Matrix Stiffness and c-SRC in Hepatic Fibrosis. Front. Physiol. 2015, 6, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, K.; Gong, Z.; Rylander, A.; Shenoy, V.B.; Janmey, P.A. Opposite responses of normal hepatocytes and hepatocellular carcinoma cells to substrate viscoelasticity. Biomater. Sci. 2020, 8, 1316–1328. [Google Scholar] [CrossRef]
- Moghe, P.V.; Ezzell, R.M.; Toner, M.; Tompkins, R.G.; Yarmush, M.L. Role of β1 Integrin Distribution in Morphology and Function of Collagen-Sandwiched Hepatocytes. Tissue Eng. 1997, 3, 1–16. [Google Scholar] [CrossRef]
- Xiang, C.; Du, Y.; Meng, G.; Soon Yi, L.; Sun, S.; Song, N.; Zhang, X.; Xiao, Y.; Wang, J.; Yi, Z.; et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science 2019, 364, 399–402. [Google Scholar] [CrossRef]
- Yu, F.-X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Bhowmick, N.A.; Ghiassi, M.; Bakin, A.; Aakre, M.; Lundquist, C.A.; Engel, M.E.; Arteaga, C.L.; Moses, H.L. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 2001, 12, 27–36. [Google Scholar] [CrossRef]
- Wells, R.G. Tissue mechanics and fibrosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 884–890. [Google Scholar] [CrossRef] [Green Version]
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol. 2019, 81, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broders-Bondon, F.; Nguyen Ho-Bouldoires, T.H.; Fernandez-Sanchez, M.-E.; Farge, E. Mechanotransduction in tumor progression: The dark side of the force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mui, K.L.; Chen, C.S.; Assoian, R.K. The mechanical regulation of integrin–cadherin crosstalk organizes cells, signaling and forces. J. Cell Sci. 2016, 129, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruitt, B.L.; Dunn, A.R.; Weis, W.I.; Nelson, W.J. Mechano-transduction: From molecules to tissues. PLoS Biol. 2014, 12, e1001996. [Google Scholar] [CrossRef]
- Han, M.K.L.; de Rooij, J. Converging and Unique Mechanisms of Mechanotransduction at Adhesion Sites. Trends Cell Biol. 2016, 26, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Mammoto, T.; Ingber, D.E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 2012, 125, 3061–3073. [Google Scholar] [CrossRef] [Green Version]
- Popa, I.; Gutzman, J.H. The extracellular matrix–myosin pathway in mechanotransduction: From molecule to tissue. Emerg. Top. Life Sci. 2018, 2, 727–737. [Google Scholar]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, B.D.; Yap, A.S. Towards a Dynamic Understanding of Cadherin-Based Mechanobiology. Trends Cell Biol. 2015, 25, 803–814. [Google Scholar] [CrossRef]
- Olsen, A.L.; Bloomer, S.A.; Chan, E.P.; Gaça, M.D.A.; Georges, P.C.; Sackey, B.; Uemura, M.; Janmey, P.A.; Wells, R.G. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G110–G118. [Google Scholar] [CrossRef] [Green Version]
- Charrier, E.E.; Pogoda, K.; Wells, R.G.; Janmey, P.A. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat. Commun. 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlak, J.; Singh, P.K.; Rittelmeyer, I. Regulation of Liver Enriched Transcription Factors in Rat Hepatocytes Cultures on Collagen and EHS Sarcoma Matrices. PLoS ONE 2015, 10, e0124867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrader, J.; Gordon-Walker, T.T.; Aucott, R.L.; van Deemter, M.; Quaas, A.; Walsh, S.; Benten, D.; Forbes, S.J.; Wells, R.G.; Iredale, J.P. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 2011, 53, 1192–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Shin, J.; Park, H.-M.; Kim, Y.-G.; Kim, B.-G.; Oh, J.-W.; Cho, S.-W. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules 2014, 15, 206–218. [Google Scholar] [CrossRef]
- Lewis, P.L.; Su, J.; Yan, M.; Meng, F.; Glaser, S.S.; Alpini, G.D.; Green, R.M.; Sosa-Pineda, B.; Shah, R.N. Complex bile duct network formation within liver decellularized extracellular matrix hydrogels. Sci. Rep. 2018, 8, 12220. [Google Scholar] [CrossRef]
- Williams, C.; Sullivan, K.; Black, L.D., 3rd. Partially Digested Adult Cardiac Extracellular Matrix Promotes Cardiomyocyte Proliferation In Vitro. Adv. Healthc. Mater. 2015, 4, 1545–1554. [Google Scholar] [CrossRef] [Green Version]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lanza, R.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123983701. [Google Scholar]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Szczesny, S.E.; Caliari, S.R.; Charrier, E.E.; Chaudhuri, O.; Cao, X.; Lin, Y.; Mauck, R.L.; Janmey, P.A.; Burdick, J.A.; et al. Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc. Natl. Acad. Sci. USA 2018, 115, E2686–E2695. [Google Scholar] [CrossRef] [Green Version]
