Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies
Abstract
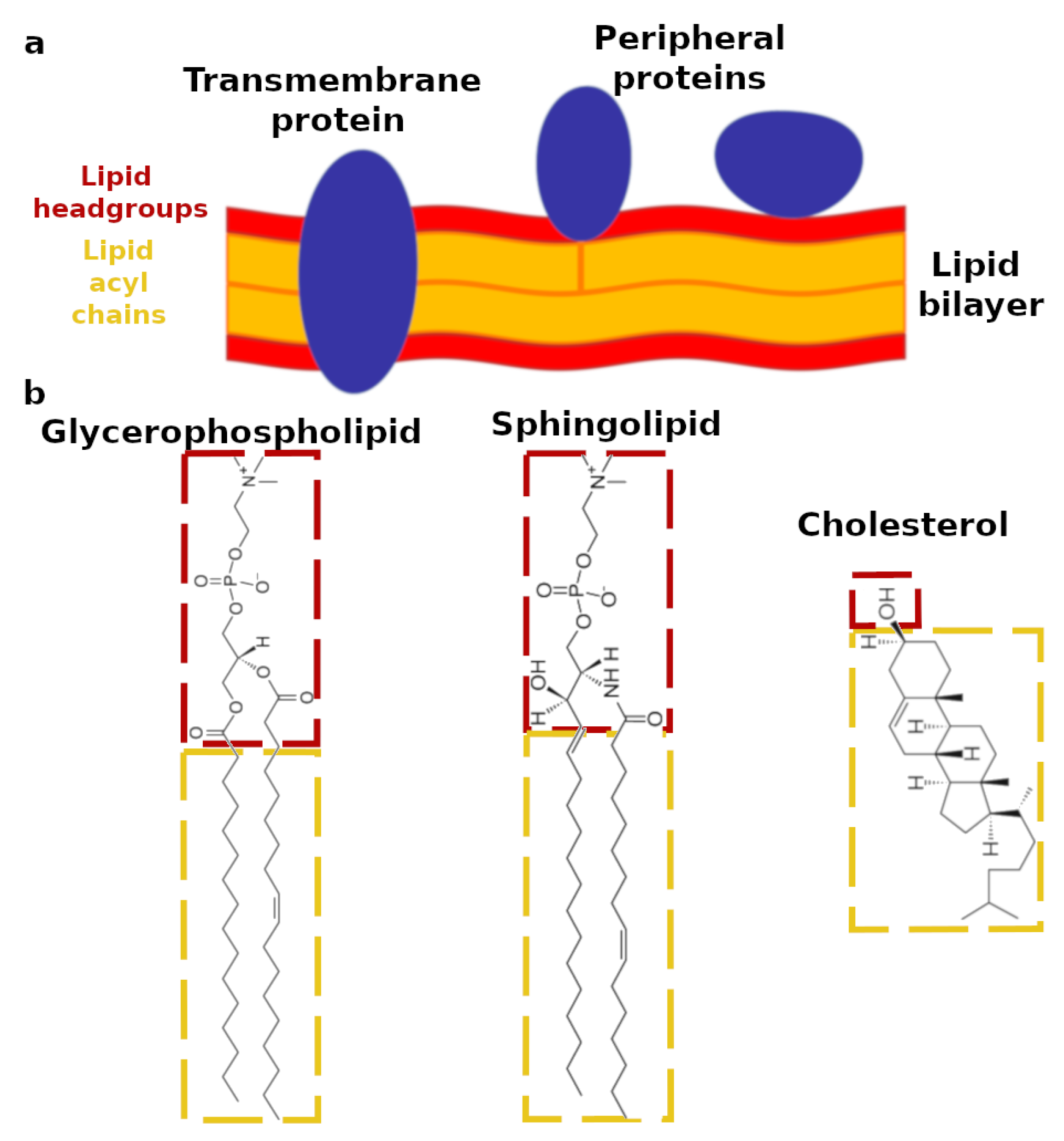
:1. Introduction
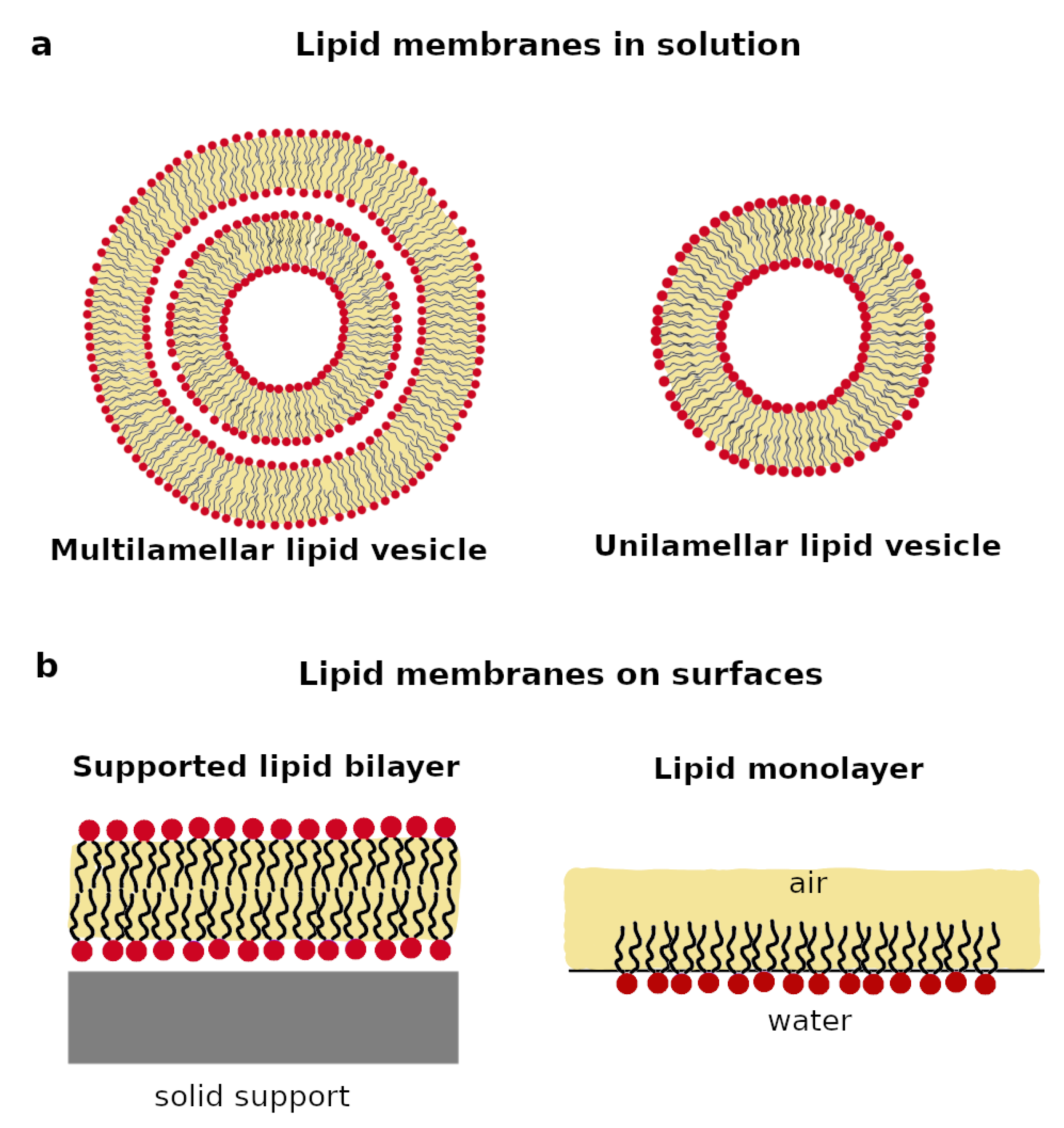
2. Design of Biomimetic Lipid Membranes
2.1. Lipid Membranes in Solution
2.2. Lipid Membrane on Surfaces
3. Applications of Biomimetic Lipid Membranes to Investigate Protein-Lipid or Drug-Lipid Interactions
3.1. Protein-Lipid Interactions

3.2. Drug-Lipid Interactions

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lombard, J. Once upon a time the cell membranes: 175 years of cell boundary research. Biol. Direct. 2014, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- De la Serna, J.B.; Schutz, G.J.; Eggeling, C.; Cebecauer, M. There is no simple model of the plasma membrane organization. Front. Cell. Dev. Biol. 2016, 4, 106. [Google Scholar]
- Lundbaek, J.A.; Collingwood, S.A.; Ingólfsson, H.I.; Kapoor, R.; Andersen, O.S. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J. R. Soc. Interface 2010, 7, 373–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandis, A.Z.; Wenk, M.R. Membrane lipids as signaling molecules. Curr. Opin. Lipidol. 2007, 18, 121–129. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [PubMed] [Green Version]
- Harayama, T.; Riezman, H. Understanding the diversity of the membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; de Kroon, A.I.P. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R.; van Meer, G. Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 2010, 22, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.A.; Cheng, K.H.; Somerharju, P. Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proc. Natl. Acad. Sci. USA 1998, 95, 4964–4969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symons, J.L.; Cho, K.; Chang, J.T.; Du, G.; Waxham, M.N.; Hancock, J.F.; Levental, I.; Levental, K.R. Lipidomic atlas of mammalian cell membranes reveals hierarchical variation induced by culture conditions, subcellular membranes, and cell lineages. Soft Matter 2021. [Google Scholar] [CrossRef]
- Kobayashi, T.; Menon, A.K. Transbilayer lipid asymmetry. Curr. Biol. 2018, 28, R386–R391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson Wood, W.; Igbavboa, U.; Müller, W.E.; Eckert, G.P. Cholesterol asymmetry in synaptic plasma membranes. J. Neurochem. 2011, 116, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daleke, D. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lip. Res. 2003, 44, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivel, T.; Ramseyer, C.; Yesylevskyy, S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 2019, 9, 5627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevers, E.M.; Williamson, P.L. Getting to the outer leaflet: Physiology of phosphatidylserine exposure at the plasma membrane. Physiol. Rev. 2016, 96, 605–645. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta 2004, 1666, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Siontorou, C.G.; Nikoleli, G.-P.; Nikolelis, D.P.; Karapetis, S.K. Artificial Lipid Membranes: Past, Present, and Future. Membranes 2017, 7, 38. [Google Scholar] [CrossRef]
- Pabst, G.; Kucerka, N.; Nieh, M.; Katsaras, J. Liposomes, Lipid Bilayers and Model Membranes From Basic Research to Application; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2004. [Google Scholar]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane lipid phase transitions and phase organization studied by Fourier transform infrared spectroscopy. Biochim. Biophys. Acta 2013, 1828, 2347–2358. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.N.A.H.; Mannock, D.A.; McElhaney, R.N. Differential Scanning Calorimetry in the Study of Lipid Phase Transitions in Model and Biological Membranes. In Methods in Membrane Lipids; Dopico, A.M., Ed.; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Boughter, C.T.; Monje-Galvan, V.; Im, W.; Klauda, J.B. Influence of Cholesterol on Phospholipid Bilayer Structure and Dynamics. J. Phys. Chem. B 2016, 120, 11761–11772. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Lipid—protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta 2003, 1612, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial Peptides: Interaction With Model and Biological Membranes and Synergism With Chemical Antibiotics. Front. Chem. 2018, 6, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Veatch, S.L.; Polozov, I.V.; Gawrisch, K.; Keller, S.L. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 2004, 86, 2910–2922. [Google Scholar] [CrossRef] [Green Version]
- Brzustowicz, M.R.; Brunger, A.T. X-ray scattering from unilamellar lipid vesicles. J. Appl. Cryst. 2005, 38, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Berti, D.; Caminati, G.; Baglioni, P. Functional liposomes and supported lipid bilayers: Towards the complexity of biological archetypes. Phys. Chem. Chem. Phys. 2011, 13, 8769–8782. [Google Scholar] [CrossRef] [Green Version]
- Clifton, L.A.; Campbell, R.A.; Sebastiani, F.; Campos-Terán, J.; Gonzalez-Martinez, J.F.; Björklund, S.; Sotres, J.; Cárdenas, M. Design and use of model membranes to study biomolecular interactions using complementary surface-sensitive techniques. Adv. Colloid Interface Sci. 2020, 277, 102118. [Google Scholar] [CrossRef]
- Liu, G.; Hou, S.; Tong, P.; Li, J. Liposomes: Preparation, Characteristics, and Application Strategies in Analytical Chemistry. Crit. Rev. Anal. 2020, 1–21. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Nele, V.; Holme, M.N.; Kauscher, U.; Thomas, M.R.; Doutch, J.J.; Stevens, M.M. Effect of Formulation Method, Lipid Composition, and PEGylation on Vesicle Lamellarity: A Small-Angle Neutron Scattering Study. Langmuir 2019, 35, 6064–6074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baykal-Caglar, E.; Hassan-Zadeh, E.; Saremi, B.; Huang, J. Preparation of giant unilamellar vesicles from damp lipid film for better lipid compositional uniformity. Biochim. Biophys. Acta 2012, 1818, 2598–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitiello, G.; Luchini, A.; DÉrrico, G.; Santamaria, R.; Capuozzo, A.; Irace, C.; Montesarchio, D.; Paduano, L. Cationic liposomes as efficient nanocarriers for the drug delivery of an anticancer cholesterol-based ruthenium complex. J. Mater. Chem. B 2015, 3, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, G.; Silipo, A.; Mangiapia, G.; Vitiello, G.; Radulescu, A.; Molinaro, A.; Lanzetta, R.; Paduano, L. Characterization of liposomes formed by lipopolysaccharides from Burkholderia cenocepacia, Burkholderia multivorans and Agrobacterium tumefaciens: From the molecular structure to the aggregate architecture. Phys. Chem. Chem. Phys. 2010, 12, 13574–13585. [Google Scholar] [CrossRef]
- Acampora, F.; Marzaioli, A.M.; Capuozzo, A.; Appavou, M.S.; Campanella, A.; D’Errico, G.; Irace, C.; Montesarchio, D.; Musumeci, D.; Szekely, N.K.; et al. Lipooligosaccharides as Amphiphiles to Build Liposomes for Effective Drug Delivery: The Case of Anticancer Ruthenium Complex-Based Aggregates. ChemistrySelect 2016, 1, 2129. [Google Scholar] [CrossRef]
- Zhu, T.F.; Szostak, J.W. Preparation of large monodisperse vesicles. PLoS ONE 2009, 4, e5009. [Google Scholar] [CrossRef]
- Mui, B.; Chow, L.; Hope, M.J. Extrusion Technique to Generate Liposomes of Defined Size. Methods Enzymol. 2003, 367, 3–14. [Google Scholar]
- De Moraes, M.L.; Caseli, L. Supramolecular Systems. In Nanostructures; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: New York, NY, USA, 2017; pp. 33–52. [Google Scholar]
- Okur, H.I.; Tarun, O.B.; Roke, S. Chemistry of Lipid Membranes from Models to Living Systems: A Perspective of Hydration, Surface Potential, Curvature, Confinement and Heterogeneity. J. Am. Chem. Soc. 2019, 141, 12168–12181. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [Green Version]
