Strong and Elastic Chitosan/Silk Fibroin Hydrogels Incorporated with Growth-Factor-Loaded Microspheres for Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Results
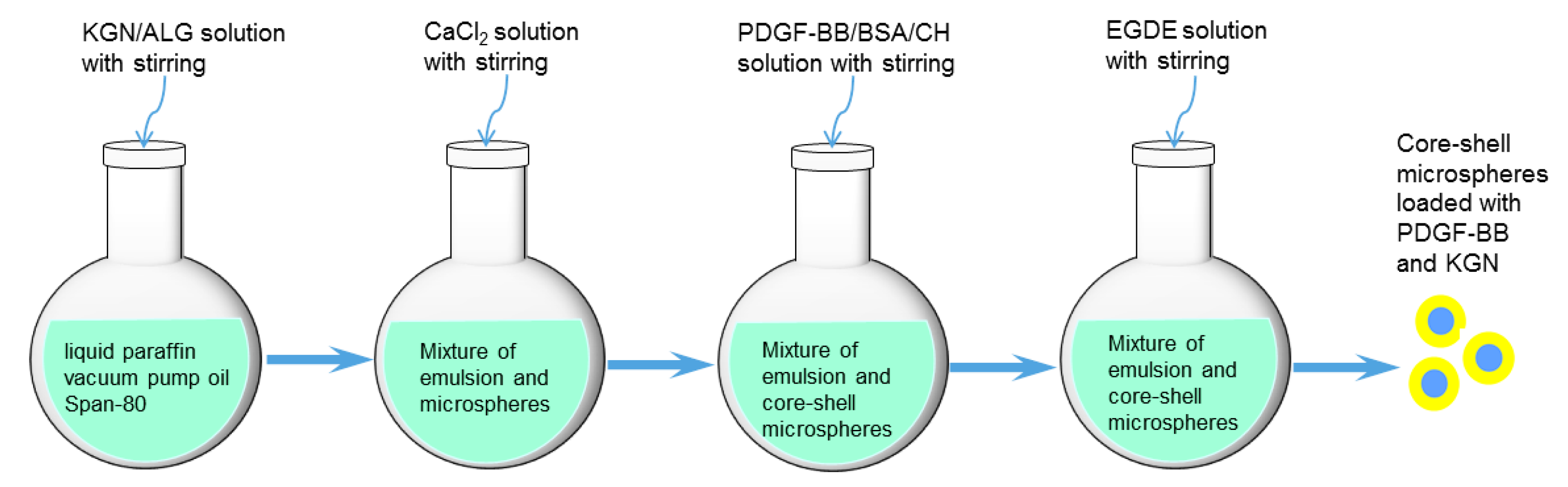
2.1. Parameters of Microspheres
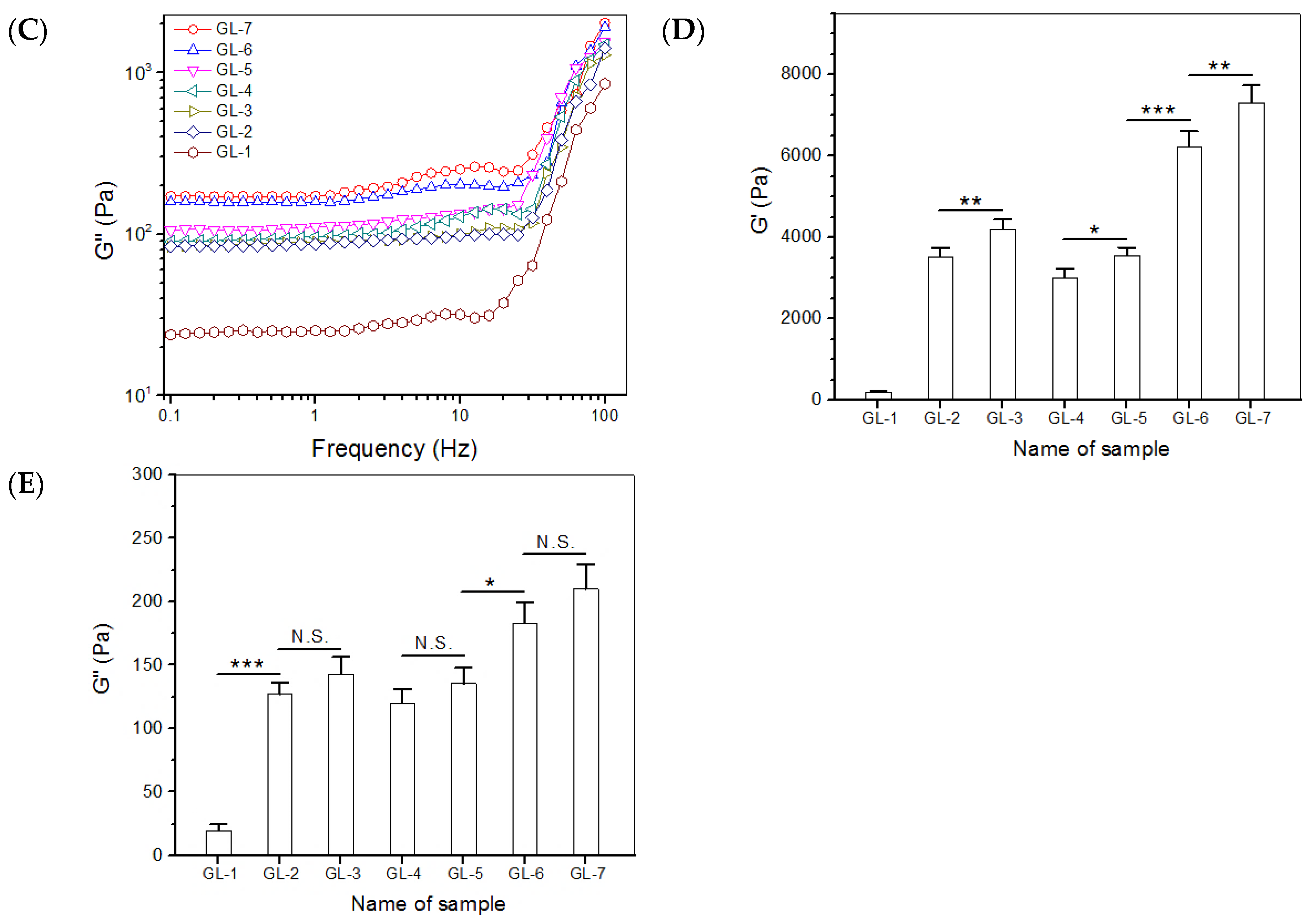
2.2. Major Parameters for Gels Embedded with BLANK-M Microspheres
2.3. Compressive Mechanical Evaluation
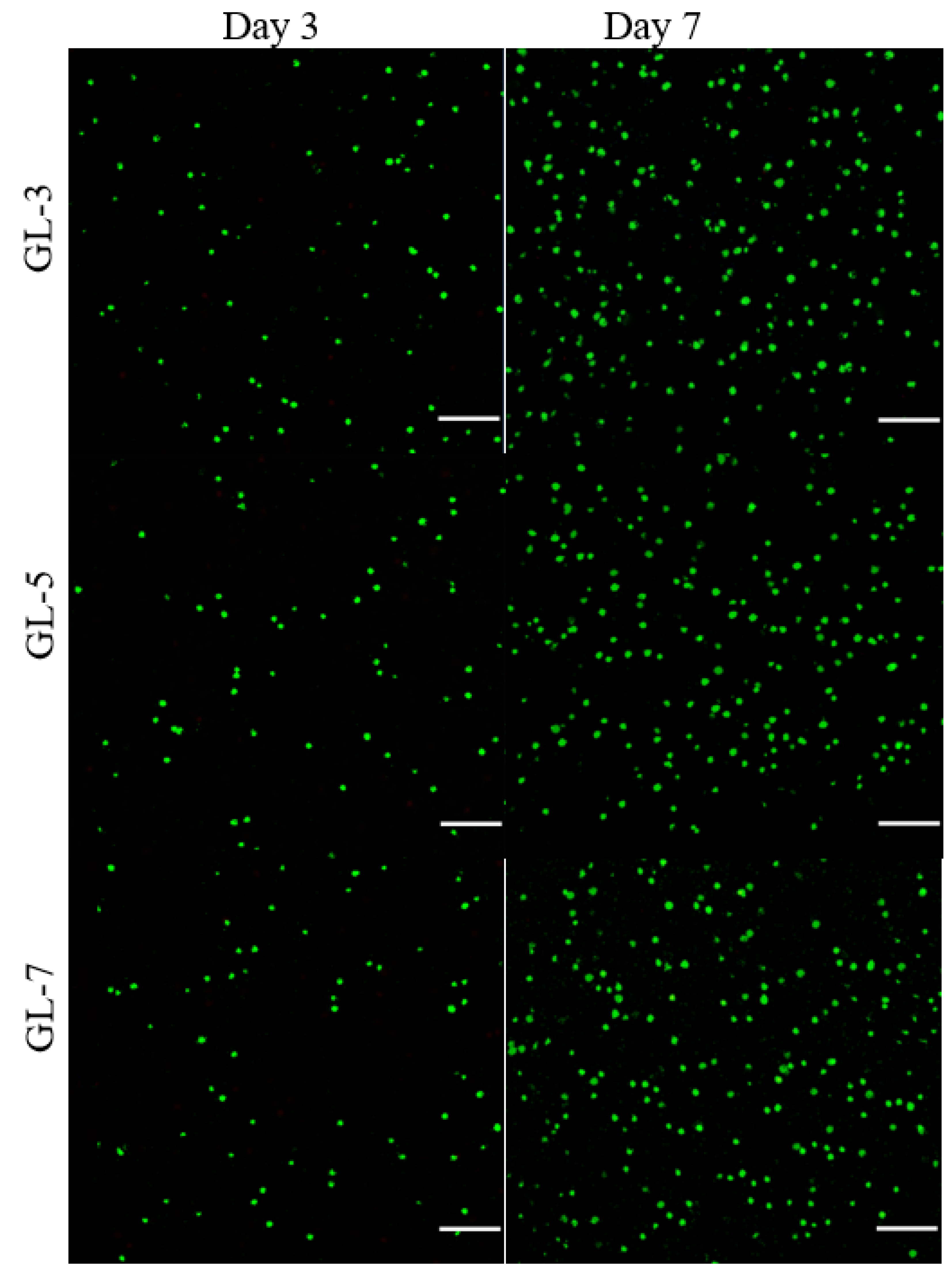
2.4. Cell Growth and Matrix Deposition
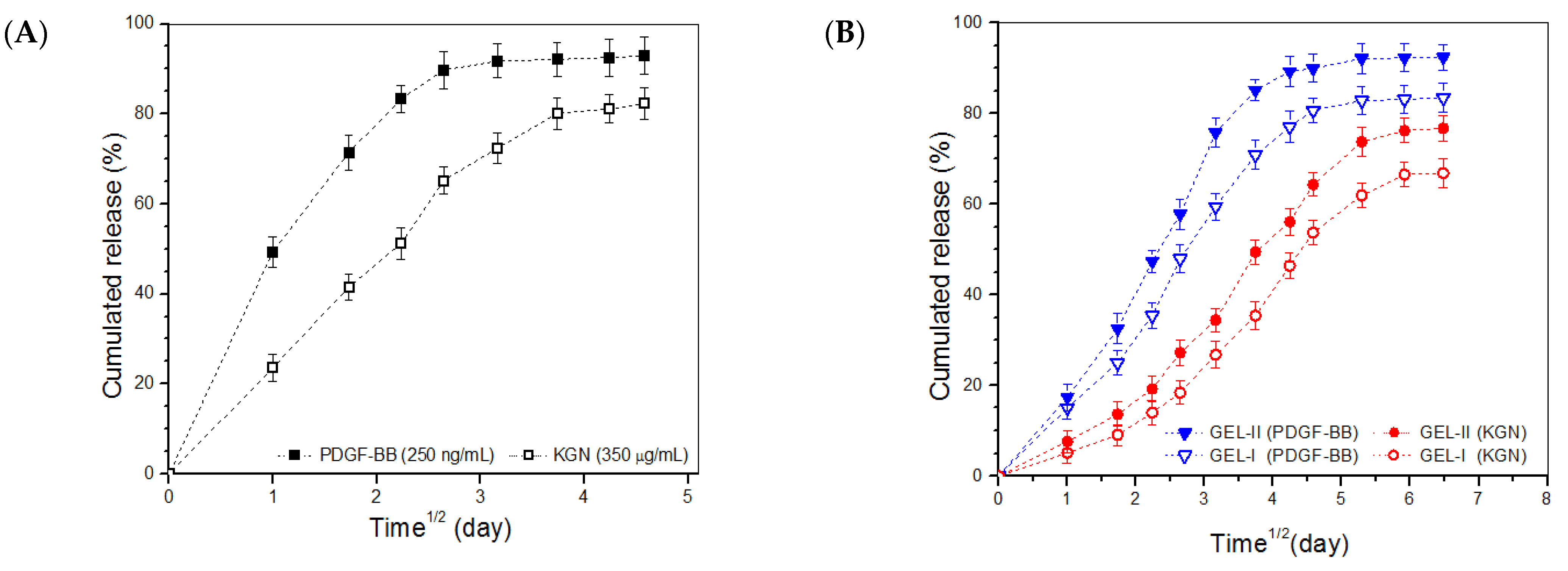
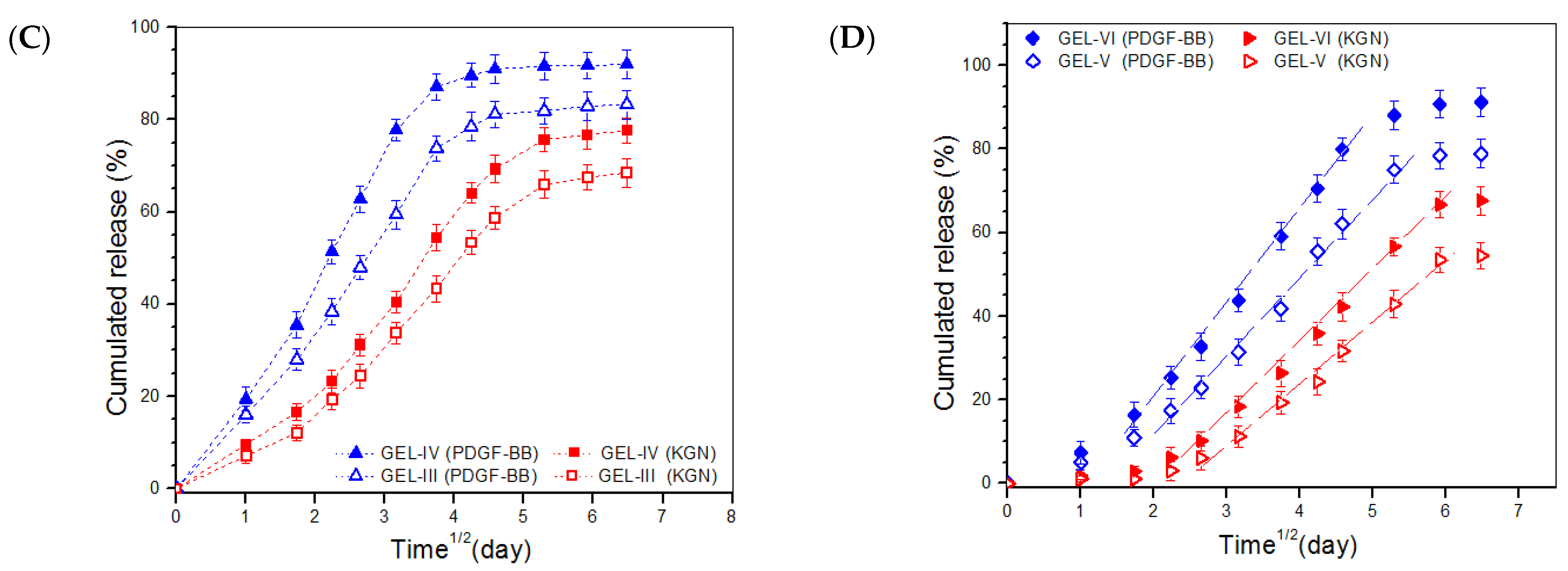
2.5. In Vitro Release
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Core-Shell Microspheres with Load of KGN and PDGF-BB
4.3. Characterization
4.4. Preparation of Composite Solutions
4.5. Rheological Measurements
4.6. Mechanical Tests
4.7. In Vitro Release Assessments
4.8. Cell Culture
4.9. Bioactivity Assessment
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.; Jiang, C.-C. Repair of articular cartilage defects: Review and perspectives. J. Formos. Med. Assoc. 2009, 108, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 77, 167–182. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Park, W.H.; Kim, M.H. Chemically cross-linked silk fibroin hydrogel with enhanced elastic properties, biodegradability, and biocompatibility. Int. J. Nanomed. 2016, 11, 2967–2978. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S.; Papolla, C. A comparative investigation of Bombyx mori silk fibroin hydrogels generated by chemical and enzymatic cross-linking. Biotechnol. Appl. Biochem. 2017, 64, 771–781. [Google Scholar] [CrossRef]
