Biological Evaluation of Oil-in-Water Microemulsions as Carriers of Benzothiophene Analogues for Dermal Applications
Abstract
:1. Introduction
2. Materials and Methods
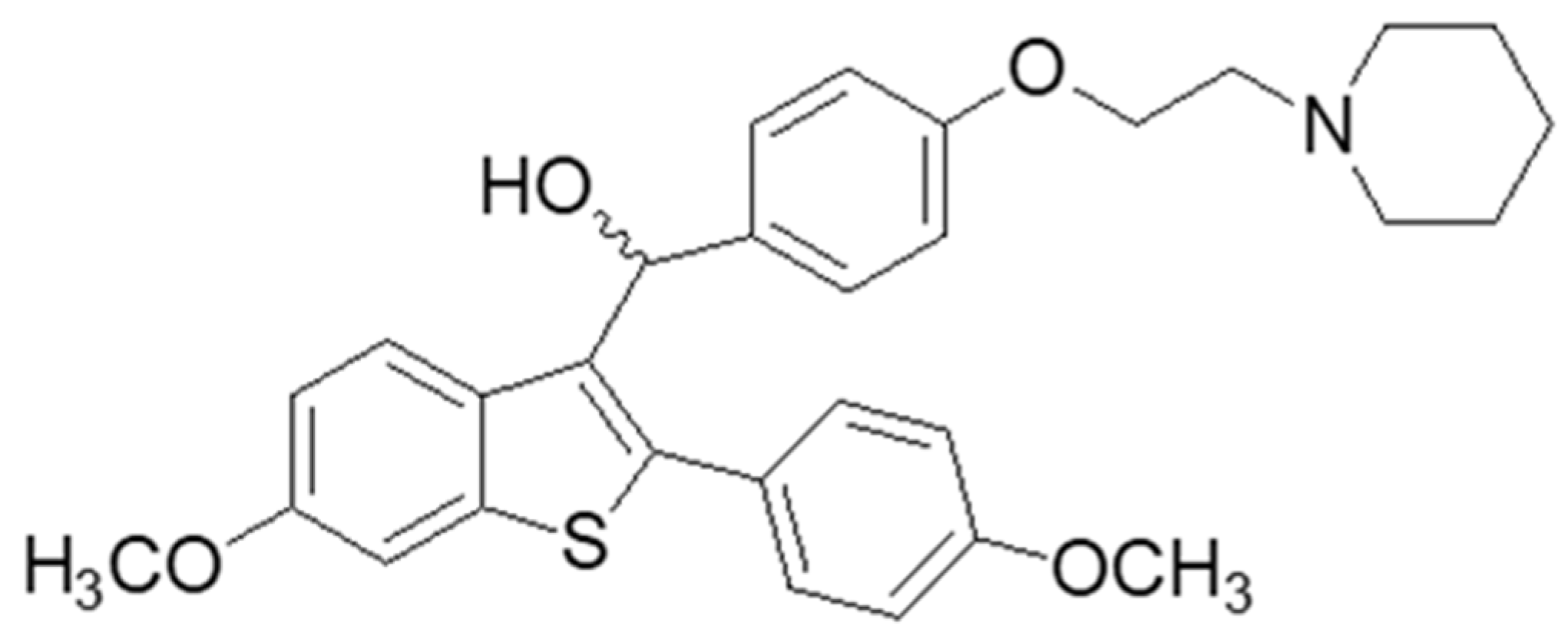
2.1. Materials
2.2. Cell Lines, Culture, and Treatments
2.3. Cell Survival and Cytotoxicity Assays
2.4. Western Blotting
2.5. Ex Vivo Permeation Study
2.6. Differential Tape Stripping
2.7. Quantification of DPS-2
3. Results
3.1. In Vitro Evaluation
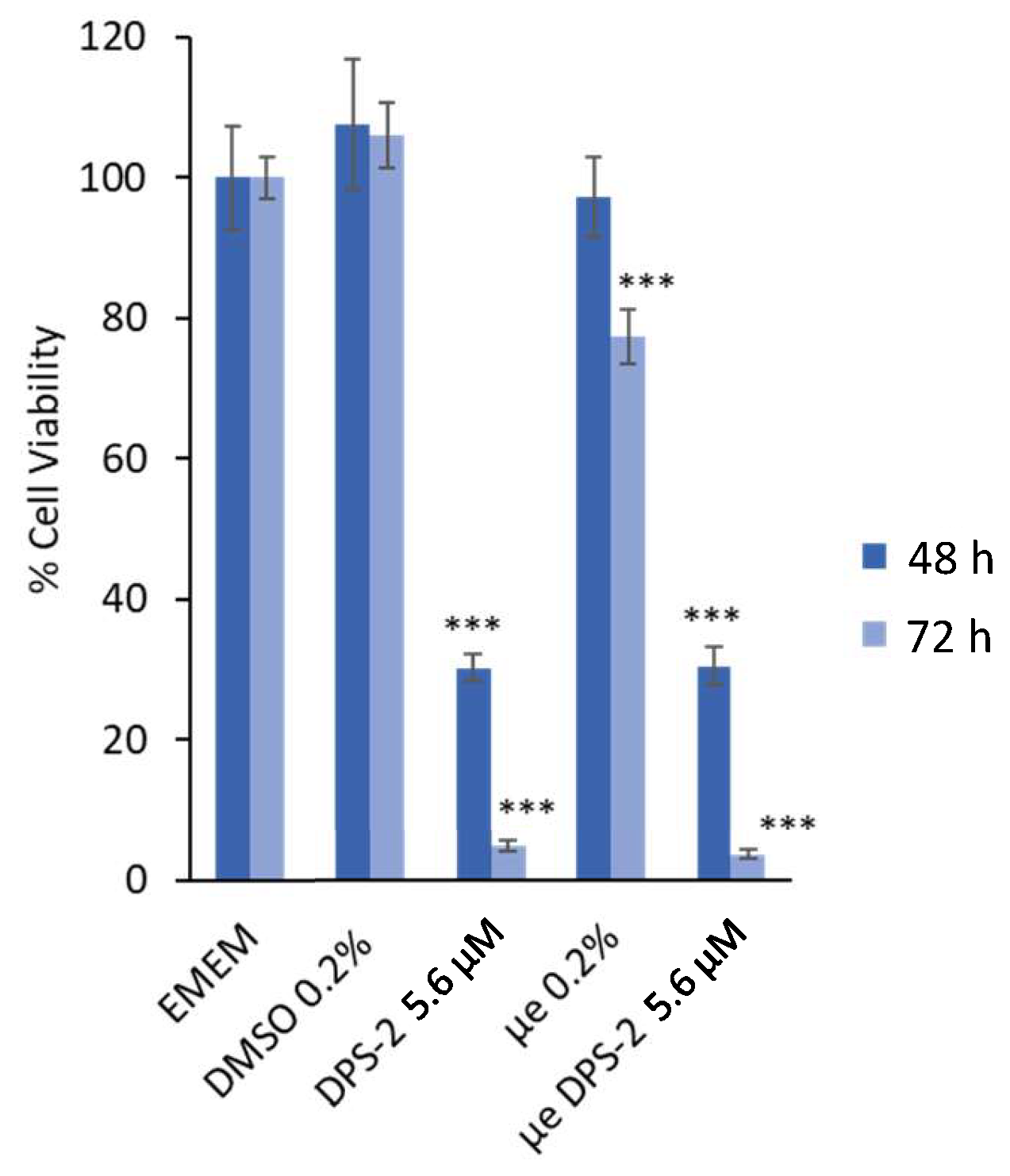
3.1.1. Cell Proliferation and Cytotoxicity Assays
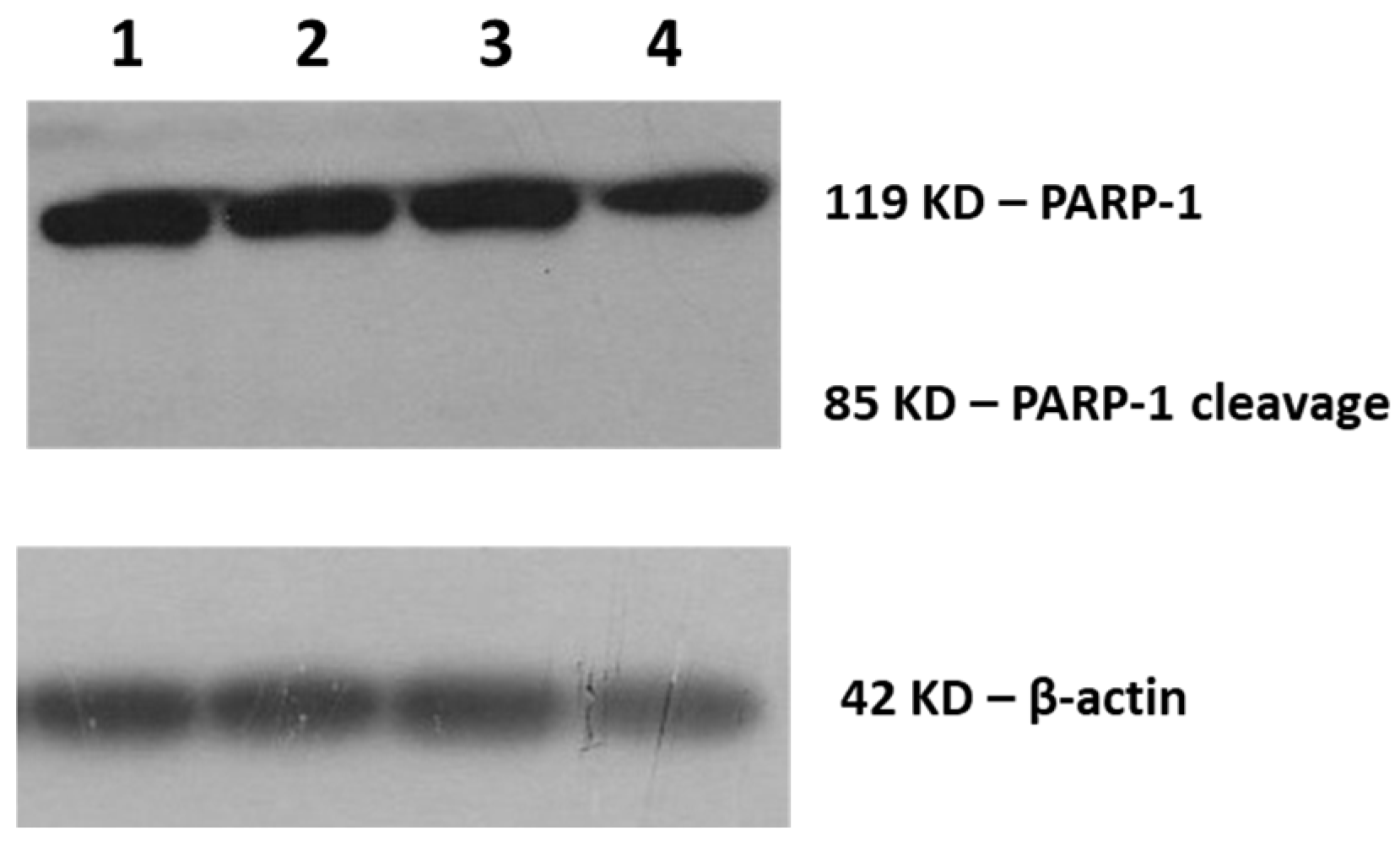
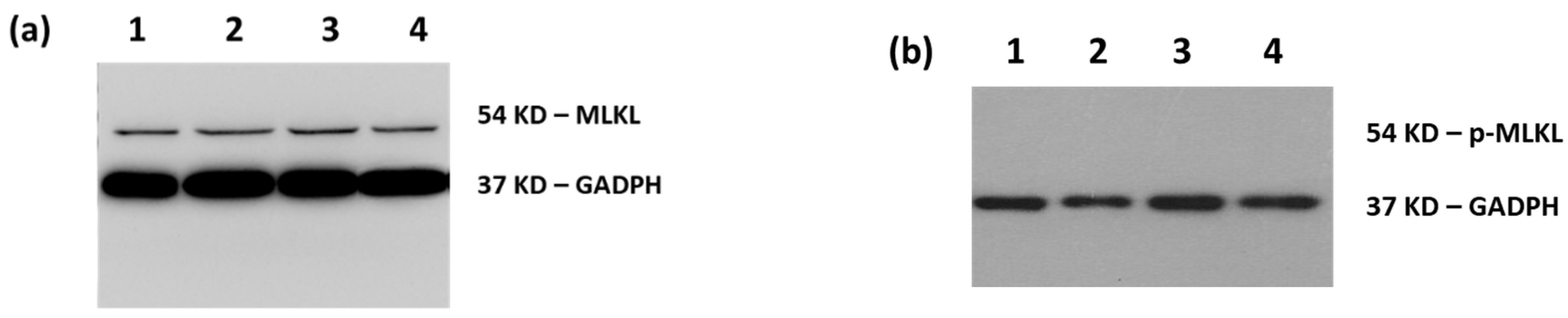
3.1.2. Molecular Analysis by Western Blotting
3.2. Ex Vivo Evaluation
3.2.1. Ex Vivo Permeation Study
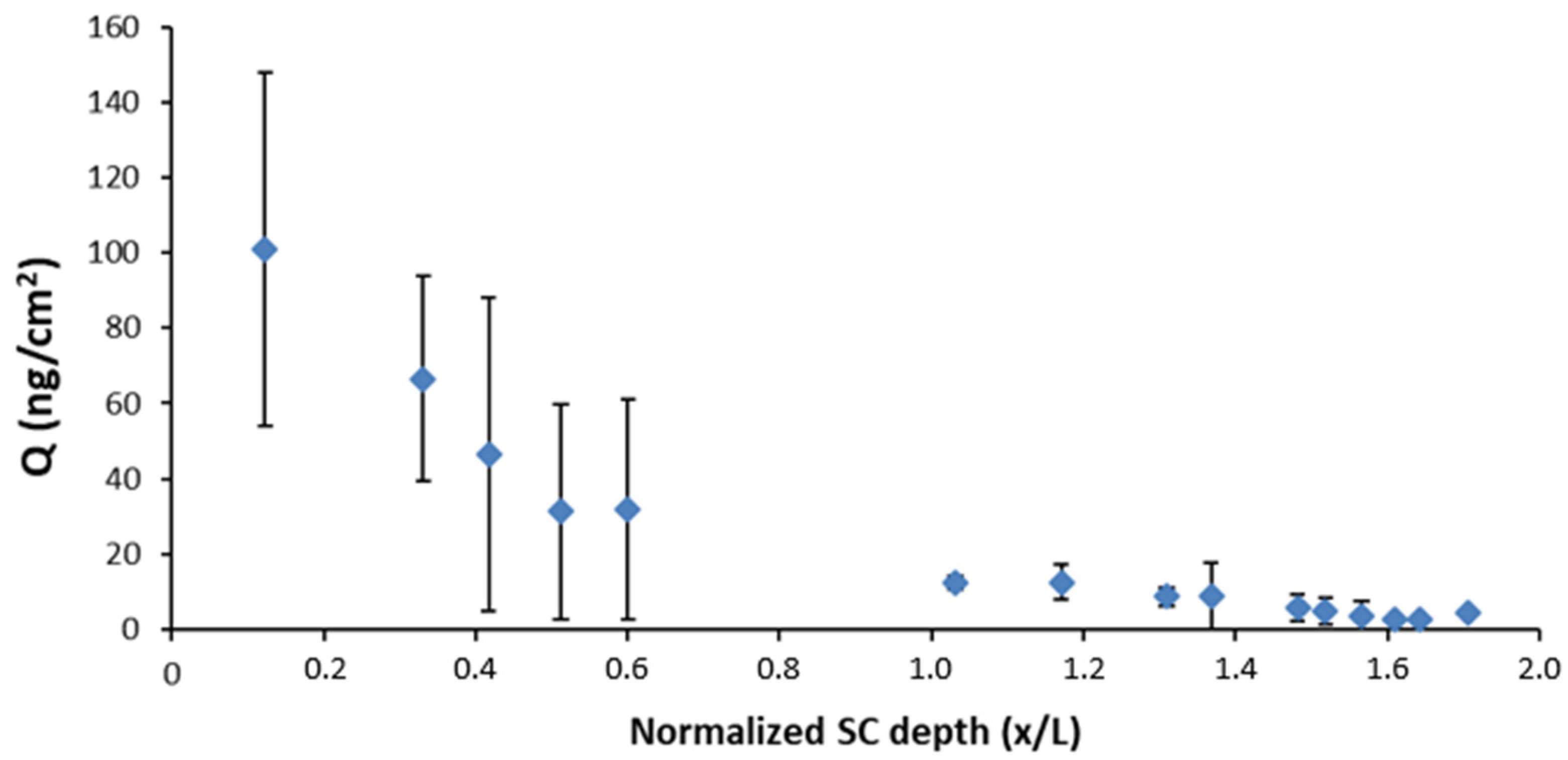
3.2.2. Differential Tape Stripping
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moshikur, R.M.; Chowdhury, M.R.; Moniruzzaman, M.; Goto, M. Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem. 2020. [Google Scholar] [CrossRef]
- Rawat, M.; Singh, D.; Saraf, S.; Saraf, S. Nanocarriers: Promising Vehicle for Bioactive Drugs. Biol. Pharm. Bull. 2006, 29, 1790–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullotti, E.; Yeo, Y.; Gullotti, E.; Yeo, Y. Extracellularly Activated Nanocarriers: A New Paradigm of Tumor Targeted Drug Delivery reviews Extracellularly Activated Nanocarriers: A New Paradigm of Tumor Targeted Drug Delivery. Mol. Pharm. 2009, 6, 1041–1051. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Moulik, S.P. Biocompatible microemulsions and their prospective uses in drug delivery. J. Pharm. Sci. 2008, 97, 22–45. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Fanun, M. Microemulsions as delivery systems. Curr. Opin. Colloid Interface Sci. 2012, 17, 306–313. [Google Scholar] [CrossRef]
