Measuring Photonics in Photosynthesis: Combined Micro-Fourier Image Spectroscopy and Pulse Amplitude Modulated Chlorophyll Fluorimetry at the Micrometre-Scale
Abstract
:1. Introduction
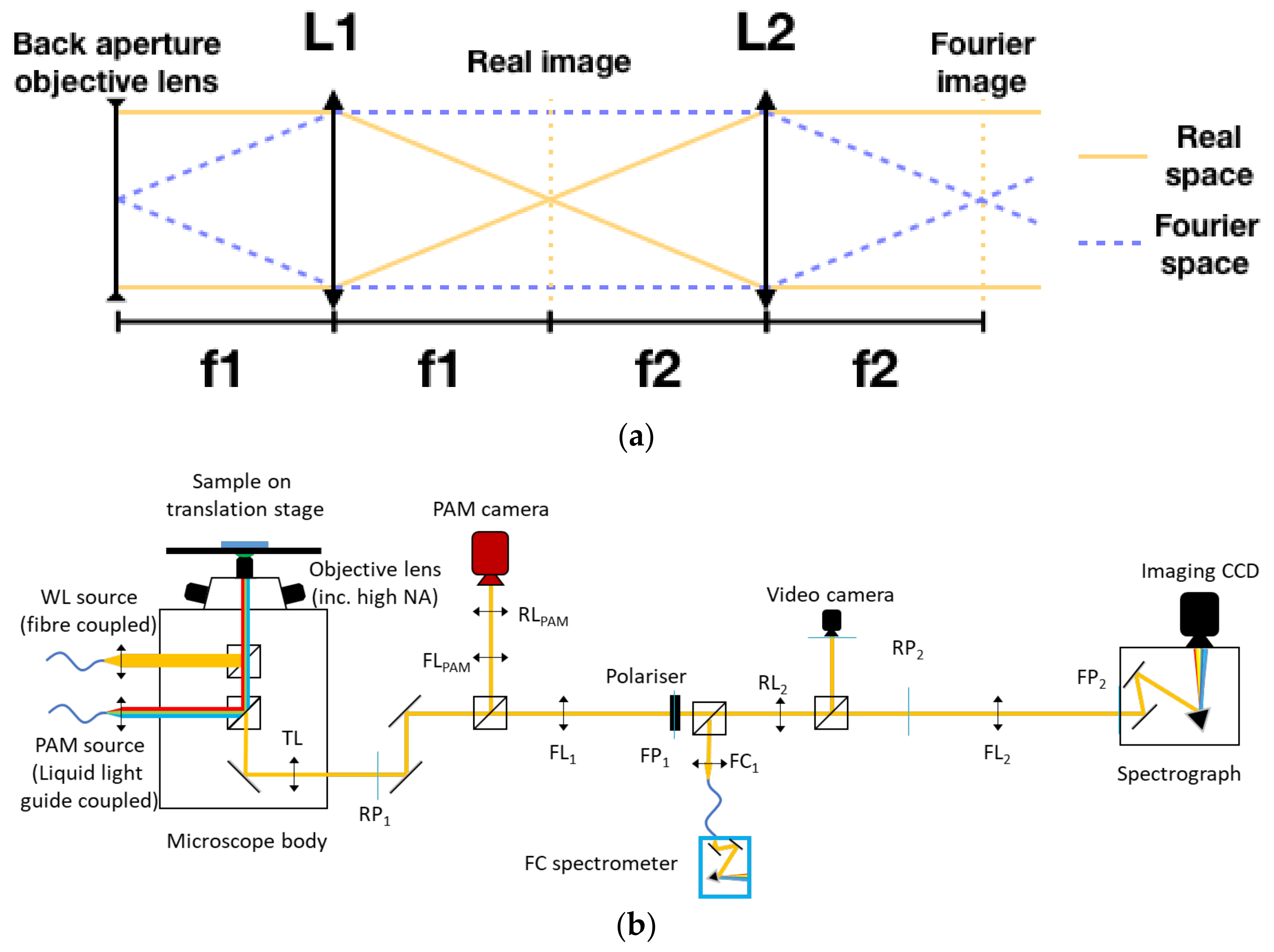
2. Materials and Methods
3. Experimental Demonstration of Capabilities
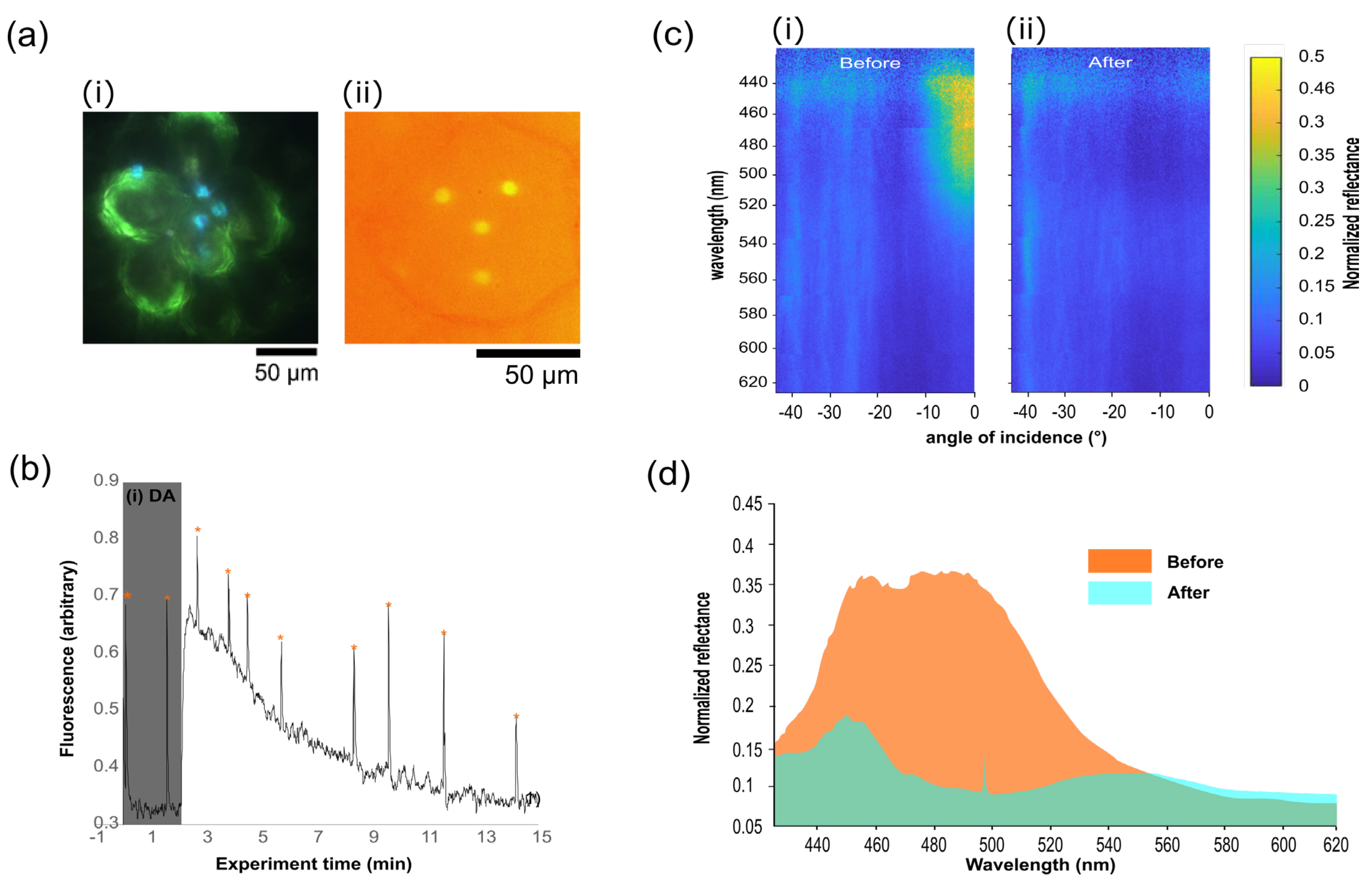
3.1. FIS and PAM Combined to Study Photosynthesis in Diatoms Featuring Photonic Crystal Girdles
3.2. Iridoplasts for FIS-PAM Test on Photosynthetic Photonic Organelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolle, M.; Salgard-Cunha, P.M.; Scherer, M.R.J.; Huang, F.; Vukusic, P.; Mahajan, S.; Baumberg, J.J.; Steiner, U. Mimicking the Colourful Wing Scale Structure of the Papilio blumei Butterfly. Nat. Nanotechnol. 