- Kang, G.D.; Lee, K.H.; Ki, C.S.; Park, Y.H. Crosslinking reaction of phenolic side chains in silk fibroin by tyrosinase. Fibers Polym. 2004, 5, 234–238. [Google Scholar] [CrossRef]
- Vega, S.; Kwon, M.; Burdick, J. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Shin, J.; Marks, R.; Hopkins, M.; Kim, T.; Park, H.; Alsberg, E. Highly elastic and tough interpenetrating polymer network-structured hybrid hydrogels for cyclic mechanical loading-enhanced tissue engineering. Chem. Mater. 2017, 29, 8425–8432. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft materials by design: Unconventional polymer networks give extreme properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering-Part II: Challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng. Part B 2013, 19, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, R.; Guldberg, R.E. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv. Healthc. Mater. 2019, 8, 1801000. [Google Scholar] [CrossRef] [Green Version]
- Wieman, T.J.; Smiell, J.M.; Su, Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 1998, 21, 822–827. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, C.H.; Schwartz, Z.; Niederauer, G.G.; Carnes, D.L., Jr.; Dean, D.D.; Boyan, B.D. pretreatment with platelet derived growth factor-BB modulates the ability of ostochondral resting zone chondrocytes incorporated into PLA/PGA scaffolds to form new cartilage in vivo. Biomaterials 2000, 21, 49–61. [Google Scholar] [CrossRef]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A stem cell–based approach to cartilage repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, J.C.; Forlino, A. Replenishing cartilage from endogenous stem cells. N. Engl. J. Med. 2012, 366, 2522–2524. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Cirelli, J.A.; Giannobile, W.V. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin. Drug Deliv. 2006, 3, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.H.; Ross-Murphy, S.B. Structural and mechanical properties of biopolymer gels. Adv. Polym. Sci. 1987, 83, 57–192. [Google Scholar]
- Tekari, A.; Luginbuehl, R.; Hofstetter, W.; Egli, R.J. Chondrocytes expressing intracellular collagen type II enter the cell cycle and co-express collagen type I in monolayer culture. J. Orthop. Res. 2014, 32, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lazebnik, M.; Detamore, M. Hyaline cartilage cells outperform mandibular condylar cartilage cells in a TMJ fibrocartilage tissue engineering application. Osteoarthr. Cartil. 2009, 17, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedlaender, G.E.; Lin, S.; Solchaga, L.A.; Snel, L.B.; Lynch, S.E. The role of recombinant human plate-let-derived growth factor-BB (rhPDGF-BB) in orthopaedic bone repair and regeneration. Curr. Pharm. Des. 2013, 19, 3384–3390. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, T.; Zheng, N.; Zhou, Y.; Hogan, M.V.; Wang, J.H. The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat achilles tendon enthuses. Bone Jt. Res. 2017, 6, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, S.; Liang, Y.; Liu, Q. Combination