- Ravi, T.P.U.; Padma, T. Nanoemulsions for drug delivery through different routes. Res. Biotechnol. 2011, 2, 1–13. [Google Scholar]
- Schroeder, A.; Heller, D.A.; Winslow, M.M.; Dahlman, J.E.; Pratt, G.W.; Langer, R.; Jacks, T.; Anderson, D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 2012, 12, 39–50. [Google Scholar] [CrossRef]
- Karasulu, E.; Karaca, B.; Alparslan, L.; Karasulu, H.Y. Places of microemulsion and emulsion in cancer therapy: In vitro and in vivo evaluation. In Microemulsions; Fanun, M., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 313–330. [Google Scholar]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Williams, R.O.; Taft, D.R.; McConville, J.T. Strategies of delivery for cancer chemotherapy. In Advanced Drug Formulation Design to Optimize Therapeutic Outcomes; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Spernath, A.; Aserin, A. Microemulsions as carriers for drugs and nutraceuticals. Adv. Colloid Interface Sci. 2006, 128, 47–64. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable drug encapsulation in micelles and microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Theochari, I.; Xenakis, A.; Papadimitriou, V. Nanocarriers for effective drug delivery. Smart Nanocontain. 2020, 315–341. [Google Scholar] [CrossRef]
- Theochari, I.; Papadimitriou, V.; Papahatjis, D.; Assimomytis, N.; Pappou, E.; Pratsinis, H.; Xenakis, A.; Pletsa, V. Oil-In-Water Microemulsions as Hosts for Benzothiophene-Based Cytotoxic Compounds: An Effective Combination. Biomimetics 2018, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulielmaki, M.; Assimomytis, N.; Rozanc, J.; Taki, E.; Christodoulou, I.; Alexopoulos, L.G.; Zoumpourlis, V.; Pintzas, A.; Papahatjis, D. DPS-2: A Novel Dual MEK/ERK and PI3K/AKT Pathway Inhibitor with Powerful Ex Vivo and In Vivo Anticancer Properties. Transl. Oncol. 2019, 12, 932–950. [Google Scholar] [CrossRef]