2010, 5, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Wilts, B.D.; Leertouwer, H.L.; Stavenga, D.G. Imaging Scatterometry and Microspectrophotometry of Lycaenid Butterfly Wing Scales with Perforated Multilayers. J. R. Soc. Interface 2009, 6, S185–S192. [Google Scholar] [CrossRef] [PubMed]
- Burresi, M.; Cortese, L.; Pattelli, L.; Kolle, M.; Vukusic, P.; Wiersma, D.S.; Steiner, U.; Vignolini, S. Bright-White Beetle Scales Optimise Multiple Scattering of Light. Sci. Rep. 2015, 4, 6075. [Google Scholar] [CrossRef] [PubMed]
- Vukusic, P.; Sambles, J.R. Photonic Structures in Biology. Nature 2003, 424, 852–855. [Google Scholar] [CrossRef]
- Holt, A.L.; Vahidinia, S.; Gagnon, Y.L.; Morse, D.E.; Sweeney, A.M. Photosymbiotic Giant Clams Are Transformers of Solar Flux. J. R. Soc. Interface 2014, 11, 20140678. [Google Scholar] [CrossRef]
- Palmer, B.A.; Taylor, G.J.; Brumfeld, V.; Gur, D.; Shemesh, M.; Elad, N.; Osherov, A.; Oron, D.; Weiner, S.; Addadi, L. The Image-Forming Mirror in the Eye of the Scallop. Science 2017, 358, 1172–1175. [Google Scholar] [CrossRef]
- Freyer, P.; Wilts, B.D.; Stavenga, D.G. Reflections on Iridescent Neck and Breast Feathers of the Peacock, Pavo cristatus. Interface Focus 2019, 9, 20180043. [Google Scholar] [CrossRef]
- Freyer, P.; Wilts, B.D.; Stavenga, D.G. Cortex Thickness Is Key for the Colors of Iridescent Starling Feather Barbules With a Single, Organized Melanosome Layer. Front. Ecol. Evol. 2021, 9, 746254. [Google Scholar] [CrossRef]
- Dramatic Colour Changes in a Bird of Paradise Caused by Uniquely Structured Breast Feather Barbules. Available online: https://royalsocietypublishing.org/doi/epdf/10.1098/rspb.2010.2293 (accessed on 3 May 2022).