of kartogenin and transforming growth factor-β3 supports synovial fluid-derived mesenchymal stem cell-based cartilage regeneration. Am. J. Transl. Res. 2019, 11, 2056–2069. [Google Scholar]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusinal behavior of hydrogel in controlled release. Adv. Drug Deliver. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogel: Swelling, drug loading, and release. Pharm. Res. 1992, 9, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Caccavo, D. An overview on the mathematical modeling of hydrogels’ behavior for drug delivery systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Röderer, G.; Günther, K.-P.; Brenner, R.E. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J. Cell. Biochem. 2002, 87, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Basu, S.K.; Sa, B. Ca2+ ion cross-linked interpenetrating network matrix tablets of polyacrylamide-grafted-sodium alginate and sodium alginate for sustained release of diltiazem hydrochloride. Carbohydr. Polym. 2010, 82, 867–873. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Charaabi, S.; Picton, L.; Cerf, D.L.; Carbonnier, B. Glucose-sensitive polyelectrolyte microcapsules based on (alginate/chitosan) pair. Carbohydr. Polym. 2018, 184, 144–153. [Google Scholar] [CrossRef]
- Matricardi, P.; Meo, C.D.; Coviello, T.; Alhaique, F. Recent advances and perspectives on coated alginate microspheres for modified drug delivery. Expert Opin. Drug Deliv. 2008, 5, 417–425. [Google Scholar] [CrossRef]