- Theochari, I.; Goulielmaki, M.; Danino, D.; Papadimitriou, V.; Pintzas, A.; Xenakis, A. Drug nanocarriers for cancer chemotherapy based on microemulsions: The case of Vemurafenib analog PLX4720. Colloids Surf. B Biointerfaces 2017, 154, 350–356. [Google Scholar] [CrossRef]
- Kamble, P.; Sadarani, B.; Majumdar, A.; Bhullar, S. Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv. Sci. Technol. 2017, 41, 124–133. [Google Scholar] [CrossRef]
- Shinde, U.A.; Modani, S.H.; Singh, K.H. Design and Development of Repaglinide Microemulsion Gel for Transdermal Delivery. AAPS PharmSciTech 2018, 19, 315–325. [Google Scholar] [CrossRef]
- Shaimaa, R.; Ali, A.; Nabil, E.A.; Aly, S.R. Microemulsion loaded hydrogel as a promising vehicle for dermal delivery of the antifungal sertaconazole: Design, optimization and ex vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1351–1365. [Google Scholar] [CrossRef]
- Rastogi, V.; Yadav, P.; Verma, A.; Pandit, J.K. Ex vivo and in vivo evaluation of microemulsion based transdermal delivery of E. coli specific T4 bacteriophage: A rationale approach to treat bacterial infection. Eur. J. Pharm. Sci. 2017, 107, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Savić, V.; Todosijević, M.; Ilić, T.; Lukić, M.; Mitsou, E.; Papadimitriou, V.; Avramiotis, S.; Marković, B.; Cekić, N.; Savić, S. Tacrolimus loaded biocompatible lecithin-based microemulsions with improved skin penetration: Structure characterization and in vitro/in vivo performances. Int. J. Pharm. 2017, 529, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Kesavan, B. Effect of chitosan coating on microemulsion for effective dermal clotrimazole delivery. Pharm. Dev. Technol. 