- Mandal, K.; Aroush, D.R.-B.; Graber, Z.T.; Wu, B.; Park, C.Y.; Fredberg, J.J.; Guo, W.; Baumgart, T.; Janmey, P.A. Soft Hyaluronic Gels Promote Cell Spreading, Stress Fibers, Focal Adhesion, and Membrane Tension by Phosphoinositide Signaling, Not Traction Force. ACS Nano 2019, 13, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Gheibi, P.; Gao, Y.; Foster, E.; Son, K.J.; You, J.; Stybayeva, G.; Patel, D.; Revzin, A. Cell biology is different in small volumes: Endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels. Sci. Rep. 2016, 6, 33980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deegan, D.B.; Zimmerman, C.; Skardal, A.; Atala, A.; Shupe, T.D. Stiffness of hyaluronic acid gels containing liver extracellular matrix supports human hepatocyte function and alters cell morphology. J. Mech. Behav. Biomed. Mater. 2015, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Cuzzucoli, F.; Jiang, J.; Liang, L.-G.; Wang, Y.; Kong, M.; Zhao, X.; Cui, W.; Li, J.; Wang, S. Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing. Lab Chip 2018, 18, 3379–3392. [Google Scholar] [CrossRef]
- You, J.; Park, S.-A.; Shin, D.-S.; Patel, D.; Raghunathan, V.K.; Kim, M.; Murphy, C.J.; Tae, G.; Revzin, A. Characterizing the effects of heparin gel stiffness on function of primary hepatocytes. Tissue Eng. Part A 2013, 19, 2655–2663. [Google Scholar] [CrossRef]
- Caligaris, C.; Vázquez-Victorio, G.; Sosa-Garrocho, M.; Ríos-López, D.G.; Marín-Hernández, A.; Macías-Silva, M. Actin-cytoskeleton polymerization differentially controls the stability of Ski and SnoN co-repressors in normal but not in transformed hepatocytes. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 1832–1841. [Google Scholar] [CrossRef]
- Tse, J.R.; Engler, A.J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 2010, 47, 10–16, Chapter 10. [Google Scholar] [CrossRef]
- Díaz-Bello, B.; Monroy-Romero, A.X.; Pérez-Calixto, D.; Zamarrón-Hernández, D.; Serna-Marquez, N.; Vázquez-Victorio, G.; Hautefeuille, M. Method for the Direct Fabrication of Polyacrylamide Hydrogels with Controlled Stiffness in Polystyrene Multiwell Plates for Mechanobiology Assays. ACS Biomater. Sci. Eng. 2019, 5, 4219–4227. [Google Scholar]
- Cretu, A.; Castagnino, P.; Assoian, R. Studying the effects of matrix stiffness on cellular function using acrylamide-based hydrogels. J. Vis. Exp. 2010. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Dominguez, A.A.; Nam, S.; Stowers, R.S.; Qi, L.S.; Chaudhuri, O. Identification of cell context-dependent YAP-associated proteins reveals β 1 and β 4 integrin mediate YAP translocation independently of cell spreading. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Vázquez-Victorio, G.; Peto-Gutiérrez, C.; Díaz-Bello, B.; Cano-Jorge, M.; Pérez-Calixto, D.; Jiménez-Escobar, A.; Espinosa-Matías, S.; Lara Martínez, R.; Courson, R.; Malaquin, L.; et al. Building a microfluidic cell culture platform with stiffness control using Loctite 3525 glue. Lab Chip 2019, 19, 3512–3525. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.; Discher, D.E. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J. Cell Sci. 2014, 127, 3005–3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.; Irianto, J.; Discher, D.E. Mechanosensing by the nucleus: From pathways to scaling relationships. J. Cell Biol. 2017, 216, 305–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxboim, A.; Irianto, J.; Swift, J.; Athirasala, A.; Shin, J.-W.; Rehfeldt, F.; Discher, D.E. Coordinated increase of nuclear tension and lamin-A with matrix stiffness outcompetes lamin-B receptor that favors soft tissue phenotypes. Mol. Biol. Cell 2017, 28, 3333–3348. [Google Scholar] [CrossRef]
- Kyrylkova, K.; Kyryachenko, S.; Leid, M.; Kioussi, C. Detection of Apoptosis by TUNEL Assay. In Odontogenesis; Kioussi, C., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 887, pp. 41–47. ISBN 9781617798597. [Google Scholar]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar] [CrossRef] [Green Version]