- Wu, H.; Liao, C.; Jiao, Q.; Wang, Z.; Cheng, W.; Wan, Y. Fabrication of core–shell microspheres using alginate and chitosan–polycaprolactone for controlled release of vascular endothelial growth factor. React. Funct. Polym. 2012, 72, 427–437. [Google Scholar] [CrossRef]
- Misirli, Y.; Ozturk, E.; Kursaklioglu, H.; Denkbas, E.B. Preparation and characterization of Mitomycin-C loaded chitosan-coated alginate microspheres for chemoembolization. J. Microencapsul. 2005, 22, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Neufeld, R.J.; Vilela, S.; Ribeiro, A.; Veiga, F. Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J. Microencapsul. 2006, 23, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Busilacchi, A.; Gigante, A.; Mattioli-Belmonte, M.; Manzotti, S.; Muzzarelli, R.A. Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr. Polym. 2013, 98, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef] [Green Version]
- Ngoenkam, J.; Faikrua, A.; Yasothornsrikul, S.; Viyoch, J. Potential of an injectable chitosan/starch/b-glycerol phosphate hydrogel for sustaining normal chondrocyte function. Int. J. Pharm. 2010, 391, 115–124. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Shi, Y.; Wan, Y. Rheological, mechanical and degradable properties of injectable chitosan/silk fibroin/hydroxyapatite/glycerophosphate hydrogels. J. Mech. Behav. Biomed. Mater. 2016, 64, 161–172. [Google Scholar] [CrossRef]