- Melcrová, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar] [CrossRef]
- Poyton, M.F.; Sendecki, A.M.; Cong, X.; Cremer, P.S. Cu2+ Binds to Phosphatidylethanolamine and Increases Oxidation in Lipid Membranes. J. Am. Chem. Soc. 2016, 138, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Doktorova, M.; Heberle, F.A.; Eicher, B.; Standaert, F.; Katsaras, J.; London, E.; Pabst, G.; Marquardt, D. Preparation of asymmetric phospholipid vesicles for use as cell membrane models. Nat. Protoc. 2018, 13, 2086–2101. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; London, E. Ordered raft domains induced by outer leaflet sphingomyelin in cholesterol-rich asymmetric vesicles. Biophys. J. 2015, 108, 2212–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, H.; Spindler, S.; Bonakdar, N.; Wang, C.; Sandoghdar, V. Production of Isolated Giant Unilamellar Vesicles under High Salt Concentrations. Front. Physiol. 2017, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horger, K.S.; Estes, D.J.; Capone, R.; Mayer, M. Films of Agarose Enable Rapid Formation of Giant Liposomes in Solutions of Physiologic Ionic Strength. J. Am. Chem. Soc. 2009, 131, 1810–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberger, A.; Tsai, F.C.; Koenderink, G.H.; Schmidt, T.F.; Itri, R.; Meier, W.; Schmatko, T.; Schröder, A.; Marques, C. Gel-assisted formation of giant unilamellar vesicles. Biophys. J. 2013, 105, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Angelova, M.I.; Dimitrov, D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Méléard, P.; Bagatolli, L.A.; Pott, T. Giant Unilamellar Vesicle Electroformation: From Lipid Mixtures to Native Membranes Under Physiological Conditions. Methods Enzymol. 2009, 465, 161–176. [Google Scholar]
- Aimon, S.; Callan-Jones, A.; Berthaud, A.; Pinot, M.; Toombes, G.E.S.; Bassereau, P. Membrane Shape Modulates Transmembrane Protein Distribution. Dev. Cell 2014, 28, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. [Google Scholar] [CrossRef] [Green Version]
- Levental, I.; Grzybek, M.; Simons, K. Raft domains of variable properties and compositions in plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2011, 108, 11411–11416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinkühler, J.; Sezgin, E.; Urbančič, I.; Eggeling, C.; Dimova, R. Mechanical properties of plasma membrane vesicles correlate with lipid order, viscosity and cell density. Commun. Biol. 2019, 2, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezgin, E.; Kaiser, H.J.; Baumgart, T.; Schwille, P.; Simons, K.; Levental, I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012, 7, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Giner-Casares, J.J.; Brezesinski, G.; Möhwald, H. Langmuir monolayers as unique physical models. Curr. Opin. Colloid Interface Sci. 2014, 19, 176–182. [Google Scholar] [CrossRef]
- Campbell, R.A.; Saaka, Y.; Shao, Y.; Gerelli, Y.; Cubitt, R.; Nazaruk, E.; Matyszewska, D.; Lawrence, M.J. Structure of surfactant and phospholipid monolayers at the air–water interface modeled from neutron reflectivity data. J. Colloid Int. Sci. 2018, 531, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Stefaniu, C.; Brezesinski, G.; Möhwald, H. Langmuir monolayers as models to study processes at membrane surfaces. Adv. Colloid Interface Sci. 2014, 208, 197–213. [Google Scholar] [CrossRef]
- Scomparin, C.; Lecuyer, S.; Ferreira, M.; Charitat, T.; Tinland, B. Diffusion in supported lipid bilayers: Influence of substrate and preparation technique on the internal dynamics. Eur. Phys. J. E 2009, 28, 211–220. [Google Scholar] [CrossRef]
