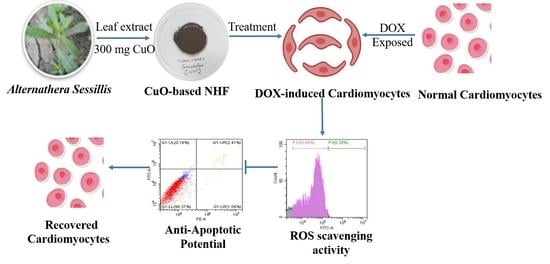
Preparation of Novel Nanoformulation to Enhance Efficacy in the Treatment of Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Method
2.1. Collection and Identification of Plants
2.2. Optimization and Formulation of Nano Herbomineral in Modified Siddha Approach
2.3. Physiochemical and Compound Identification
2.4. In-Vitro Drug Release Study
2.5. Cell Culture and Assessment of Cell Viability
2.6. Apoptosis and Cell Death Estimation
2.7. Intracellular ROS Generation Estimation Using Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. UV-Vis Spectra Studies
3.2. XRD and FTIR Analysis of Nano Herboformulation
3.3. Size and Shape Determination of Nano Herboformulation
3.4. Zeta Potential and Particle Size of Nano Herboformulation
3.5. GC-MS Analysis of Nano Herboformulation
3.6. In-Vitro Phytochemical Release Study of NHF Formulation
3.7. Cell Viability, Oxidative Stress, and Apoptosis in Doxorubicin-Induced h9c2 Cell Line
3.7.1. Dose-Response Curve Using MTT Assay—Cell Viability Assay
3.7.2. NHF in Oxidative Stress Induced by DOX-Treatment in H9c2 Cells
3.7.3. NHF in Apoptosis Induced by DOX-Treatment in H9c2 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhou, J.; Liu, L.; Huang, C.; Zhou, D.; Fu, L. Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, Danio rerio. Carbohydr. Polym. 2016, 141, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2019, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sundarrajan, S.; Arumugam, M. A systems pharmacology perspective to decipher the mechanism of action of Parangichakkai chooranam, a Siddha formulation for the treatment of psoriasis. Biomed. Pharmacother. 2017, 88, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-L.; Li, X.-G.; Zhu, F.; Lei, C.-L. Structural Characterization of Nanoparticles Loaded with Garlic Essential Oil and Their Insecticidal Activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem. 2009, 57, 10156–10162. [Google Scholar] [CrossRef] [PubMed]
- Marles, R.; Farnsworth, N. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Jain, K.K. Biomarkers for Drug Discovery and Development. In The Handbook of Biomarkers; Springer: Berlin/Heidelberg, Germany, 2017; pp. 113–145. [Google Scholar]
- Green, P.S.; Leeuwenburgh, C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2002, 1588, 94–101. [Google Scholar] [CrossRef]
- Someya, A.; Akiyama, T.; Misumi, M.; Tanaka, N. Interaction of anthracycline antibiotics with actin and heavy meromyosin. Biochem. Biophys. Res. Commun. 1978, 85, 1542–1550. [Google Scholar] [CrossRef]
- Morey, T.E.; Varshney, M.; Flint, J.A.; Rajasekaran, S.; Shah, D.O.; Dennis, D.M. Treatment of Local Anesthetic-Induced Cardiotoxicity Using Drug Scavenging Nanoparticles. Nano Lett. 2004, 4, 757–759. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, E.; Zhou, L.; Dong, Z.; Liang, P.; Weng, Q.; Yang, M. Thrombopoietin protects H9C2 cells from excessive autophagy and apoptosis in doxorubicin-induced cardiotoxicity. Oncol. Lett. 2017, 15, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Sayles, H.; Mikuls, T.R.; Michaud, K. Minocycline and doxycycline therapy in community patients with rheumatoid arthritis: Prescribing patterns, patient-level determinants of use, and patient-reported side effects. Arthritis Res. Ther. 2011, 13, R168. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-K.; Yang, H.J.; Ma, J.Y. Lotus (Nelumbo nucifera Gaertn.) leaf water extracts suppress influenza a viral infection via inhibition of neuraminidase and hemagglutinin. J. Funct. Foods 2022, 91, 105019. [Google Scholar] [CrossRef]