- Mirahmadi, F.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A.; Bonakdar, S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 4786–4794. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, B.; Liu, W.; Wang, P.; Lv, X.; Chen, S.; Liu, H.; Shao, Z. Articular cartilage regeneration: The role of endogenous mesenchymal stem/progenitor cell recruitment and migration. Semin. Arthritis Rheum. 2020, 50, 198–208. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Yuan, Z.; Fu, L.; Jiang, S.; Gao, C.; Wang, F.; Zha, K.; Tian, G.; Sun, Z.; et al. Endogenous cell recruitment strategy for articular cartilage regeneration. Acta Biomater. 2020, 114, 31–52. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Structure and ionic conductivity of a series of di-o-butyrylchitosan membranes. J. Appl. Polym. Sci. 2004, 94, 2309–2323. [Google Scholar] [CrossRef]
- Liu, J.; Fang, Q.; Lin, H.; Yu, X.; Zheng, H.; Wan, Y. Alginate-poloxamer/silk fibroin hydrogels with covalently and physically cross-linked networks for cartilage tissue engineering. Carbohydr. Polym. 2020, 247, 116593. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; Sun, J.; Chrzanowski, V.; Buschmann, M. A multivalent assay to detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Anal. Biochem. 2002, 300, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
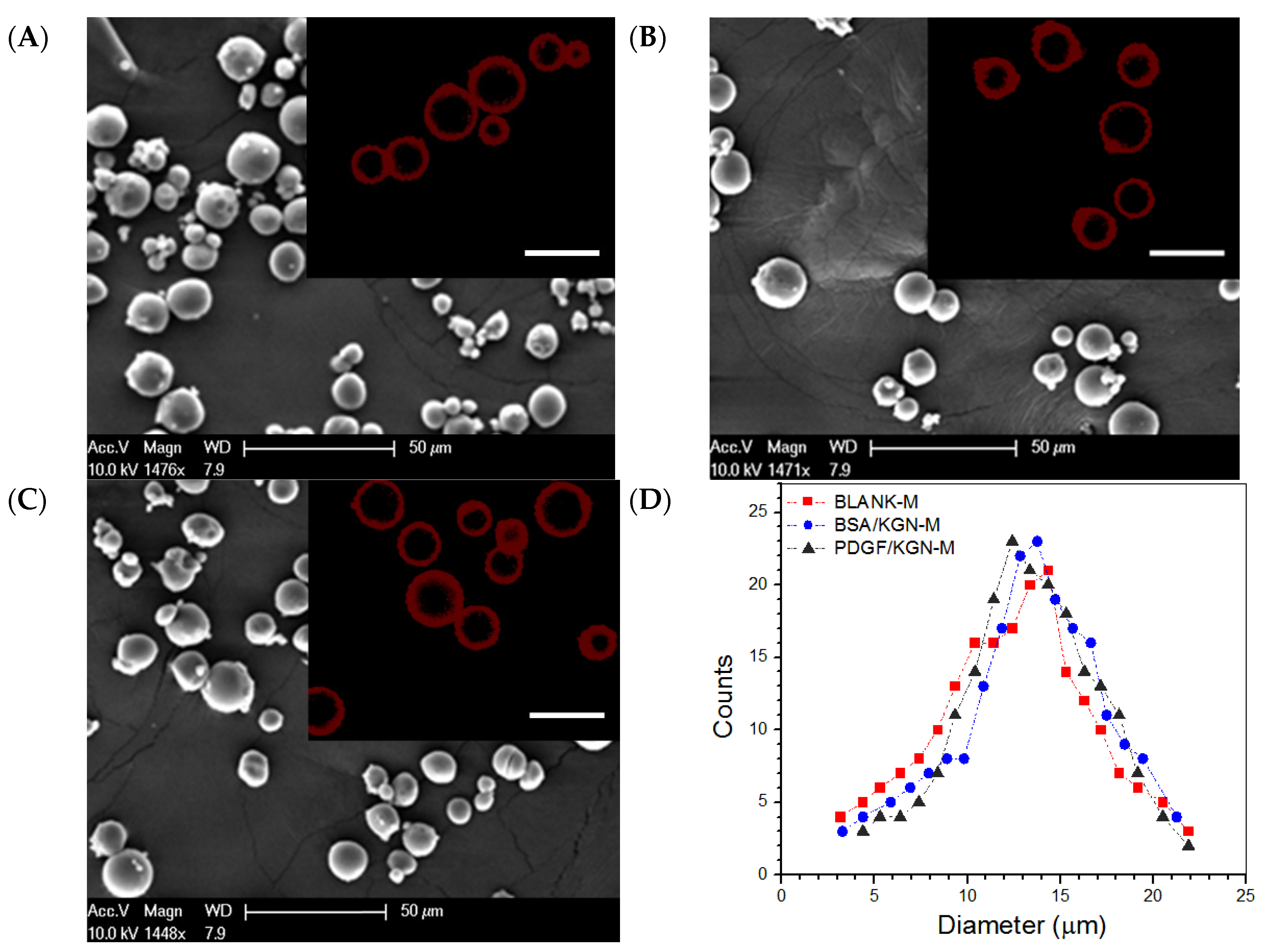
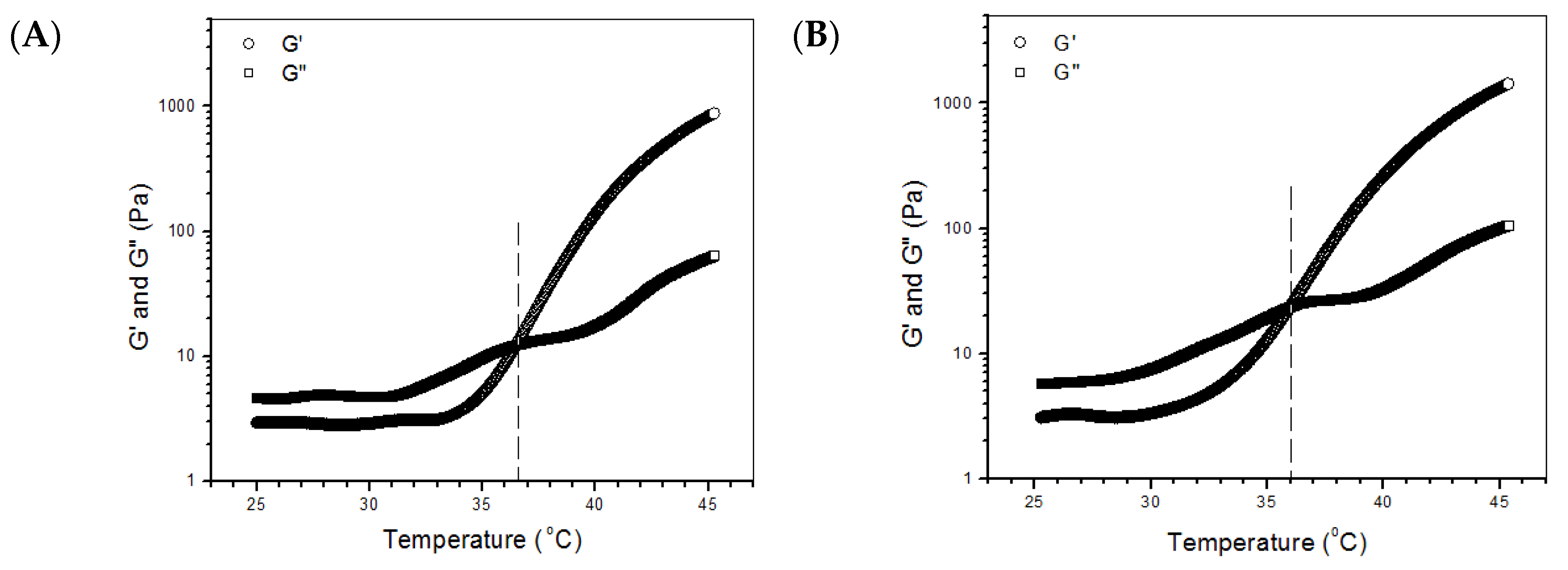
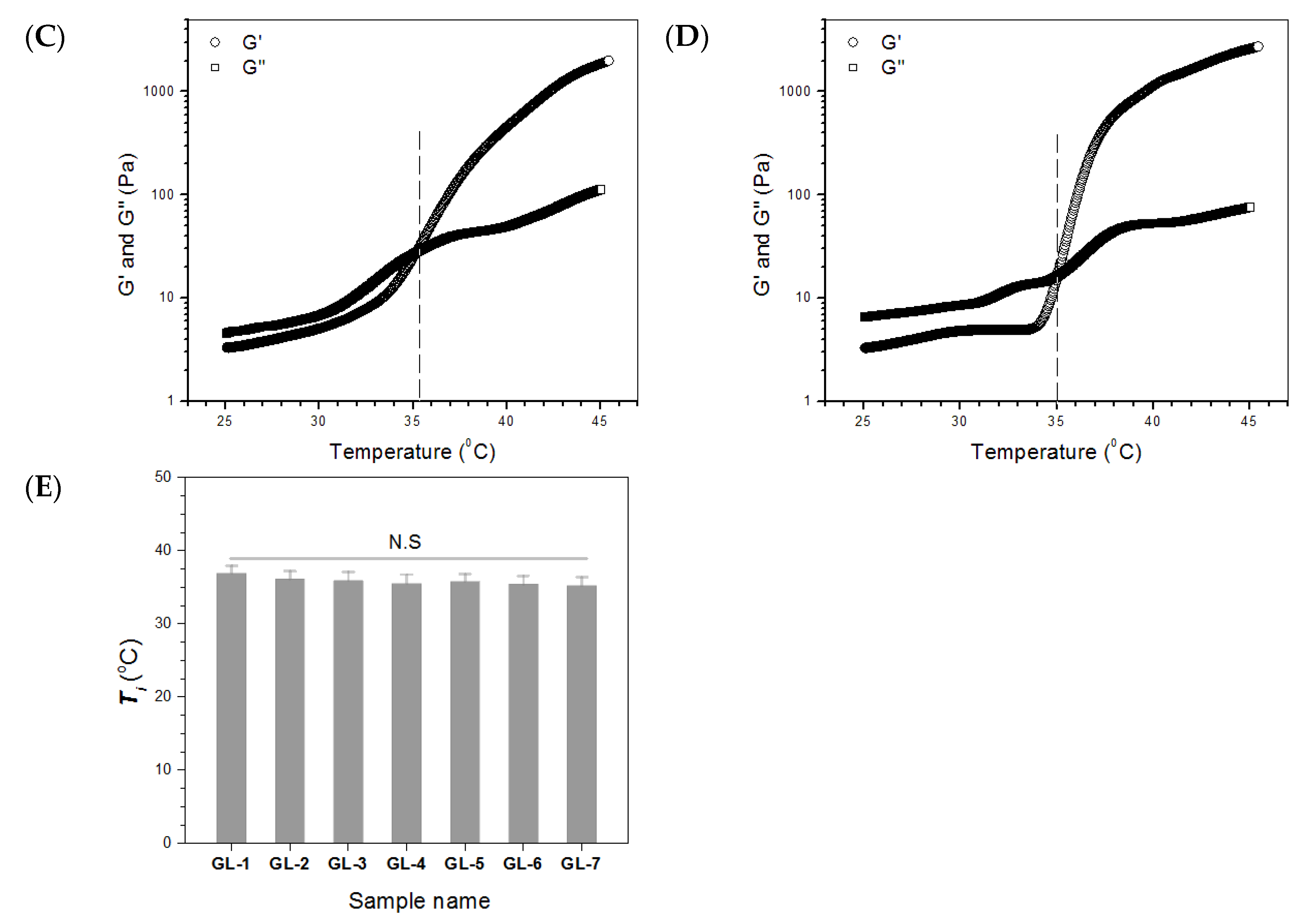
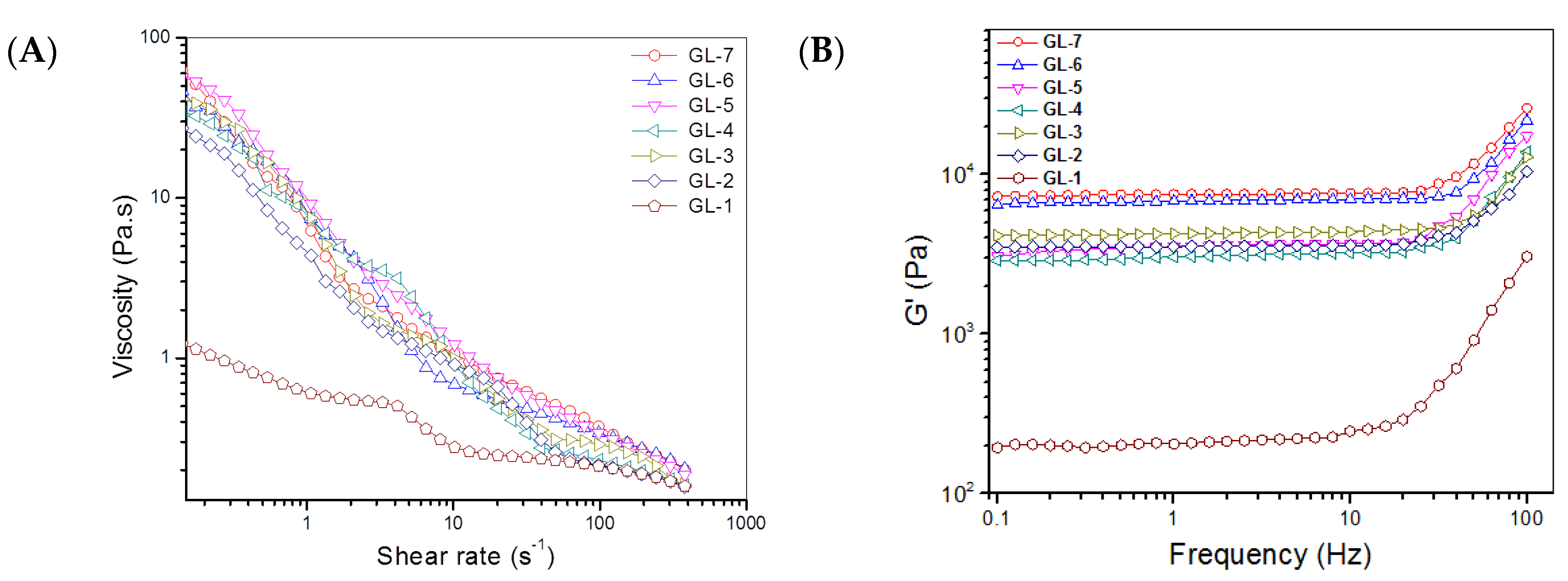


| Sample Name | EE for BSA (%) | DL for BSA (μg/mg) | EE for PDGF-BB (%) | DL for PDGF-BB (ng/mg) | EE for KGN (%) | DL for KGN (μg/mg) | Mean Size (μm) | Mean Thickness of Shell Layer (μm) |