- Vollhardt, D.; Fainerman, V.B. Characterisation of phase transition in adsorbed monolayers at the air–water interface. Adv. Colloid Interface Sci. 2010, 154, 1–19. [Google Scholar] [CrossRef]
- Daear, W.; Mahadeo, M.; Prenner, E.J. Applications of Brewster angle microscopy from biological materials to biological systems. Biochim. Biophys. Acta 2017, 1859, 1749–1766. [Google Scholar] [CrossRef]
- Hazell, G.; Gee, A.P.; Arnold, T.; Edler, K.J.; Lewis, S.E. Langmuir monolayers composed of single and double tail sulfobetaine lipids. J. Colloid Int. Sci. 2016, 474, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Barraza, K.M.; Beauchamp, J.L. Cholesterol provides nonsacrificial protection of membrane lipids from chemical damage at air-water interface. Proc. Natl. Acad. Sci. USA 2018, 115, 3255–3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catapano, E.R.; Natale, P.; Monroy, F.; López-Montero, I. The enzymatic sphingomyelin to ceramide conversion increases the shear membrane viscosity at the air-water interface. Adv. Colloid Interface Sci. 2017, 247, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, J.; Ventrici de Souza, J.F.; Dang, A.T.; Liu, G.; Kuhl, T.L. Preparation and Characterization of Solid-Supported Lipid Bilayers Formed by Langmuir–Blodgett. Langmuir 2018, 34, 15622–15639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model cell membranes: Techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Curr. Opin. Colloid Interface Sci. 2013, 18, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, T.K.; Cárdenas, M. Understanding the formation of supported lipid bilayers via vesicle fusion—A case that exemplifies the need for the complementary method approach. Biointerphases 2016, 11, 020801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchini, A.; Gerelli, Y.; Fragneto, G.; Nylander, T.; Pálsson, G.K.; Appavou, M.S.; Paduano, L. Neutron Reflectometry reveals the interaction between functionalized SPIONs and the surface of lipid bilayers. Colloids Surf. B 2017, 151, 76–87. [Google Scholar] [CrossRef]
- Rondelli, V.; Brocca, P.; Tranquilli, N.; Fragneto, G.; Del Favero, E.; Cantù, L. Building a biomimetic membrane for neutron reflectivity investigation: Complexity, asymmetry and contrast. Biophys. Chem. 2017, 229, 135–141. [Google Scholar] [CrossRef]
- Andersson, J.; Köper, I.; Knoll, W. Tethered Membrane Architectures—Design and Applications. Front. Mater. 2018, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Andersson, J.; Köper, I. Tethered and Polymer Supported Bilayer Lipid Membranes: Structure and Function. Membranes 2016, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Fragneto, G.; Charitat, T.; Daillant, J. Floating lipid bilayers: Models for physics and biology. Eur. Biophys. J. 2012, 41, 863–874. [Google Scholar] [CrossRef]
- Dickson, E.J.; Hille, B. Understanding phosphoinositides: Rare, dynamic, and essential membrane phospholipids. Biochem. J. 2019, 476, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Luchini, A.; Nzulumike, A.N.O.; Lind, T.K.; Nylander, T.; Barker, R.; Arleth, L.; Mortensen, K.; Cárdenas, M. Towards biomimics of cell membranes: Structural effect of phosphatidylinositol triphosphate (PIP3) on a lipid bilayer. Colloids Surf. B 2019, 173, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Gerelli, Y. Phase Transitions in a Single Supported Phospholipid Bilayer: Real-Time Determination by Neutron Reflectometry. Phys. Rev. Lett. 2019, 122, 248101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerelli, Y.; Porcar, L.; Lombardi, L.; Fragneto, G. Lipid Exchange and Flip-Flop in Solid Supported Bilayers. Langmuir 2013, 29, 12762–12769. [Google Scholar] [CrossRef] [PubMed]