- Rajendra, V.K.P.; Kurapati, S.; Balineni, S.K.; Gogineni, N.T.T. A blend of Sphaeranthus indicus flower head and Terminalia chebula fruit extracts reduces fatty liver and improves liver function in non-alcoholic, overweight adults. Funct. Foods Health Dis. 2022, 12, 361. [Google Scholar] [CrossRef]
- Jayakodi, S.; Shanmugam, R.; Almutairi, B.O.; Almutairi, M.H.; Mahboob, S.; Kavipriya, M.; Gandusekar, R.; Nicoletti, M.; Govindarajan, M. Azadirachta indica-wrapped copper oxide nanoparticles as a novel functional material in cardiomyocyte cells: An ecotoxicity assessment on the embryonic development of Danio rerio. Environ. Res. 2022, 212, 113153. [Google Scholar] [CrossRef]
- Santhosh Kumar, J.; Shanmugam, V. Green synthesis of copper oxide nanoparticles from magnolia champaca floral extract and its antioxidant & toxicity assay using Danio Rerio. Int. J. Recent Technol. Eng. 2020, 8, 5444–5449. [Google Scholar]
- Uthamarayan, C. Siddha Maruthuvanga Churukkam. 1953. Available online: https://www.tknsiddha.com/medicine/siddha-books-free/ (accessed on 30 October 2022).
- Shailaja, R.; Sugunthan, S. Concept of nanotechnology in siddha medical literature. World J. Pharm. Res. 2016, 5, 276–284. [Google Scholar]
- Onódi, Z.; Visnovitz, T.; Kiss, B.; Hambalkó, S.; Koncz, A.; Ágg, B.; Váradi, B.; Tóth, V.É.; Nagy, R.N.; Gergely, T.G. Systematic transcriptomic and phenotypic characterization of human and murine cardiac myocyte cell lines and primary cardiomyocytes reveals serious limitations and low resemblances to adult cardiac phenotype. J. Mol. Cell. Cardiol. 2022, 165, 19–30. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Khotina, V.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Mikhaleva, L.M.; Orekhov, A.N. The Role of Mitochondrial DNA Mutations in Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 952. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Jin, S.; Ye, K. Nanoparticle-Mediated Drug Delivery and Gene Therapy. Biotechnol. Prog. 2007, 23, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.; Grebe, R.; Merges, C.; Smith, J.; McLeod, S.; Leary, J.; Lutty, G. Nanoparticle tethered biosensors for autoregulated gene therapy in hyperoxic endothelium. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 276. [Google Scholar] [CrossRef]
- Perugini, P.; Simeoni, S.; Scalia, S.; Genta, I.; Modena, T.; Conti, B.; Pavanetto, F. Effect of nanoparticle encapsulation on the photostability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. Int. J. Pharm. 2002, 246, 37–45. [Google Scholar] [CrossRef]
- Czupryna, J.; Tsourkas, A. Suicide gene delivery by calcium phosphate nanoparticles: A novel method of targeted therapy for gastric cancer. Cancer Biol. Ther. 2006, 5, 1691–1692. [Google Scholar] [CrossRef]
- Jayakodi, S.; Shanmugam, V.K. Statistical Optimization of Copper Oxide Nanoparticles Using Response Surface Methodology and Box–Behnken Design Towards In Vitro and In Vivo Toxicity Assessment. Biointerface Res. Appl. Chem. 2020, 11, 10027–10039. [Google Scholar]