|---|---|---|---|---|---|---|---|---|
| BLANK-M | − | − | − | − | − | − | 13.1 ± 1.71 | 2.94 ± 0.19 |
| BSA/KGN-M | 85.7 ± 4.12 | 2.48 ± 0.16 | − | − | 87.4 ± 2.39 | 34.96 ± 1.38 | 12.7 ± 1.57 | 3.17 ± 0.16 |
| PDGF/KGN-M | 86.3 ± 3.86 | 2.44 ± 0.19 | 87.4 ± 1.81 | 25.89 ± 0.61 | 88.1 ± 2.63 | 35.24 ± 1.51 | 13.5 ± 1.62 | 3.06 ± 0.12 |
| Sample Name | CH (w/v%) | SF (w/v%) | Genipin (w/v%) | TYR (μL) (c) | BLANK-M MPs (w/v%) (d) | pH | Gelation Time at 37 °C (sec) (e) |
|---|---|---|---|---|---|---|---|
| GL-1 (b) | 2.0 | − | − | − | − | 6.94 ± 0.06 | 580 ± 23 |
| GL-2 | 2.0 | 1.0 | 0.05 | − | 1.0 | 7.03 ± 0.08 | 295 ± 19 |
| GL-3 | 2.0 | 1.0 | 0.05 | − | 2.0 | 7.01 ± 0.07 | 260 ± 16 |
| GL-4 | 2.0 | 1.0 | − | 20 | 1.0 | 7.09 ± 0.06 | 285 ± 25 |
| GL-5 | 2.0 | 1.0 | − | 20 | 2.0 | 7.11 ± 0.09 | 255 ± 19 |
| GL-6 | 2.0 | 1.0 | 0.05 | 20 | 1.0 | 7.07 ± 0.08 | 230 ± 11 |
| GL-7 | 2.0 | 1.0 | 0.05 | 20 | 2.0 | 7.14 ± 0.09 | 205 ± 10 |
| Sample Name | CH (w/v%) | SF (w/v%) | Genipin (w/v%) | TYR (μL) (b) | PDGF/KGN-M MPs (w/v%) (c) | PDGF-BB Content in Gel (ng/mL) | KGN Content in Gel (μg/mL) |
|---|---|---|---|---|---|---|---|
| GEL-I | 2.0 | 1.0 | 0.05 | − | 1.0 | 258.9 ± 6.13 | 352.4 ± 15.13 |
| GEL-II | 2.0 | 1.0 | 0.05 | − | 2.0 | 517.8 ± 12.26 | 703.8 ± 30.26 |
| GEL-III | 2.0 | 1.0 | − | 20 | 1.0 | 258.9 ± 6.13 | 352.4 ± 15.13 |
| GEL-IV | 2.0 | 1.0 | − | 20 | 2.0 | 517.8 ± 12.26 | 703.8 ± 30.26 |
| GEL-V | 2.0 | 1.0 | 0.05 | 20 | 1.0 | 258.9 ± 6.13 | 352.4 ± 15.13 |
| GEL-VI | 2.0 | 1.0 | 0.05 | 20 | 2.0 | 517.8 ± 12.26 | 703.8 ± 30.26 |
