Characterization of Decellularized Extracellular Matrix from Milkfish (Chanos chanos) Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Decellularization of Milkfish Skin
2.2. Histological Staining
2.3. DNA Extraction and Quantification
2.4. Composition Analysis Using Attenuated Total Reflectance—Fourier Transform Infrared (ATR–FTIR) Spectroscopy
2.5. Physical and Mechanical Properties
2.5.1. Tensile Strength
2.5.2. Hydrophilicity
2.5.3. Water Absorption
2.6. Thermal Degradation and Denaturation Profile
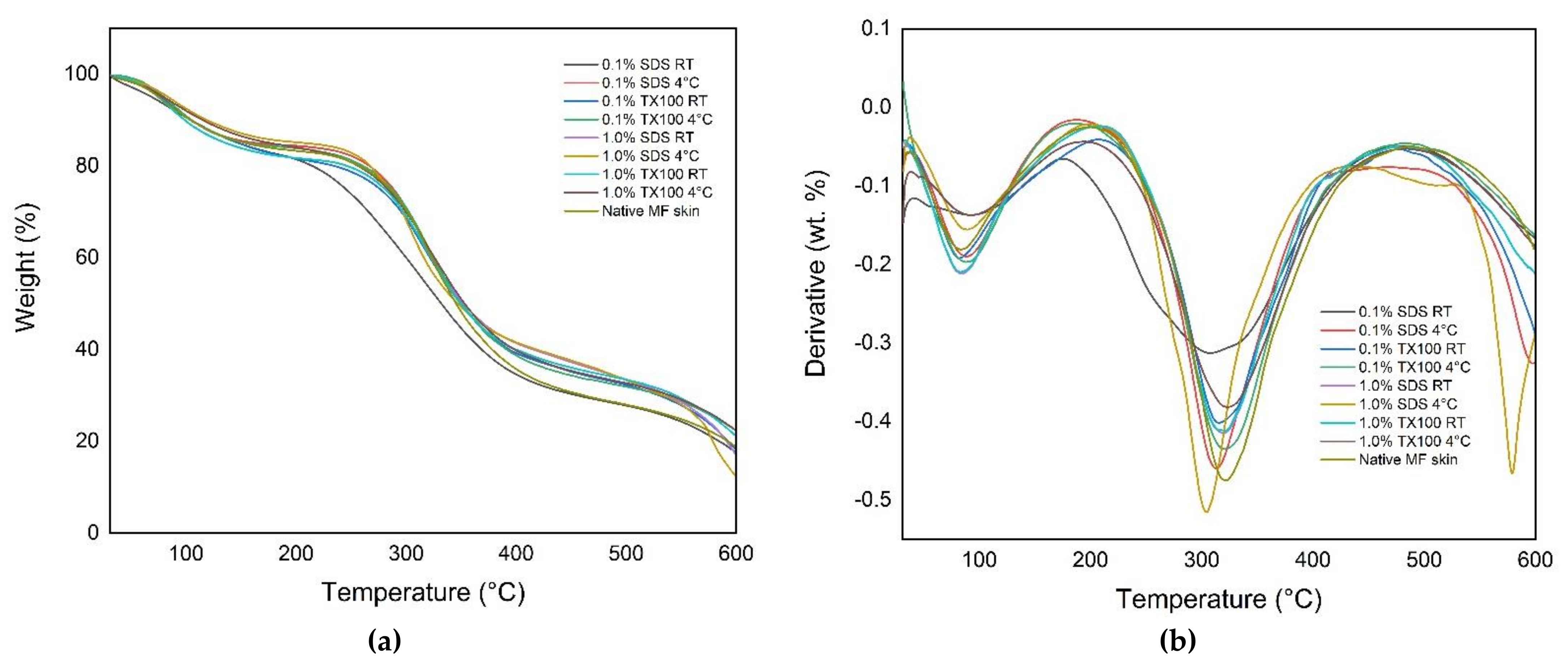
2.6.1. Thermogravimetric Analysis (TGA)
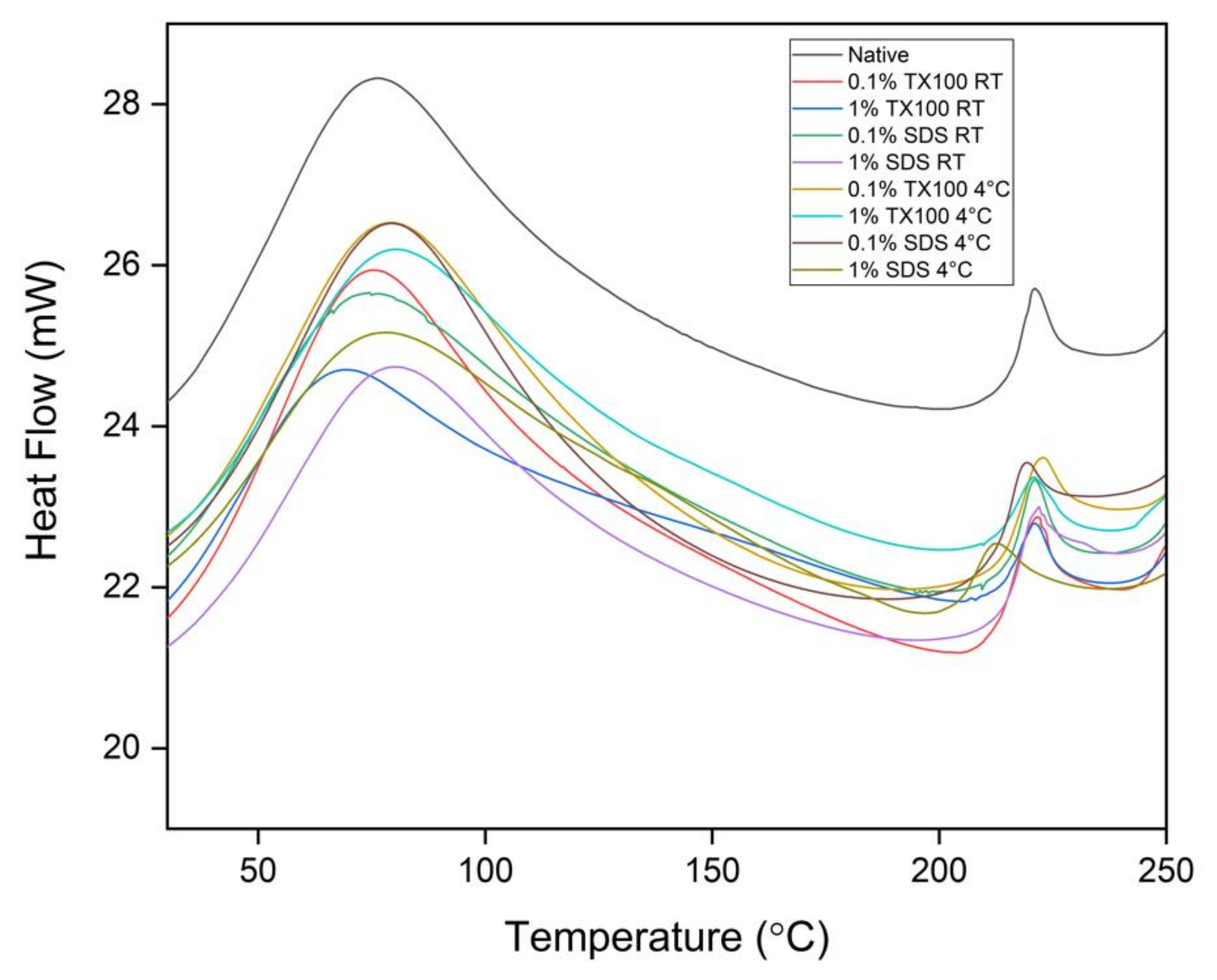
2.6.2. Differential Scanning Calorimetry (DSC)
