Inspired by the Nature: A Post-printed Strategy to Efficiently Elaborate Parahydrophobic Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Elaboration
2.1.1. Surface Modelling
2.1.2. Surface Printing
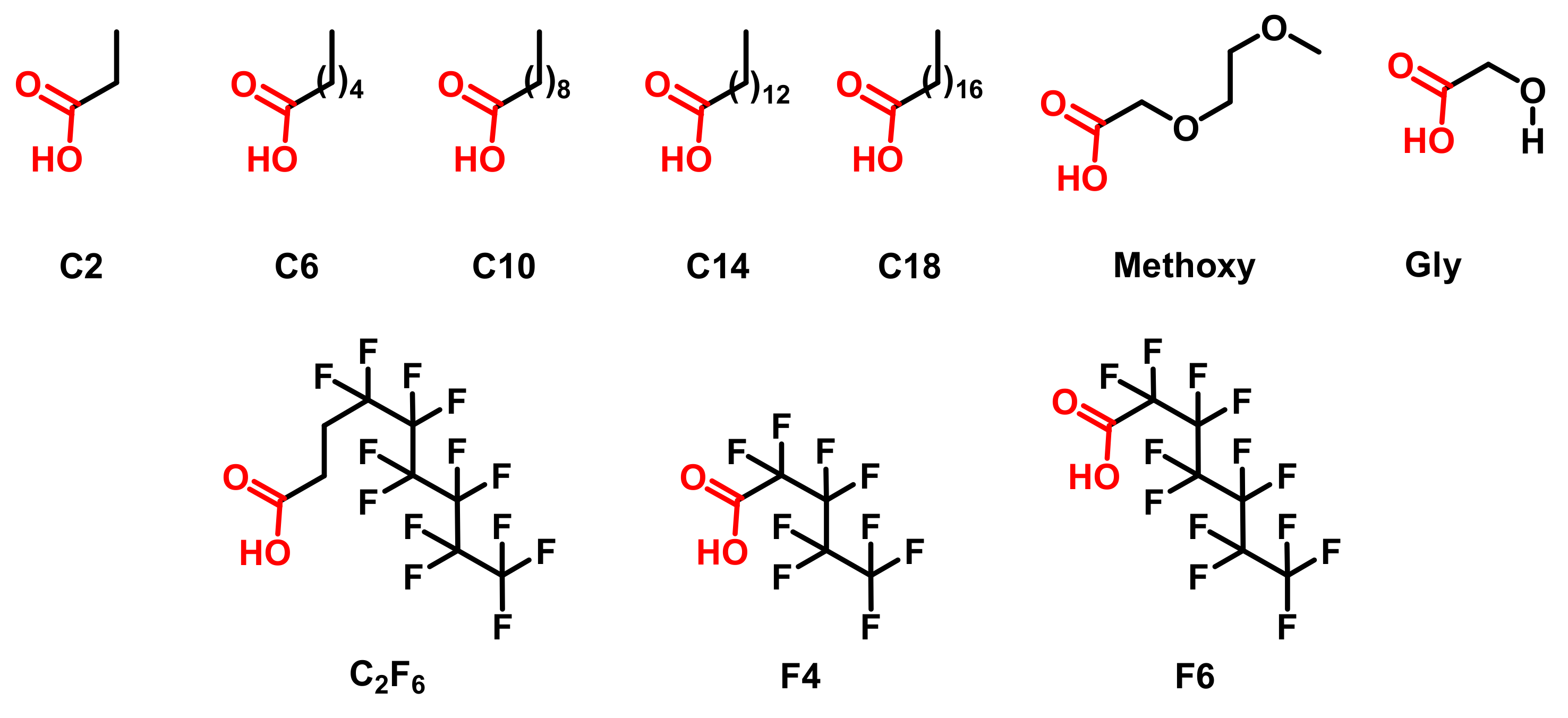
2.2. Surface Functionalization
2.3. Surface Roughness
2.4. Surface Morphologies
2.5. Surface Wettability
3. Results
3.1. Surface Elaboration
3.2. Surface Functionalization
3.3. Surfaces Roughness
3.4. Surface Morphology
3.5. Surface Wettability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, H.; Zhao, S.; Zhang, X.; Wen, B.; Su, Z. The Future of Freshwater Access: Functional Material-Based Nano-Membranes for Desalination. Mater. Today Energy 2021, 22, 100856. [Google Scholar] [CrossRef]
- Al-Ibrahim, A.M. Seawater Desalination: The Strategic Choice for Saudi Arabia. Desalin. Water Treat. 2013, 51, 1–4. [Google Scholar] [CrossRef]
- Al-Sahlawi, M.A. Seawater Desalination in Saudi Arabia: Economic Review and Demand Projections. Desalination 1999, 123, 143–147. [Google Scholar] [CrossRef]
- Einav, R.; Harussi, K.; Perry, D. The Footprint of the Desalination Processes on the Environment. Desalination 2003, 152, 141–154. [Google Scholar] [CrossRef]
- Uddin, S.; Al Ghadban, A.N.; Khabbaz, A. Localized Hyper Saline Waters in Arabian Gulf from Desalination Activity—An Example from South Kuwait. Environ. Monit. Assess. 2011, 181, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Yiannacou, K.; Karjalainen, M.; Lahtonen, K.; Valden, M.; Sariola, V. Large-Scale Efficient Water Harvesting Using Bioinspired Micro-Patterned Copper Oxide Nanoneedle Surfaces and Guided Droplet Transport. Nanoscale Adv. 2019, 1, 4025–4040. [Google Scholar] [CrossRef]
- Bitonto, M.G.D.; Angelotti, A.; Zanelli, A. Fog and Dew Harvesting: Italy and Chile in Comparison. Int. J. Innov. Res. Dev. 2020, 9, 132–140. [Google Scholar] [CrossRef]
- Knapczyk-Korczak, J.; Szewczyk, P.K.; Ura, D.P.; Bailey, R.J.; Bilotti, E.; Stachewicz, U. Improving Water Harvesting Efficiency of Fog Collectors with Electrospun Random and Aligned Polyvinylidene Fluoride (PVDF) Fibers. Sustain. Mater. Technol. 2020, 25, e00191. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Kleingartner, J.; Oliphant, A.; Bowman, M.; Torregrosa, A.; Weiss-Penzias, P.S.; Zhang, B.J.; Sorensen, D.; Cohen, R.E.; McKinley, G.H. Fog Water Collection Effectiveness: Mesh Intercomparisons. Aerosol Air Qual. Res. 2018, 18, 270–283. [Google Scholar] [CrossRef]