| Sample (a) | k | n | r2 |
|---|---|---|---|
| PDGF/KGN-M (PGDE-BB) | 50.11 | 0.28 | 0.9721 |
| PDGF/KGN-M (KGN) | 24.54 | 0.44 | 0.9784 |
| GEL-II (PGDE-BB) | 17.78 | 0.56 | 0.9816 |
| GEL-I (PGDE-BB) | 14.45 | 0.58 | 0.9892 |
| GEL-II (KGN) | 7.17 | 0.68 | 0.9828 |
| GEL-I (KGN) | 4.51 | 0.76 | 0.9840 |
| GEL-IV (PGDE-BB) | 19.95 | 0.55 | 0.9860 |
| GEL-III (PGDE-BB) | 15.84 | 0.55 | 0.9943 |
| GEL-IV (KGN) | 9.01 | 0.64 | 0.9847 |
| GEL-III (KGN) | 6.54 | 0.69 | 0.9869 |
| GEL-VI (PGDE-BB) | 7.94 | 0.71 | 0.9816 |
| GEL-V (PGDE-BB) | 4.98 | 0.79 | 0.9897 |
| GEL-VI (KGN) | 2.18 | 0.95 | 0.9763 |
| GEL-V (KGN) | 1.46 | 0.99 | 0.9703 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Q.; Tian, D.; Zhang, Y.; Wang, C.; Wan, Y.; Wu, J. Strong and Elastic Chitosan/Silk Fibroin Hydrogels Incorporated with Growth-Factor-Loaded Microspheres for Cartilage Tissue Engineering. Biomimetics 2022, 7, 41. https://doi.org/10.3390/biomimetics7020041
Min Q, Tian D, Zhang Y, Wang C, Wan Y, Wu J. Strong and Elastic Chitosan/Silk Fibroin Hydrogels Incorporated with Growth-Factor-Loaded Microspheres for Cartilage Tissue Engineering. Biomimetics. 2022; 7(2):41. https://doi.org/10.3390/biomimetics7020041
Chicago/Turabian StyleMin, Qing, Danlei Tian, Yuchen Zhang, Congcong Wang, Ying Wan, and Jiliang Wu. 2022. "Strong and Elastic Chitosan/Silk Fibroin Hydrogels Incorporated with Growth-Factor-Loaded Microspheres for Cartilage Tissue Engineering" Biomimetics 7, no. 2: 41. https://doi.org/10.3390/biomimetics7020041