2.7. Residual Detergents Determination
2.8. Statistical Analysis
3. Results
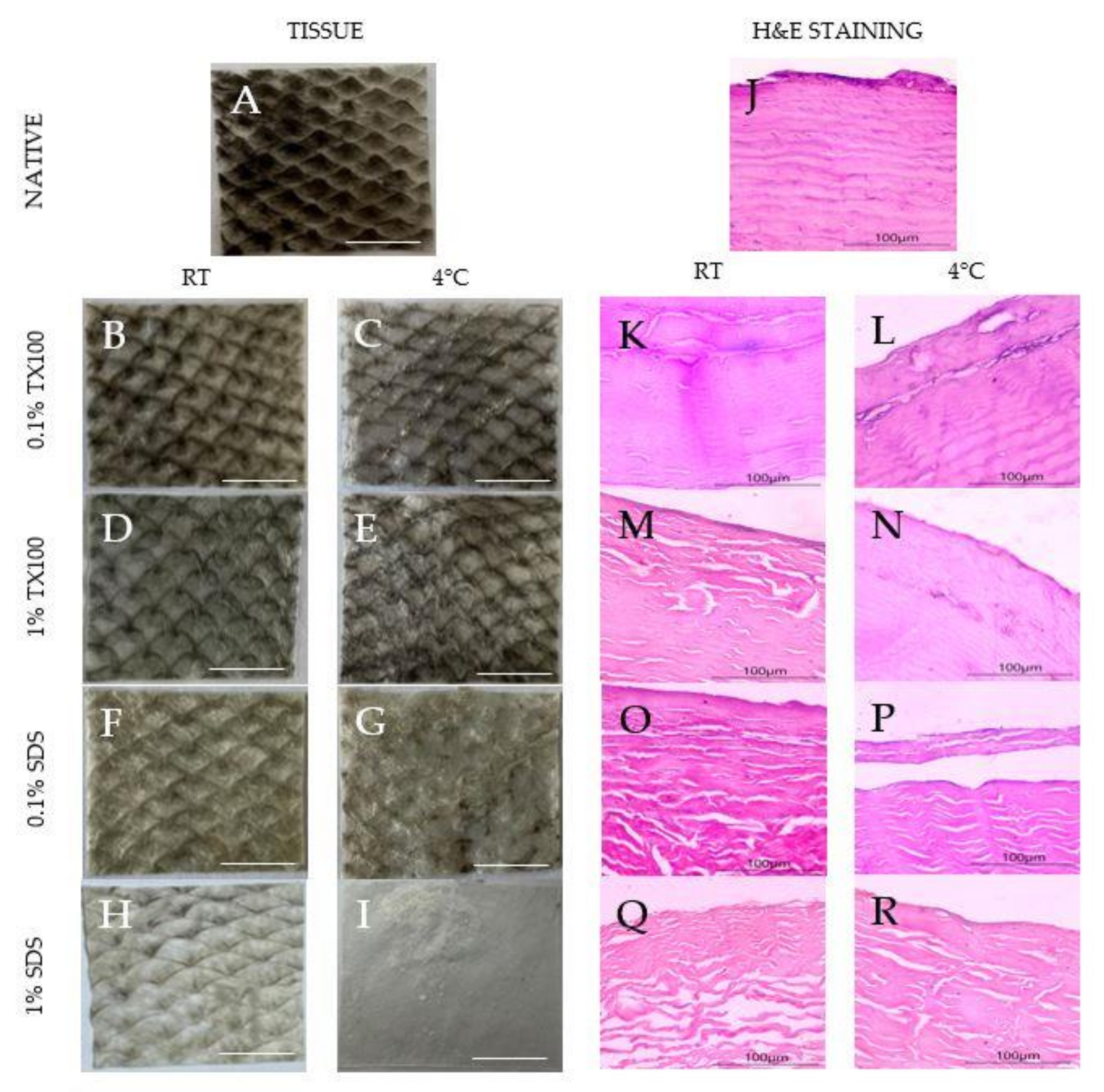
3.1. Visual Assessment
3.2. Histological Staining
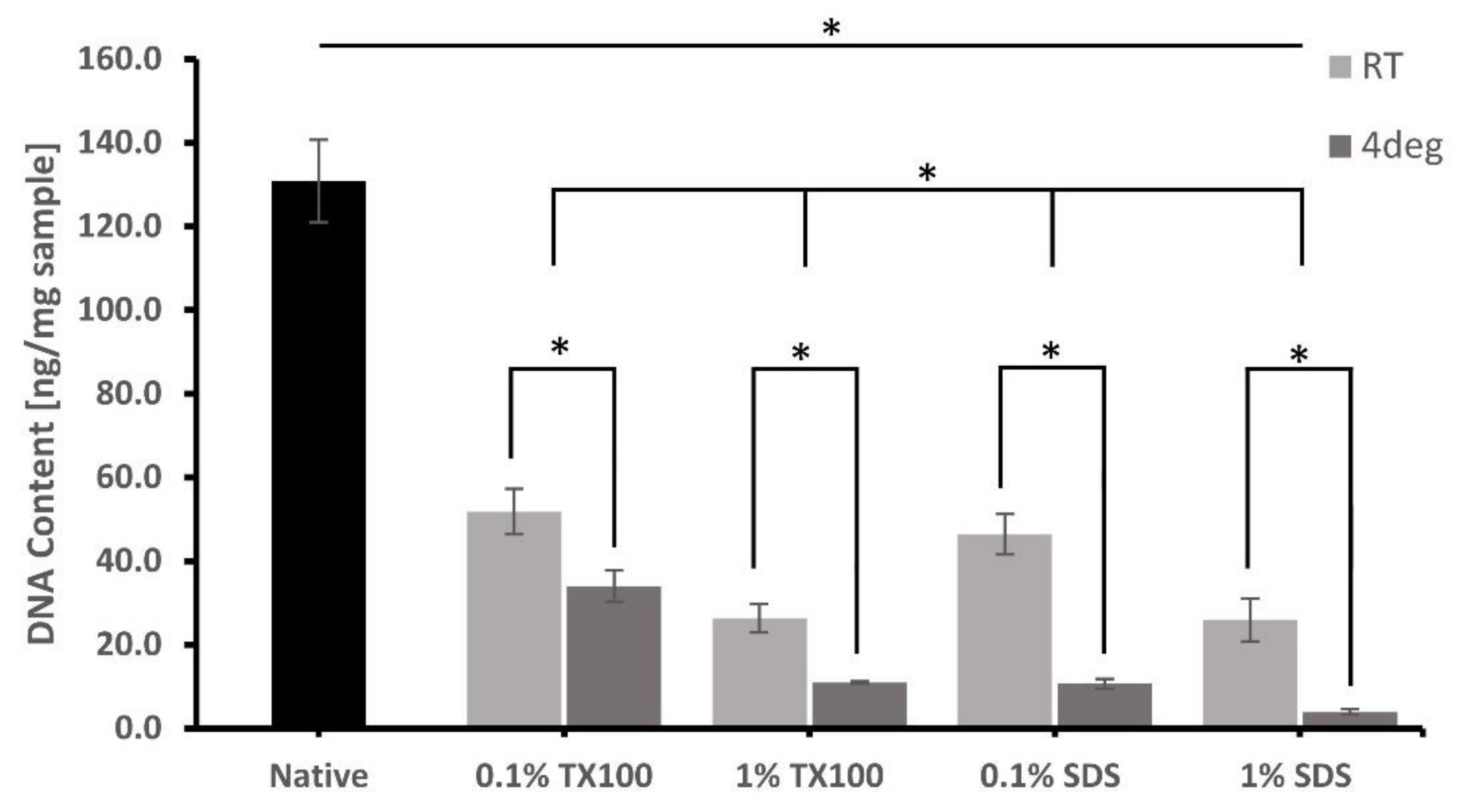