- De Ghellinck, A.; Fragneto, G.; Laux, V.; Haertlein, M.; Jouhet, J.; Sferrazza, M.; Wacklin, H. Lipid polyunsaturation determines the extent of membrane structural changes induced by Amphotericin B in Pichia pastoris yeast. Biochem. Biophys. Acta 2015, 1848, 2317–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchini, A.; Sebastiani, F.; Tidemand, F.G.; Batchu, K.C.; Campana, M.; Fragneto, G.; Cárdenas, M.; Arleth, L. Peptide discs as precursors of biologically relevant supported lipid bilayers. J. Colloid Interface Sci. 2020, 585, 376–385. [Google Scholar] [CrossRef]
- Grillitsch, K.; Tarazona, P.; Klug, L.; Wriessnegger, T.; Zellnig, G.; Leitner, E.; Feussner, I.; Daum, G. Isolation and characterization of the plasma membrane from the yeast Pichia pastoris. Biochem. Biophys. Acta 2014, 1838, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Möller, I.; Seeger, S. Solid supported lipid bilayers from artificial and natural lipid mixtures—long-term stable, homogeneous and reproducible. J. Mater. Chem. B 2015, 3, 6046–6056. [Google Scholar] [CrossRef]
- Saliba, A.E.; Vonkova, I.; Gavin, A.C. The systematic analysis of protein–lipid interactions comes of age. Nat. Rev. Mol. Cell Biol. 2015, 16, 753–761. [Google Scholar] [CrossRef]
- Zahidul, I.M.; Jahangir, A.; Tamba, Y.; Karal, M.A.S.; Yamazaki, M. The single GUV method for revealing the functions of antimicrobial, pore-forming toxin, and cell-penetrating peptides or proteins. Phys. Chem. Chem. Phys. 2014, 30, 15752–15767. [Google Scholar]
- Cheng, B.; Li, Y.; Ma, L.; Wang, Z.; Petersen, R.B.; Zheng, L.; Chen, Y.; Huang, K. Interaction between amyloidogenic proteins and biomembranes in protein misfolding diseases: Mechanisms, contributors, and therapy. Biochem. Biophys. Acta 2018, 1860, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Luchini, A.; Arleth, L. Protocol for Investigating the Interactions Between Intrinsically Disordered Proteins and Membranes by Neutron Reflectometry. Methods Mol. Biol. 2020, 2141, 569–584. [Google Scholar] [PubMed]
- Elderdfi, M.; Sikorski, A.F. Langmuir-monolayer methodologies for characterizing protein-lipid interactions. Chem. Phys. Lip. 2018, 212, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Overington, J.; Al-Lazikani, B.; Hopkins, A. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease—Lessons and emerging principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef] [Green Version]
- Gilmozzi, V.; Gentile, G.; Castelo Rueda, M.P.; Hicks, A.A.; Pramstaller, P.P.; Zanon, A.; Lévesque, M.; Pichler, I. Interaction of Alpha-Synuclein With Lipids: Mitochondrial Cardiolipin as a Critical Player in the Pathogenesis of Parkinson’s Disease. Front. Neurosci. 2020, 14, 578993. [Google Scholar] [CrossRef]
- Hannestad, J.K.; Rocha, S.; Agnarsson, B.; Zhdanov, Z.P.; Wittung-Stafshede, P.; Höök, F. Single-vesicle imaging reveals lipid-selective and stepwise membrane disruption by monomeric α-synuclein. Proc. Natl. Acad. Sci. USA 2020, 117, 14178–14186. [Google Scholar] [CrossRef]
- Wildburger, N.C.; Hartke, A.; Schidlitzki, A.; Richter, F. Current Evidence for a Bidirectional Loop Between the Lysosome and Alpha-Synuclein Proteoforms. Front. Cell Dev. Biol. 2020, 8, 1372. [Google Scholar] [CrossRef]
- Galvagnion, C.; Buell, A.K.; Meisl, G.; Michaels, T.C.T.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 2015, 11, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Hellstrand, E.; Grey, M.; Ainalem, M.L.; Ankner, J.; Forsyth, V.T.; Fragneto, G.; Haertlein, M.; Dauvergne, M.T.; Nilsson, H.; Brundin, P.; et al. Adsorption of α-synuclein to supported lipid bilayers: Positioning and role of electrostatics. ACS Chem. Neurosci. 2013, 16, 1339–1351. [Google Scholar] [CrossRef] [Green Version]