- de Rivera, J.D. Aerodynamic Collection Efficiency of Fog Water Collectors. Atmos. Res. 2011, 102, 335–342. [Google Scholar] [CrossRef]
- Yue, G.; Wang, Y.; Li, D.; Hou, L.; Cui, Z.; Li, Q.; Wang, N.; Zhao, Y. Bioinspired Surface with Special Wettability for Liquid Transportation and Separation. Sustain. Mater. Technol. 2020, 25, e00175. [Google Scholar] [CrossRef]
- Nørgaard, T.; Dacke, M. Fog-Basking Behaviour and Water Collection Efficiency in Namib Desert Darkling Beetles. Front. Zool. 2010, 7, 23. [Google Scholar] [CrossRef]
- Chakrabarti, U.; Paoli, R.; Chatterjee, S.; Megaridis, C.M. Importance of Body Stance in Fog Droplet Collection by the Namib Desert Beetle. Biomimetics 2019, 4, 59. [Google Scholar] [CrossRef]
- Hamilton, W.J.; Seely, M.K. Fog Basking by the Namib Desert Beetle, Onymacris Unguicularis. Nature 1976, 262, 284–285. [Google Scholar] [CrossRef]
- Ward, D.; Seely, M.K. Adaptation and Constraint in the Evolution of the Physiology and Behavior of the Namib Desert Tenebrionid Beetle Genus Onymacris. Evolution 1996, 50, 1231. [Google Scholar] [CrossRef]
- Mitchell, D.; Henschel, J.R.; Hetem, R.S.; Wassenaar, T.D.; Strauss, W.M.; Hanrahan, S.A.; Seely, M.K. Fog and Fauna of the Namib Desert: Past and Future. Ecosphere 2020, 11, e02996. [Google Scholar] [CrossRef]
- Malik, F.T.; Clement, R.M.; Gethin, D.T.; Beysens, D.; Cohen, R.E.; Krawszik, W.; Parker, A.R. Dew Harvesting Efficiency of Four Species of Cacti. Bioinspir. Biomim. 2015, 10, 036005. [Google Scholar] [CrossRef]
- Godeau, G.; Laugier, J.-P.; Orange, F.; Godeau, R.-P.; Guittard, F.; Darmanin, T. A Travel in the Echeveria Genus Wettability’s World. Appl. Surf. Sci. 2017, 411, 291–302. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A Multi-Structural and Multi-Functional Integrated Fog Collection System in Cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef]
- Gurera, D.; Bhushan, B. Passive Water Harvesting by Desert Plants and Animals: Lessons from Nature. Philos. Trans. R. Soc. A 2020, 378, 20190444. [Google Scholar] [CrossRef]
- Konrad, W.; Burkhardt, J.; Ebner, M.; Roth-Nebelsick, A. Leaf Pubescence as a Possibility to Increase Water Use Efficiency by Promoting Condensation. Ecohydrology 2015, 8, 480–492. [Google Scholar] [CrossRef]