2016, 2, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Sood, J.; Sapra, B.; Tiwary, A.K. Microemulsion Transdermal Formulation for Simultaneous Delivery of Valsartan and Nifedipine: Formulation by Design. AAPS PharmSciTech 2017, 18, 1901–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Michniak-Kohn, B.B. Investigation of microemulsion and microemulsion gel formulations for dermal delivery of clotrimazole. Int. J. Pharm. 2018, 536, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Zhang, K.; Zhang, H.; He, Z.; Xia, Q.; Zhao, J.; Feng, N. Co-delivery of evodiamine and rutaecarpine in a microemulsion-based hyaluronic acid hydrogel for enhanced analgesic effects on mouse pain models. Int. J. Pharm. 2017, 528, 100–106. [Google Scholar] [CrossRef]
- Yehia, N.D.; Hathout, R.; Attia, M.R.; Elmazar, A.D.; Mortada, M.M. Anti-tumor efficacy of an integrated Methyl Dihydrojasmonate transdermal microemulsion system targeting breast cancer cells: In-vitro and In-vivo studies. Colloids Surf. B Biointerfaces 2017, 155, 512–521. [Google Scholar] [CrossRef]
- Cao, M.; Ren, L.; Chen, G. Formulation Optimization and Ex Vivo and In Vivo Evaluation of Celecoxib Microemulsion-Based Gel for Transdermal Delivery. AAPS PharmSciTech 2017, 18, 1960–1971. [Google Scholar] [CrossRef]
- Parra, A.; Clares, B.; Rosselló, A.; Garduño-Ramírez, M.L.; Abrego, G.; García, M.L.; Calpena, A.C. Ex vivo permeation of carprofen from nanoparticles: A comprehensive study through human, porcine and bovine skin as anti-inflammatory agent. Int. J. Pharm. 2016, 501, 10–17. [Google Scholar] [CrossRef]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Mushroom ethanolic extracts as cosmeceuticals ingredients: Safety and ex vivo skin permeation studies. Food Chem. Toxicol. 