- Guidetti, G.; Levy, G.; Matzeu, G.; Finkelstein, J.M.; Levin, M.; Omenetto, F.G. Unmixing Octopus Camouflage by Multispectral Mapping of Octopus Bimaculoides’ Chromatic Elements. Nanophotonics 2021, 10, 2441–2450. [Google Scholar] [CrossRef]
- Deravi, L.F.; Magyar, A.P.; Sheehy, S.P.; Bell, G.R.R.; Mäthger, L.M.; Senft, S.L.; Wardill, T.J.; Lane, W.S.; Kuzirian, A.M.; Hanlon, R.T.; et al. The Structure–Function Relationships of a Natural Nanoscale Photonic Device in Cuttlefish Chromatophores. J. R. Soc. Interface 2014, 11, 20130942. [Google Scholar] [CrossRef]
- Kolle, M.; Steiner, U. Structural Color in Animals. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 2514–2527. ISBN 978-90-481-9751-4. [Google Scholar]
- Middleton, R.; Sinnott-Armstrong, M.; Ogawa, Y.; Jacucci, G.; Moyroud, E.; Rudall, P.J.; Prychid, C.; Conejero, M.; Glover, B.J.; Donoghue, M.J.; et al. Viburnum Tinus Fruits Use Lipids to Produce Metallic Blue Structural Color. Curr. Biol. 2020, 30, 3804–3810. [Google Scholar] [CrossRef]
- Vignolini, S.; Rudall, P.J.; Rowland, A.V.; Reed, A.; Moyroud, E.; Faden, R.B.; Baumberg, J.J.; Glover, B.J.; Steiner, U. Pointillist Structural Color in Pollia Fruit. Proc. Natl. Acad. Sci. USA 2012, 109, 15712–15715. [Google Scholar] [CrossRef]
- Ren, H.; Xiao, T.; Zhang, Q.; Liu, Z. Photosynthesis-Inspired Bifunctional Energy-Harvesting Devices That Convert Light and Salinity Gradients into Electricity. Chem. Commun. 2018, 54, 12310–12313. [Google Scholar] [CrossRef]
- Chenu, A.; Scholes, G.D. Coherence in Energy Transfer and Photosynthesis. Annu. Rev. Phys. Chem. 2015, 66, 69–96. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig-Adams, B. Regulation of Photosynthetic Light Energy Capture, Conversion, and Dissipation in Leaves of Higher Plants. In Ecophysiology of Photosynthesis, Springer Study Edition; Schulze, E.-D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 17–47. ISBN 978-3-642-79354-7. [Google Scholar]
- Minagawa, J. Dynamic Reorganization of Photosynthetic Supercomplexes during Environmental Acclimation of Photosynthesis. Front. Plant Sci. 2013, 4, 513. [Google Scholar] [CrossRef]
- Klimant, I.; Kühl, M.; Glud, R.N.; Holst, G. Optical Measurement of Oxygen and Temperature in Microscale: Strategies and Biological Applications. Sens. Actuators B: Chem. 1997, 38, 29–37. [Google Scholar] [CrossRef]
- Trampe, E.; Hansen, P.J.; Kuhl, M. A Comparison of Photosynthesis Measurements by O2 Evolution, 14C Assimilation, and Variable Chlorophyll Fluorescence during Light Acclimatization of the Diatom Coscinodiscus Granii. ALGAE 2015, 30, 103–119. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Wangpraseurt, D.; Lichtenberg, M.; Jacques, S.L.; Larkum, A.W.D.; Kühl, M. Optical Properties of Corals Distort Variable Chlorophyll Fluorescence Measurements. Plant Physiol. 2019, 179, 1608–1619. [Google Scholar] [CrossRef]
- Capretti, A.; Ringsmuth, A.K.; Velzen, J.F.; van Rosnik, A.; Croce, R.; Gregorkiewicz, T. Nanophotonics of Higher-Plant Photosynthetic Membranes. Light: Sci. Appl. 2019, 8, 5. [Google Scholar] [CrossRef]
- Jacobs, M.; Lopez-Garcia, M.; Phrathep, O.-P.; Lawson, T.; Oulton, R.; Whitney, H.M. Photonic Multilayer Structure of Begonia Chloroplasts Enhances Photosynthetic Efficiency. Nat. Plants 2016, 2, 16162. [Google Scholar] [CrossRef]
- Masters, N.J.; Lopez-Garcia, M.; Oulton, R.; Whitney, H.M. Characterization of Chloroplast Iridescence in Selaginella Erythropus. J. R. Soc. Interface 2018, 15, 20180559. [Google Scholar] [CrossRef]