- Cholak, E.; Bugge, K.; Khondker, A.; Gauger, K.; Pedraz-Cuesta, E.; Pedersen, M.E.; Bucciarelli, S.; Vestergaard, B.; Pedersen, S.F.; Rheinstädter, M.C.; et al. Avidity within the N-terminal anchor drives α-synuclein membrane interaction and insertion. FASEB J. 2020, 34, 7462–7482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, T.; Eliezer, D. Membrane interactions of intrinsically disordered proteins: The example of alpha-synuclein. Biochem. Biophys. Acta 2019, 1867, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Tosatto, L.; Andrighetti, A.O.; Plotegher, N.; Antonini, V.; Tessari, I.; Ricci, L.; Bubacco, L.; Dalla Serra, M. Alpha-synuclein pore forming activity upon membrane association. Biochem. Biophys. Acta 2012, 1818, 2876–2883. [Google Scholar] [CrossRef] [PubMed]
- Perissinotto, F.; Rondelli, V.; Parisse, P.; Tormena, N.; Zunino, A.; Almásy, L.; Merkel, D.G.; Bottyán, L.; Sajti, S.; Casalis, L. GM1 Ganglioside role in the interaction of Alpha-synuclein with lipid membranes: Morphology and structure. Biophys. Chem. 2019, 255, 106272. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Rigacci, S.; Stefani, M. Amyloid Aggregation: Role of Biological Membranes and the Aggregate–Membrane System. J. Phys. Chem. Lett. 2014, 5, 517–527. [Google Scholar] [CrossRef]
- Lindberg, D.J.; Wesén, E.; Björkeroth, J.; Rocha, S.; Esbjörner, E.K. Lipid membranes catalyse the fibril formation of the amyloid-β (1–42) peptide through lipid-fibril interactions that reinforce secondary pathways. Biochem. Biophys. Acta 2017, 1859, 1921–1929. [Google Scholar] [CrossRef]
- Martel, A.; Antony, L.; Gerelli, Y.; Porcar, L.; Fluitt, A.; Hoffmann, K.; Kiesel, I.; Vivaudou, M.; Fragneto, G.; de Pablo, J.J. Membrane Permeation versus Amyloidogenicity: A Multitechnique Study of Islet Amyloid Polypeptide Interaction with Model Membranes. J. Am. Chem. Soc. 2017, 139, 137–148. [Google Scholar] [CrossRef]
- Staneva, G.; Watanabe, C.; Puff, N.; Yordanova, V.; Seigneuret, M.; Angelova, M.I. Amyloid-β Interactions with Lipid Rafts in Biomimetic Systems: A Review of Laboratory Methods. Methods Mol. Biol. 2021, 2187, 47–86. [Google Scholar]
- Dies, H.; Toppozini, L.; Rheinstädter, M.C. Amyloid-β The Interaction between Amyloid-β Peptides and Anionic Lipid Membranes Containing Cholesterol and Melatonin. PLoS ONE 2014, 9, e99124. [Google Scholar] [CrossRef] [Green Version]
- Vitiello, G.; Di Marino, S.; D’Ursi, A.M.; D’Errico, G. Omega-3 Fatty Acids Regulate the Interaction of the Alzheimer’s Aβ(25–35) Peptide with Lipid Membranes. Langmuir 2013, 9, 14239–14245. [Google Scholar] [CrossRef] [Green Version]
- Emendato, A.; Spadaccini, R.; De Santis, A.; Guerrini, R.; D’Errico, G.; Picone, D. Preferential interaction of the Alzheimer peptide Aβ-(1-42) with Omega-3-containing lipid bilayers: Structure and interaction studies. FEBS Lett. 2016, 590, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Hendus-Altenburger, R.; Vogensen, J.; Pedersen, E.S.; Luchini, A.; Araya-Secchi, R.; Bendsoe, A.H.; Prasad, N.S.; Prestel, A.; Cardenas, M.; Pedraz-Cuesta, E.; et al. The intracellular lipid-binding domain of human Na+/H+ exchanger 1 forms a lipid-protein co-structure essential for activity. Commun. Biol. 2020, 3, 731. [Google Scholar] [CrossRef] [PubMed]
- Weissenhorn, W.; Hinz, A.; Gaudin, Y. Virus membrane fusion. FEBS Lett. 2007, 581, 2150–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlino, A.; Vitiello, G.; Grimaldi, M.; Sica, F.; Busi, E.; Basosi, R.; D’Ursi, A.M.; Fragneto, G.; Paduano, L.; D’Errico, G. Destabilization of lipid membranes by a peptide derived from glycoprotein gp36 of feline immunodeficiency virus: A combined molecular dynamics/experimental study. J. Phys. Chem. B 2012, 116, 401–412. [Google Scholar] [CrossRef]
- Vitiello, G.; Fragneto, G.; Petruk, A.A.; Falanga, A.; Galdiero, S.; D’Ursi, A.M.; Merlino, A.; D’Errico, G. Cholesterol modulates the fusogenic activity of a membranotropic domain of the FIV glycoprotein gp36. Soft Matter 2013, 9, 6442–6456. [Google Scholar] [CrossRef]