- Marmur, A. Hydro- Hygro- Oleo- Omni-Phobic? Terminology of Wettability Classification. Soft Matter 2012, 8, 6867. [Google Scholar] [CrossRef]
- Bormashenko, E.; Stein, T.; Pogreb, R.; Aurbach, D. “Petal Effect” on Surfaces Based on Lycopodium: High-Stick Surfaces Demonstrating High Apparent Contact Angles. J. Phys. Chem. C 2009, 113, 5568–5572. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Chaule, S.; Hwang, J.; Ha, S.; Kang, J.; Yoon, J.; Jang, J. Rational Design of a High Performance and Robust Solar Evaporator via 3D-Printing Technology. Adv. Mater. 2021, 33, 2102649. [Google Scholar] [CrossRef]
- Khalil, A.; Ahmed, F.E.; Hilal, N. The Emerging Role of 3D Printing in Water Desalination. Sci. Total Environ. 2021, 790, 148238. [Google Scholar] [CrossRef]
- Wei, Q.; Li, H.; Liu, G.; He, Y.; Wang, Y.; Tan, Y.E.; Wang, D.; Peng, X.; Yang, G.; Tsubaki, N. Metal 3D Printing Technology for Functional Integration of Catalytic System. Nat. Commun. 2020, 11, 4098. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D Printing of Conducting Polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D Printing of PCL/Nano-Hydroxyapatite Scaffolds Derived from Biogenic Sources for Bone Tissue Engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- Jacquot, C.; Middelkoop, V.; Köckritz, A.; Pohar, A.; Bienert, R.; Kellici, S.; Bărăgău, I.-A.; Venezia, B.; Gavriilidis, A.; Likozar, B.; et al. 3D Printed Catalytic Reactors for Aerobic Selective Oxidation of Benzyl Alcohol into Benzaldehyde in Continuous Multiphase Flow. Sustain. Mater. Technol. 2021, 30, e00329. [Google Scholar] [CrossRef]
- Godeau, G.; Darmanin, T.; Guittard, F. Staudinger Vilarassa Reaction: A Powerful Tool for Surface Modification and Superhydrophobic Properties. J. Colloid Interface Sci. 2015, 457, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Godeau, G.; Darmanin, T.; Guittard, F. Ante versus Post-Functionalization to Control Surface Structures with Superhydrophobic and Superoleophobic Properties. RSC Adv. 2015, 5, 63945–63951. [Google Scholar] [CrossRef]
- Ramos Chagas, G.; Xie, X.; Darmanin, T.; Steenkeste, K.; Gaucher, A.; Prim, D.; Méallet-Renault, R.; Godeau, G.; Amigoni, S.; Guittard, F. Electrodeposition of Polypyrenes with Tunable Hydrophobicity, Water Adhesion, and Fluorescence Properties. J. Phys. Chem. C 2016, 120, 7077–7087. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Wang, W.; Xu, C.; Ren, L. Superhydrophobic Copper Surface Textured by Laser for Delayed Icing Phenomenon. Langmuir 2020, 36, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tu, Y.; Hu, J.; Zou, H.; Liu, G.; Lin, S.; Yang, G.; Hu, S.; Miao, L.; Mo, Y. Fabrication of Fluorinated Raspberry Particles and Their Use as Building Blocks for the Construction of Superhydrophobic Films to Mimic the Wettabilities from Lotus Leaves to Rose Petals. Polym. Chem. 2015, 6, 6746–6760. [Google Scholar] [CrossRef]
- Peng, S.; Tian, D.; Miao, X.; Yang, X.; Deng, W. Designing Robust Alumina Nanowires-on-Nanopores Structures: Superhydrophobic Surfaces with Slippery or Sticky Water Adhesion. J. Colloid Interface Sci. 2013, 409, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Highly Ordered Anodic Alumina Nanofibers Fabricated via Two Distinct Anodizing Processes. ECS Electrochem. Lett. 2015, 4, H14–H17. [Google Scholar] [CrossRef]
- Long, J.; Fan, P.; Gong, D.; Jiang, D.; Zhang, H.; Li, L.; Zhong, M. Superhydrophobic Surfaces Fabricated by Femtosecond Laser with Tunable Water Adhesion: From Lotus Leaf to Rose Petal. ACS Appl. Mater. Interfaces 2015, 7, 9858–9865. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.; Farooq, U.; Du, G.; Hou, X. A Simple Way to Achieve Superhydrophobicity, Controllable Water Adhesion, Anisotropic Sliding, and Anisotropic Wetting Based on Femtosecond-Laser-Induced Line-Patterned Surfaces. J. Mater. Chem. A 2014, 2, 5499–5507. [Google Scholar] [CrossRef]
- Chen, H.-H.; Anbarasan, R.; Kuo, L.-S.; Tsai, M.-Y.; Chen, P.-H.; Chiang, K.-F. Synthesis, Characterizations and Hydrophobicity of Micro/Nano Scaled Heptadecafluorononanoic Acid Decorated Copper Nanoparticle. Nano Micro Lett. 2010, 2, 101–105. [Google Scholar] [CrossRef]
- Yin, S.; Wu, D.; Yang, J.; Lei, S.; Kuang, T.; Zhu, B. Fabrication and Surface Characterization of Biomimic Superhydrophobic Copper Surface by Solution-Immersion and Self-Assembly. Appl. Surf. Sci. 2011, 257, 8481–8485. [Google Scholar] [CrossRef]
- Chen, X.; Kong, L.; Dong, D.; Yang, G.; Yu, L.; Chen, J.; Zhang, P. Fabrication of Functionalized Copper Compound Hierarchical Structure with Bionic Superhydrophobic Properties. J. Phys. Chem. C 2009, 113, 5396–5401. [Google Scholar] [CrossRef]
- Sasmal, A.K.; Mondal, C.; Sinha, A.K.; Gauri, S.S.; Pal, J.; Aditya, T.; Ganguly, M.; Dey, S.; Pal, T. Fabrication of Superhydrophobic Copper Surface on Various Substrates for Roll-off, Self-Cleaning, and Water/Oil Separation. ACS Appl. Mater. Interfaces 2014, 6, 22034–22043. [Google Scholar] [CrossRef]