- Ramon, C.; Cormier Maria, S.; Shanmugasundaram, N.; Robert, C.; Joo, O.; Glenn, H. Chemical Induction of Human Adipose Stromal Cells Into Hepatocyte-Like Cells under Various Differentiation Conditions. J. Stem Cell Res. Ther. 2018, 8. [Google Scholar] [CrossRef]
- Wells, R.G. Location, location, location: Cell-level mechanics in liver fibrosis. Hepatology 2016, 64, 32–33. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, M.; Monga, S.P.; Liu, Y.; Brodie, S.G.; Tang, Y.; Li, C.; Mishra, L.; Deng, C.X. Smad proteins and hepatocyte growth factor control parallel regulatory pathways that converge on beta1-integrin to promote normal liver development. Mol. Cell. Biol. 2001, 21, 5122–5131. [Google Scholar] [CrossRef] [Green Version]
- Lora, J.M.; Rowader, K.E.; Soares, L.; Giancotti, F.; Zaret, K.S. Alpha3beta1-integrin as a critical mediator of the hepatic differentiation response to the extracellular matrix. Hepatology 1998, 28, 1095–1104. [Google Scholar] [CrossRef]
- Kim, S.-H.; Akaike, T. Epidermal growth factor signaling for matrix-dependent cell proliferation and differentiation in primary cultured hepatocytes. Tissue Eng. 2007, 13, 601–609. [Google Scholar] [CrossRef]
- Speicher, T.; Siegenthaler, B.; Bogorad, R.L.; Ruppert, R.; Petzold, T.; Padrissa-Altes, S.; Bachofner, M.; Anderson, D.G.; Koteliansky, V.; Fässler, R.; et al. Knockdown and knockout of β1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat. Commun. 2014, 5, 3862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialik, J.F.; Ding, M.; Speight, P.; Dan, Q.; Miranda, M.Z.; Di Ciano-Oliveira, C.; Kofler, M.M.; Rotstein, O.D.; Pedersen, S.F.; Szászi, K.; et al. Profibrotic epithelial phenotype: A central role for MRTF and TAZ. Sci. Rep. 2019, 9, 4323. [Google Scholar] [CrossRef] [PubMed]
- de Juan, C.; Benito, M.; Fabregat, I. Regulation of albumin expression in fetal rat hepatocytes cultured under proliferative conditions: Role of epidermal growth factor and hormones. J. Cell. Physiol. 1992, 152, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Takashi, H.; Katsumi, M.; Toshihiro, A. Hepatocytes maintain their function on basement membrane formed by epithelial cells. Biochem. Biophys. Res. Commun. 2007, 359, 151–156. [Google Scholar] [CrossRef]
- Baqué, S.; Guinovart, J.J.; Ferrer, J.C. Glycogenin, the primer of glycogen synthesis, binds to actin. FEBS Lett. 1997, 417, 355–359. [Google Scholar] [CrossRef] [Green Version]
- García-Rocha, M.; Roca, A.; De La Iglesia, N.; Baba, O.; Fernández-Novell, J.M.; Ferrer, J.C.; Guinovart, J.J. Intracellular distribution of glycogen synthase and glycogen in primary cultured rat hepatocytes. Biochem. J. 2001, 357, 17–24. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Márquez, N.; Rodríguez-Hernández, A.; Ayala-Reyes, M.; Martínez-Hernández, L.O.; Peña-Rico, M.Á.; Carretero-Ortega, J.; Hautefeuille, M.; Vázquez-Victorio, G. Fibrillar Collagen Type I Participates in the Survival and Aggregation of Primary Hepatocytes Cultured on Soft Hydrogels. Biomimetics 2020, 5, 30. https://doi.org/10.3390/biomimetics5020030
Serna-Márquez N, Rodríguez-Hernández A, Ayala-Reyes M, Martínez-Hernández LO, Peña-Rico MÁ, Carretero-Ortega J, Hautefeuille M, Vázquez-Victorio G. Fibrillar Collagen Type I Participates in the Survival and Aggregation of Primary Hepatocytes Cultured on Soft Hydrogels. Biomimetics. 2020; 5(2):30. https://doi.org/10.3390/biomimetics5020030
Chicago/Turabian StyleSerna-Márquez, Nathalia, Adriana Rodríguez-Hernández, Marisol Ayala-Reyes, Lorena Omega Martínez-Hernández, Miguel Ángel Peña-Rico, Jorge Carretero-Ortega, Mathieu Hautefeuille, and Genaro Vázquez-Victorio. 2020. "Fibrillar Collagen Type I Participates in the Survival and Aggregation of Primary Hepatocytes Cultured on Soft Hydrogels" Biomimetics 5, no. 2: 30. https://doi.org/10.3390/biomimetics5020030
APA StyleSerna-Márquez, N., Rodríguez-Hernández, A., Ayala-Reyes, M., Martínez-Hernández, L. O., Peña-Rico, M. Á., Carretero-Ortega, J., Hautefeuille, M., & Vázquez-Victorio, G. (2020). Fibrillar Collagen Type I Participates in the Survival and Aggregation of Primary Hepatocytes Cultured on Soft Hydrogels. Biomimetics, 5(2), 30. https://doi.org/10.3390/biomimetics5020030