- Oliva, R.; Emendato, A.; Vitiello, G.; De Santis, A.; Grimaldi, M.; D’Ursi, A.M.; Busi, E.; Del Vecchio, P.; Petraccone, L.; D’Errico, G. On the microscopic and mesoscopic perturbations of lipid bilayers upon interaction with the MPER domain of the HIV glycoprotein gp41. Biochem. Biophys. Acta 2016, 1858, 1904–1913. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, G.; Vitiello, M.; Pedone, C.; D’Errico, G.; Galdiero, M. Role of membranotropic sequences from herpes simplex virus type I glycoproteins B and H in the fusion process. Biochem. Biophys. Acta 2010, 1798, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Vitiello, G.; Falanga, A.; Galdiero, M.; Marsh, D.; Galdiero, S.; D’Errico, G. Lipid composition modulates the interaction of peptides deriving from herpes simplex virus type I glycoproteins B and H with biomembranes. Biochem. Biophys. Acta 2011, 1808, 2517–2526. [Google Scholar] [CrossRef] [Green Version]
- Falanga, A.; Tarallo, R.; Vitiello, G.; Vitiello, M.; Perillo, E.; Cantisani, M.; D’Errico, G.; Galdiero, M.; Galdiero, S. Biophysical characterization and membrane interaction of the two fusion loops of glycoprotein B from herpes simplex type I virus. PLoS ONE 2012, 7, e32186. [Google Scholar] [CrossRef] [Green Version]
- Vitiello, G.; Falanga, A.; Petruk, A.A.; Merlino, A.; Fragneto, G.; Paduano, L.; Galdiero, S.; D’Errico, G. Fusion of raft-like lipid bilayers operated by a membranotropic domain of the HSV-type I glycoprotein gH occurs through a cholesterol-dependent mechanism. Soft Matter 2015, 11, 3003–3016. [Google Scholar] [CrossRef]
- Marquette, A.; Leborgne, C.; Schartner, V.; Salnikov, E.; Bechinger, B.; Kichler, A. Peptides derived from the C-terminal domain of HIV-1 Viral Protein R in lipid bilayers: Structure, membrane positioning and gene delivery. Biochem. Biophys. Acta 2020, 1862, 183149. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.S.; Corrêa, G.; Einicker-Lamas, M.; Coutinho-Silva, R. Host-cell lipid rafts: A safe door for micro-organisms? Biol. Cell 2010, 102, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Meher, G.; Chakraborty, H. Membrane Composition Modulates Fusion by Altering Membrane Properties and Fusion Peptide Structure. J. Membr. Biol. 2019, 252, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Seddon, A.M.; Casey, D.; Law, R.V.; Gee, A.; Templer, R.H.; Ces, O. Drug interactions with lipid membranes. Chem. Soc. Rev. 2009, 38, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.; Ribeiro, D.; Nunes, C.; Reis, S. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochem. Biophys. Acta 2016, 1858, 2231–2244. [Google Scholar] [CrossRef] [PubMed]
- Karewicz, A.; Bielska, D.; Gzyl-Malcher, B.; Kepczynski, M.; Lach, R.; Nowakowska, M. Interaction of curcumin with lipid monolayers and liposomal bilayers. Coll. Surf. B 2011, 88, 231–239. [Google Scholar] [CrossRef]
- Bourgaux, C.; Couvreur, P. Interactions of anticancer drugs with biomembranes: What can we learn from model membranes? J. Control. Release 2014, 190, 127–138. [Google Scholar] [CrossRef]
- Matyszewska, D.; Nazaruk, E.; Campbell, R.A. Interactions of anticancer drugs doxorubicin and idarubicin with lipid monolayers: New insight into the composition, structure and morphology. J. Colloid Interface Sci. 2021, 581, 403–416. [Google Scholar] [CrossRef]
- Peetla, C.; Bhave, R.; Vijayaraghavalu, S.; Stine, A.; Kooijman, E.; Labhasetwar, V. Drug Resistance in Breast Cancer Cells: Biophysical Characterization of and Doxorubicin Interactions with Membrane Lipids. Mol. Pharm. 2010, 7, 2334–2348. [Google Scholar] [CrossRef] [Green Version]