- Goessling, J.W.; Wardley, W.P.; Lopez-Garcia, M. Highly Reproducible, Bio-Based Slab Photonic Crystals Grown by Diatoms. Adv. Sci. 2020, 7, 1903726. [Google Scholar] [CrossRef]
- Fuhrmann, T.; Landwehr, S.; El Rharbi-Kucki, M.; Sumper, M. Diatoms as Living Photonic Crystals. Appl. Phys. B Lasers Opt. 2004, 78, 257–260. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- López-García, M.; Galisteo-López, J.F.; Blanco, A.; Sánchez-Marcos, J.; López, C.; García-Martín, A. Enhancement and Directionality of Spontaneous Emission in Hybrid Self-Assembled Photonic–Plasmonic Crystals. Small 2010, 6, 1757–1761. [Google Scholar] [CrossRef]
- Kurvits, J.A.; Jiang, M.; Zia, R. Comparative Analysis of Imaging Configurations and Objectives for Fourier Microscopy. J. Opt. Soc. Am. A JOSAA 2015, 32, 2082–2092. [Google Scholar] [CrossRef]
- Hwang, S.-U.; Lee, Y.-G. Simulation of an Oil Immersion Objective Lens: A Simplified Ray-Optics Model Considering Abbe’s Sine Condition. Opt. Express OE 2008, 16, 21170–21183. [Google Scholar] [CrossRef]
- Vignolini, S.; Moyroud, E.; Glover, B.J.; Steiner, U. Analysing Photonic Structures in Plants. J. R. Soc. Interface 2013, 10, 20130394–20130394. [Google Scholar] [CrossRef]
- Castillo, M.A.; Estévez-Varela, C.; Wardley, W.P.; Serna, R.; Pastoriza-Santos, I.; Núñez-Sánchez, S.; Lopez-Garcia, M. Enhanced Light Absorption in All-Polymer Biomimetic Photonic Structures by Near-Zero-Index Organic Matter. Adv. Funct. Mater. 2022, 32, 2113039. [Google Scholar] [CrossRef]
- Lopez-Garcia, M.; Masters, N.; O’Brien, H.E.; Lennon, J.; Atkinson, G.; Cryan, M.J.; Oulton, R.; Whitney, H.M. Light-Induced Dynamic Structural Color by Intracellular 3D Photonic Crystals in Brown Algae. Sci. Adv. 2018, 4, eaan8917. [Google Scholar] [CrossRef] [PubMed]
- WALZ. High Quality Instrumentation For Plant Sciences. Available online: https://www.walz.com/ (accessed on 3 May 2022).
- Goessling, J.W.; Su, Y.; Cartaxana, P.; Maibohm, C.; Rickelt, L.F.; Trampe, E.C.L.; Wangpraseurt, D.; Walby, S.L.; Wu, X.; Ellegaard, M.; et al. Structure-Based Optics of Centric Diatom Frustules: Modulation of the in Vivo Light Field for Efficient Diatom Photosynthesis. New Phytol. 2018, 219, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.-H.; Tsai, P.-Y.; Peng, C.-I.; Chen, P.-J.; Tsai, C.-C.; Yang, E.-C.; Shih, M.-C.; Chen, J.; Yang, J.-Y.; Chesson, P.; et al. Lamelloplasts and Minichloroplasts in Begoniaceae: Iridescence and Photosynthetic Functioning. J. Plant Res. 2018, 131, 655–670. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardley, W.P.; Goessling, J.W.; Lopez-Garcia, M. Measuring Photonics in Photosynthesis: Combined Micro-Fourier Image Spectroscopy and Pulse Amplitude Modulated Chlorophyll Fluorimetry at the Micrometre-Scale. Biomimetics 2022, 7, 107. https://doi.org/10.3390/biomimetics7030107
Wardley WP, Goessling JW, Lopez-Garcia M. Measuring Photonics in Photosynthesis: Combined Micro-Fourier Image Spectroscopy and Pulse Amplitude Modulated Chlorophyll Fluorimetry at the Micrometre-Scale. Biomimetics. 2022; 7(3):107. https://doi.org/10.3390/biomimetics7030107
Chicago/Turabian StyleWardley, William P., Johannes W. Goessling, and Martin Lopez-Garcia. 2022. "Measuring Photonics in Photosynthesis: Combined Micro-Fourier Image Spectroscopy and Pulse Amplitude Modulated Chlorophyll Fluorimetry at the Micrometre-Scale" Biomimetics 7, no. 3: 107. https://doi.org/10.3390/biomimetics7030107