- Swamy, M.K.; Sudipta, K.M.; Jayanta, K.; Balasubramanya, S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl. Nanosci. 2014, 5, 73–81. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green Synthesized Copper Oxide Nanoparticles Ameliorate Defence and Antioxidant Enzymes in Lens culinaris. Nanomaterials 2020, 10, 312. [Google Scholar] [CrossRef]
- Yan, W.; Petkov, V.; Mahurin, S.M.; Overbury, S.H.; Dai, S. Powder XRD analysis and catalysis characterization of ultra-small gold nanoparticles deposited on titania-modified SBA-15. Catal. Commun. 2005, 6, 404–408. [Google Scholar] [CrossRef]
- Shanmugam, V.; Jas, J.S.; Jayakodi, S. Biomimetic Copper Oxide Nanoparticles and its Validation Through In-silico Approach on Cardiac Enzymes. Curr. Nanosci. 2022, 18, 86–93. [Google Scholar] [CrossRef]
- Sabeena, G.; Rajaduraipandian, S.; Pushpalakshmi, E.; Alhadlaq, H.A.; Mohan, R.; Annadurai, G.; Ahamed, M. Green and chemical synthesis of CuO nanoparticles: A comparative study for several in vitro bioactivities and in vivo toxicity in zebrafish embryos. J. King Saud Univ.-Sci. 2022, 5, 102092. [Google Scholar]
- Nasrullah, M.; Gul, F.Z.; Hanif, S.; Mannan, A.; Naz, S.; Ali, J.S.; Zia, M. Green and Chemical Syntheses of CdO NPs: A Comparative Study for Yield Attributes, Biological Characteristics, and Toxicity Concerns. ACS Omega 2020, 5, 5739–5747. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Warheit, D.B. Characterization of nanomaterials for toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2009, 1, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.-J. Ultrasmall Gold Nanoparticles as Carriers for Nucleus-Based Gene Therapy Due to Size-Dependent Nuclear Entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, A.; Saidur, R.; Metselaar, H. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Balamurugan, B.; Mehta, B.R.; Shivaprasad, S.M. Surface-modified CuO layer in size-stabilized single-phase Cu2O nanoparticles. Appl. Phys. Lett. 2001, 79, 3176–3178. [Google Scholar] [CrossRef]
- Mozaffarnia, S.; Teimuri-Mofrad, R.; Rashidi, M.-R. Design, synthesis and biological evaluation of 2,3-dihydro-5,6-dimethoxy-1H-inden-1-one and piperazinium salt hybrid derivatives as hAChE and hBuChE enzyme inhibitors. Eur. J. Med. Chem. 2020, 191, 112140. [Google Scholar] [CrossRef]
- Calderón Guzmán, D.; Osnaya Brizuela, N.; Ortiz Herrera, M.; Juarez Olguin, H.; Hernández García, E.; Valenzuela Peraza, A.; Barragan Mejia, G. Oleic acid protects against oxidative stress exacerbated by cytarabine and doxorubicin in rat brain. Anti-Cancer Agents Med. Chem. (Former Curr. Med. Chem.-Anti-Cancer Agents) 2016, 16, 1491–1495. [Google Scholar]
- Grossini, E.; Bellofatto, K.; Farruggio, S.; Sigaudo, L.; Marotta, P.; Raina, G.; De Giuli, V.; Mary, D.; Pollesello, P.; Minisini, R.; et al. Levosimendan Inhibits Peroxidation in Hepatocytes by Modulating Apoptosis/Autophagy Interplay. PLoS ONE 2015, 10, e0124742. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, Q.; Wang, N.; Yang, Y.; Liu, J.; Yu, G.; Yang, X.; Xu, H.; Wang, H. A complex micellar system co-delivering curcumin with doxorubicin against cardiotoxicity and tumor growth. Int. J. Nanomed. 2018, 13, 4549–4561. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Rosenzweig, B.A.; Zhang, J.; Knapton, A.D.; Honchel, R.; Lipshultz, S.E.; Retief, J.; Sistare, F.D.; Herman, E.H. Early alterations in heart gene expression profiles associated with doxorubicin cardiotoxicity in rats. Cancer Chemother. Pharmacol. 2009, 66, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Kruger, A.; Kleschyov, A.L.; Kalinowski, L.; Daiber, A.; Wojnowski, L. Gp91phox-containing N.A.D. (P) H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic. Biol. Med. 2007, 42, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Nitobe, J.; Yamaguchi, S.; Okuyama, M.; Nozaki, N.; Sata, M.; Miyamoto, T.; Takeishi, Y.; Kubota, I.; Tomoike, H. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc. Res. 2003, 57, 119–128. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Beal, M.F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995, 38, 357–366. [Google Scholar] [CrossRef]












| S. No | RT. | Name of the Compound | Molecular Formula | Molecular Weight | Peak Area % |
|---|---|---|---|---|---|
| 1. | 5.87 | Ethanol, 2-phenoxy- | C8H10O2 | 138 | 14.46 |
| 2. | 9.58 | Cyclobarbital | C12H16N2O3 | 236 | 12.35 |
| 3. | 9.73 | 2-Allyl-3,6-dimethoxybenzyl alcohol | C12H16O3 | 208 | 4.71 |
| 4. | 10.07 | 1H-Inden-1-one, 2,3-dihydro-5,6- dimethoxy-3-methyl- | C12H14O3 | 206 | 13.29 |
| 5. | 11.23 | 1,2-Benzisothiazol-3-amine tbdms | C13H20N2SSi | 264 | 1.27 |
| 6. | 12.35 | 2-Myristynoyl-glycinamide | C16H28N2O2 | 280 | 2.36 |
| 7. | 14.09 | Z, E-2,13-Octadecadien-1-ol | C18H34O | 266 | 5.99 |
| 8. | 14.38 | Phen-1,4-diol, 2,3-dimethyl-5- Trifluoromethyl- | C9H9F3O2 | 206 | 7.28 |
| 9. | 15.20 | Dodecanoic acid, 10-methyl-, methyl ester | C14H28O2 | 228 | 11.81 |
| 10. | 17.52 | 6-epi-shyobunol | C15H26O | 222 | 6.11 |
| 11. | 17.99 | d-Mannitol, 1-decylsulfonyl- | C16H34O7S | 370 | 3.30 |
| 12. | 20.46 | Oleic Acid | C18H34O2 | 282 | 6.21 |
| 13. | 26.37 | Di-n-decylsulfone | C20H42O2S | 346 | 10.85 |
| Model | R2 | SST |
|---|---|---|
| Zero-order kinetic model | 0.8562 | 0.094 |
| First-order kinetic model | 0.8745 | 0.0354 |
| Korsmeyer–Peppas kinetic model | 0.9764 | 1.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayakodi, S.; Kim, H.; Menon, S.; Shanmugam, V.K.; Choi, I.; Sekhar, M.R.; Bhaskar, R.; Han, S.S. Preparation of Novel Nanoformulation to Enhance Efficacy in the Treatment of Cardiovascular Disease. Biomimetics 2022, 7, 189. https://doi.org/10.3390/biomimetics7040189
Jayakodi S, Kim H, Menon S, Shanmugam VK, Choi I, Sekhar MR, Bhaskar R, Han SS. Preparation of Novel Nanoformulation to Enhance Efficacy in the Treatment of Cardiovascular Disease. Biomimetics. 2022; 7(4):189. https://doi.org/10.3390/biomimetics7040189
Chicago/Turabian StyleJayakodi, Santhoshkumar, Hyunjin Kim, Soumya Menon, Venkat Kumar Shanmugam, Inho Choi, Medidi Raja Sekhar, Rakesh Bhaskar, and Sung Soo Han. 2022. "Preparation of Novel Nanoformulation to Enhance Efficacy in the Treatment of Cardiovascular Disease" Biomimetics 7, no. 4: 189. https://doi.org/10.3390/biomimetics7040189
APA StyleJayakodi, S., Kim, H., Menon, S., Shanmugam, V. K., Choi, I., Sekhar, M. R., Bhaskar, R., & Han, S. S. (2022). Preparation of Novel Nanoformulation to Enhance Efficacy in the Treatment of Cardiovascular Disease. Biomimetics, 7(4), 189. https://doi.org/10.3390/biomimetics7040189