2019, 127, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pany, A.; Klang, V.; Peinhopf, C.; Zecevic, A.; Ruthofer, J.; Valenta, C. Hair removal and bioavailability of chemicals: Effect of physicochemical properties of drugs and surfactants on skin permeation ex vivo. Int. J. Pharm. 2019, 567, 118477. [Google Scholar] [CrossRef] [PubMed]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig ear skin ex vivo as a model for in vivo dermatopharmacokinetic studies in man. Pharm. Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Koryllou, A.; Patrinou-Georgoula, M.; Troungos, C.; Pletsa, V. Cell death induced by N-methyl-N-nitrosourea, a model SN1 methylating agent, in two lung cancer cell lines of human origin. Apoptosis 2009, 14, 1121–1133. [Google Scholar] [CrossRef]
- OECD. OECD Guidline for the Testing of Chemicals; Skin Absorption: In Vitro Method (Test No. 428); OECD Publishing: Paris, France, 2004. [Google Scholar]
- Committee for Medicinal Products for Human Use (CHMP), European Medicine Agency. Draft Guideline on Quality and Equivalence of Topical Products; Committee for Medicinal Products for Human Use (CHMP), European Medicine Agency: London, UK, 2018. [Google Scholar]
- Pantelic, I.; Ilic, T.; Markovic, B.; Savic, S.; Lukic, M.; Savic, S. A stepwise protocol for drug permeation assessment that combines heat-separated porcine ear epidermis and vertical diffusion cells. Hem. Ind. Ind. 2017, 72, 47–53. [Google Scholar] [CrossRef]
- Ilić, T.; Savić, S.; Batinić, B.; Marković, B.; Schmidberger, M.; Lunter, D.; Savić, M.; Savić, S. Combined use of biocompatible nanoemulsions and solid microneedles to improve transport of a model NSAID across the skin: In vitro and in vivo studies. Eur. J. Pharm. Sci. 2018, 125, 110–119. [Google Scholar] [CrossRef]