- Nunes, C.; Brezesinski, G.; Lopes, D.; Lima, J.L.F.C.; Reis, S.; Lúcio, M. Lipid–Drug Interaction: Biophysical Effects of Tolmetin on Membrane Mimetic Systems of Different Dimensionality. J. Phys. Chem. B 2011, 115, 12615–12623. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Heinbockel, L.; Suwalsky, M.; Garidel, P.; Brandenburg, K. Biophysical study of the non-steroidal anti-inflammatory drugs (NSAID) ibuprofen, naproxen and diclofenac with phosphatidylserine bilayer membranes. Biochem. Biophys. Acta 2016, 1858, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Kremkow, J.; Luck, M.; Huster, D.; Müller, P.; Scheidt, H.A. Membrane Interaction of Ibuprofen with Cholesterol-Containing Lipid Membranes. Biomolecules 2020, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Khajeh, A.; Modarress, H. The influence of cholesterol on interactions and dynamics of ibuprofen in a lipid bilayer. Biochem. Biophys. Acta 2014, 1838, 2431–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Sendecki, A.M.; Pullanchery, S.; Huang, D.; Yang, T.; Cremer, P.S. Multistep Interactions between Ibuprofen and Lipid Membranes. Langmuir 2018, 34, 10782–10792. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Mamontov, E.; Ohl, M.; Tyagi, M. Incorporation of aspirin modulates the dynamical and phase behavior of the phospholipid membrane. Phys. Chem. Chem. Phys. 2017, 19, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Nunes, C.; Amenitsch, H.; Reis, S. Resveratrol Interaction with Lipid Bilayers: A Synchrotron X-ray Scattering Study. Langmuir 2016, 32, 12914–12922. [Google Scholar] [CrossRef]
- Nur, S.; Nur, F.; Alsamarah, A.; Chatterjee, P.; Nur, N.; Moreno, J.; Luo, L.; Lambros, M. Interaction of Resveratrol with Lipid Membranes. Biophys. J. 2016, 110, 411a. [Google Scholar] [CrossRef]
- Kruszewski, M.; Kusaczuk, M.; Kotyńska, J.; Gál, M.; Krętowski, R.; Cechowska-Pasko, M.; Naumowicz, M. The effect of quercetin on the electrical properties of model lipid membranes and human glioblastoma cells. Bioelectrochemistry 2018, 124, 133–141. [Google Scholar] [CrossRef]
- Smirnova, I.A.; Ädelroth, P.; Brzezinski, P. Extraction and liposome reconstitution of membrane proteins with their native lipids without the use of detergents. Sci. Rep. 2018, 8, 14950. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, I.L.; Kemmer, G.C.; Pomorski, T.G. Membrane protein reconstitution into giant unilamellar vesicles: A review on current techniques. Eur. Biophys. J. 2017, 46, 103–119. [Google Scholar] [CrossRef]
- Luchini, A.; Tidemand, F.G.; Johansen, N.T.; Campana, M.; Sotres, J.; Ploug, M.; Cárdenas, M.; Arleth, L. Peptide Disc Mediated Control of Membrane Protein Orientation in Supported Lipid Bilayers for Surface-Sensitive Investigations. Anal. Chem. 2020, 92, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Pace, H.; Nyström, L.S.; Gunnarsson, A.; Eck, E.; Monson, C.; Geschwindner, S.; Snijder, A.; Höök, F. Preserved Transmembrane Protein Mobility in Polymer-Supported Lipid Bilayers Derived from Cell Membranes. Anal. Chem. 2015, 87, 9194–9203. [Google Scholar] [CrossRef] [PubMed]
- Hatty, C.R.; Le Brun, A.P.; Lake, V.; Clifton, L.A.; Liu, G.J.; James, M.; Banati, R.B. Investigating the interactions of the 18kDa translocator protein and its ligand PK11195 in planar lipid bilayers. Biochem. Biophys. Acta 2014, 1838, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchini, A.; Vitiello, G. Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies. Biomimetics 2021, 6, 3. https://doi.org/10.3390/biomimetics6010003
Luchini A, Vitiello G. Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies. Biomimetics. 2021; 6(1):3. https://doi.org/10.3390/biomimetics6010003
Chicago/Turabian StyleLuchini, Alessandra, and Giuseppe Vitiello. 2021. "Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies" Biomimetics 6, no. 1: 3. https://doi.org/10.3390/biomimetics6010003
APA StyleLuchini, A., & Vitiello, G. (2021). Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies. Biomimetics, 6(1), 3. https://doi.org/10.3390/biomimetics6010003