- Ciffréo, L.; Marchand, C.; Szczepanski, C.R.; Medici, M.-G.; Godeau, G. Bioinspired and Post-Functionalized 3D-Printed Surfaces with Parahydrophobic Properties. Biomimetics 2021, 6, 71. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Guo, Z. Superhydrophobic Sand Grains Structured with Aligned Cu(OH) 2 Nano-Needles for Efficient Oily Water Treatment. Mater. Des. 2017, 135, 377–384. [Google Scholar] [CrossRef]
- Chaudhary, A.; Barshilia, H.C. Nanometric Multiscale Rough CuO/Cu(OH) 2 Superhydrophobic Surfaces Prepared by a Facile One-Step Solution-Immersion Process: Transition to Superhydrophilicity with Oxygen Plasma Treatment. J. Phys. Chem. C 2011, 115, 18213–18220. [Google Scholar] [CrossRef]











| PVB | GVB | LMG | NMG | |
|---|---|---|---|---|
| Cu | 139.4 ± 0.4 | 138.9 ± 0.3 | 130.4 ± 2.0 | 122.9 ± 0.4 |
| Cu(OH)2 | 146.2 ± 2.8 | 128.9 ± 1.2 | 139.3 ± 1.3 | 144.7 ± 1.0 |
| C2 | 150.1 ± 0.4 | 154.4 ± 1.1 | 144.0 ± 1.2 | 149.6 ± 2.0 |
| C6 | 155.7 ± 0.5 | 157.1 ± 1.9 | 155.3 ± 0.3 | 155.1 ± 0.4 |
| C10 | 154.7 ± 1.3 | 157.8 ± 0.7 | 155.7 ± 2.5 | 151.7 ± 3.8 |
| C14 | 156.6 ± 0.6 | 157.0 ± 2.6 | 155.3 ± 2.1 | 154.7 ± 1.9 |
| C18 | 155.8 ± 1.0 | 156.1 ± 2.1 | 152.2 ± 2.2 | 154.0 ± 1.5 |
| C2F4 | 147.0 ± 2.6 | 156.0 ± 1.1 | 145.4 ± 2.9 | 150.7 ± 0.3 |
| F4 | 144.9 ± 0.5 | 150.2 ± 1.6 | 144.5 ± 0.4 | 149.0 ± 1.0 |
| F6 | 152.1 ± 0.2 | 149.4 ± 1.8 | 150.1 ± 0.5 | 151.8 ± 1.0 |
| Gly | N/A | N/A | N/A | N/A |
| Methoxy | 146.2 ± 1.8 | 133.1 ± 0.7 | 130.9 ± 1.4 | 136.2 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.Q.; Szczepanski, C.R.; Medici, M.G.; Godeau, G. Inspired by the Nature: A Post-printed Strategy to Efficiently Elaborate Parahydrophobic Surfaces. Biomimetics 2022, 7, 122. https://doi.org/10.3390/biomimetics7030122
Campos JQ, Szczepanski CR, Medici MG, Godeau G. Inspired by the Nature: A Post-printed Strategy to Efficiently Elaborate Parahydrophobic Surfaces. Biomimetics. 2022; 7(3):122. https://doi.org/10.3390/biomimetics7030122
Chicago/Turabian StyleCampos, Jordy Queiros, Caroline R. Szczepanski, Marie Gabrielle Medici, and Guilhem Godeau. 2022. "Inspired by the Nature: A Post-printed Strategy to Efficiently Elaborate Parahydrophobic Surfaces" Biomimetics 7, no. 3: 122. https://doi.org/10.3390/biomimetics7030122