- Vlachy, N.; Touraud, D.; Heilmann, J.; Kunz, W. Determining the cytotoxicity of catanionic surfactant mixtures on HeLa cells. Colloids Surf. B Biointerfaces 2009, 70, 278–280. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [Green Version]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Ménissier-De Murcia, J. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis: Lesson from an uncleavable mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A.; et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Ren, J.; Huang, D.; He, W.T.; Song, Y.; Yang, C.; Li, W.; Zheng, X.; Chen, P.; et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014, 24, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, Y.; Declercq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W.; et al. MLKL Compromises Plasma Membrane Integrity by Binding to Phosphatidylinositol Phosphates. Cell Rep. 2014, 7, 971–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Ski. Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef]
- Desmet, E.; van Gele, M.; Lambert, J. Topically applied lipid- and surfactant-based nanoparticles in the treatment of skin disorders. Expert Opin. Drug Deliv. 2017, 14, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef]
- He, C.-X.; He, Z.-G.; Gao, J.-Q. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2010, 7, 445–460. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123, 369–385. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Azeem, A.; Ahmad, F.J.; Khar, R.K.; Pathan, S.A.; Khan, Z.I. Microemulsions: A Novel Approach to Enhanced Drug Delivery. Recent Pat. Drug Deliv. Formul. 2008, 2, 238–257. [Google Scholar] [CrossRef]
- de Moura, M.; van Houten, B. Mechanims of chromosomal instability in melanoma. Environ. Mol. Mutagen. 2010, 405, 391–405. [Google Scholar] [CrossRef]
- Csányi, E.; Bakonyi, M.; Kovács, A.; Budai-Szűcs, M.; Csóka, I.; Berkó, S. Development of Topical Nanocarriers for Skin Cancer Treatment Using Quality by Design Approach. Curr. Med. Chem. 2019, 26, 6440–6458. [Google Scholar] [CrossRef] [PubMed]
- Todo, H. Transdermal permeation of drugs in various animal species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teichmann, A.; Heuschkel, S.; Jacobi, U.; Presse, G.; Neubert, R.H.H.; Sterry, W.; Lademann, J. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur. J. Pharm. Biopharm. 2007, 67, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Sirirak, T. Development and skin penetration pathway evaluation of microemulsions for enhancing the dermal delivery of celecoxib. Colloids Surf. B Biointerfaces 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Mak, W.C.; Jung, S.; Knorr, F.; Meinke, M.C.; Richter, H.; Rühl, E.; Cheung, K.Y.; Tran, N.B.N.N.; Lademann, J. Do nanoparticles have a future in dermal drug delivery? J. Control. Release 2017, 246, 174–182. [Google Scholar] [CrossRef]
- Islam, M.R.; Chowdhury, M.R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic liquid-in-oil microemulsions prepared with biocompatible choline carboxylic acids for improving the transdermal delivery of a sparingly soluble drug. Pharmaceutics 2020, 12, 392. [Google Scholar] [CrossRef]
- Todosijević, M.N.; Brezesinski, G.; Savić, S.D.; Neubert, R.H.H. Sucrose esters as biocompatible surfactants for penetration enhancement: An insight into the mechanism of penetration enhancement studied using stratum corneum model lipids and Langmuir monolayers. Eur. J. Pharm. Sci. 2017, 99, 161–172. [Google Scholar] [CrossRef]
- Pajić, N.B.; Ilić, T.; Nikolić, I.; Dobričić, V.; Pantelić, I.; Savić, S. Alkyl polyglucoside-based adapalene-loaded microemulsions for targeted dermal delivery: Structure, stability and comparative biopharmaceutical characterization with a conventional dosage form. J. Drug Deliv. Sci. Technol. 2019, 54, 101245. [Google Scholar] [CrossRef]

| Sample | % of Cell Death | ±SD |
|---|---|---|
| Not treated | 4.0 | 0.4 |
| DMSO/DPS-2 5.6 μM | 6.9 | 0.9 |
| O/W microemulsion empty | 3.6 | 0.6 |
| O/W microemulsion loaded/DPS-2 5.6 μM | 12.2 | 0.6 |
| Sample | % of Cell Death | ±SD |
|---|---|---|
| Not treated | 8.7 | 0.5 |
| DMSO/DPS-2 5.6 μM | 9.8 | 0.4 |
| O/W microemulsion empty | 9.0 | 0.6 |
| O/W microemulsion loaded/DPS-2 5.6 μM | 18.7 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theochari, I.; Ilic, T.; Nicolic, I.; Dobricic, V.; Tenchiou, A.; Papahatjis, D.; Savic, S.; Xenakis, A.; Papadimitriou, V.; Pletsa, V. Biological Evaluation of Oil-in-Water Microemulsions as Carriers of Benzothiophene Analogues for Dermal Applications. Biomimetics 2021, 6, 10. https://doi.org/10.3390/biomimetics6010010
Theochari I, Ilic T, Nicolic I, Dobricic V, Tenchiou A, Papahatjis D, Savic S, Xenakis A, Papadimitriou V, Pletsa V. Biological Evaluation of Oil-in-Water Microemulsions as Carriers of Benzothiophene Analogues for Dermal Applications. Biomimetics. 2021; 6(1):10. https://doi.org/10.3390/biomimetics6010010
Chicago/Turabian StyleTheochari, Ioanna, Tanja Ilic, Ines Nicolic, Vladimir Dobricic, Alia Tenchiou, Demetris Papahatjis, Snezana Savic, Aristotelis Xenakis, Vassiliki Papadimitriou, and Vasiliki Pletsa. 2021. "Biological Evaluation of Oil-in-Water Microemulsions as Carriers of Benzothiophene Analogues for Dermal Applications" Biomimetics 6, no. 1: 10. https://doi.org/10.3390/biomimetics6010010
APA StyleTheochari, I., Ilic, T., Nicolic, I., Dobricic, V., Tenchiou, A., Papahatjis, D., Savic, S., Xenakis, A., Papadimitriou, V., & Pletsa, V. (2021). Biological Evaluation of Oil-in-Water Microemulsions as Carriers of Benzothiophene Analogues for Dermal Applications. Biomimetics, 6(1), 10. https://doi.org/10.3390/biomimetics6010010