3.3. DNA Extraction and Quantification
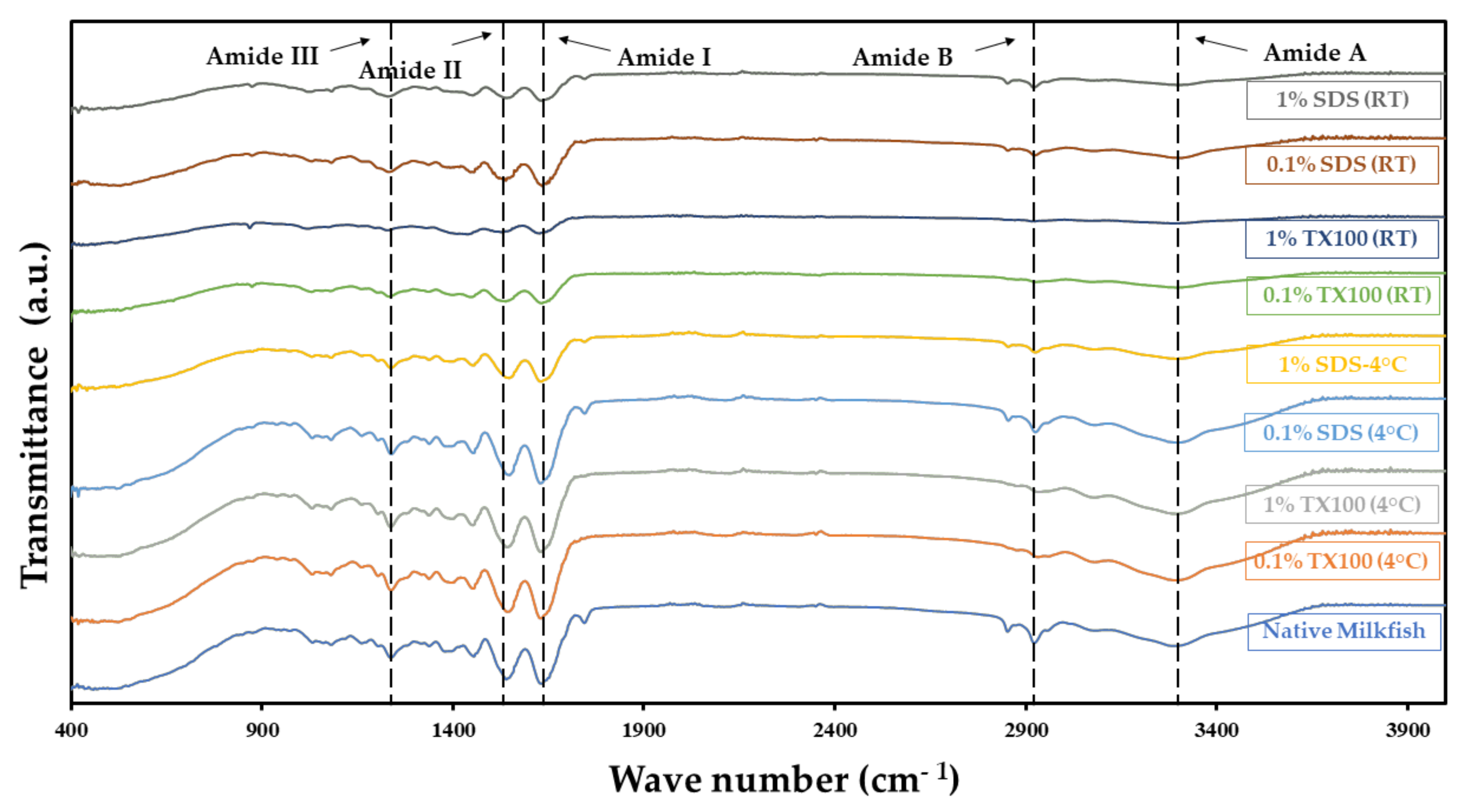
3.4. Composition Analysis Using Attenuated Total Reflectance—Fourier Transform Infrared (ATR–FTIR) Spectroscopy
3.5. Physical and Mechanical Properties
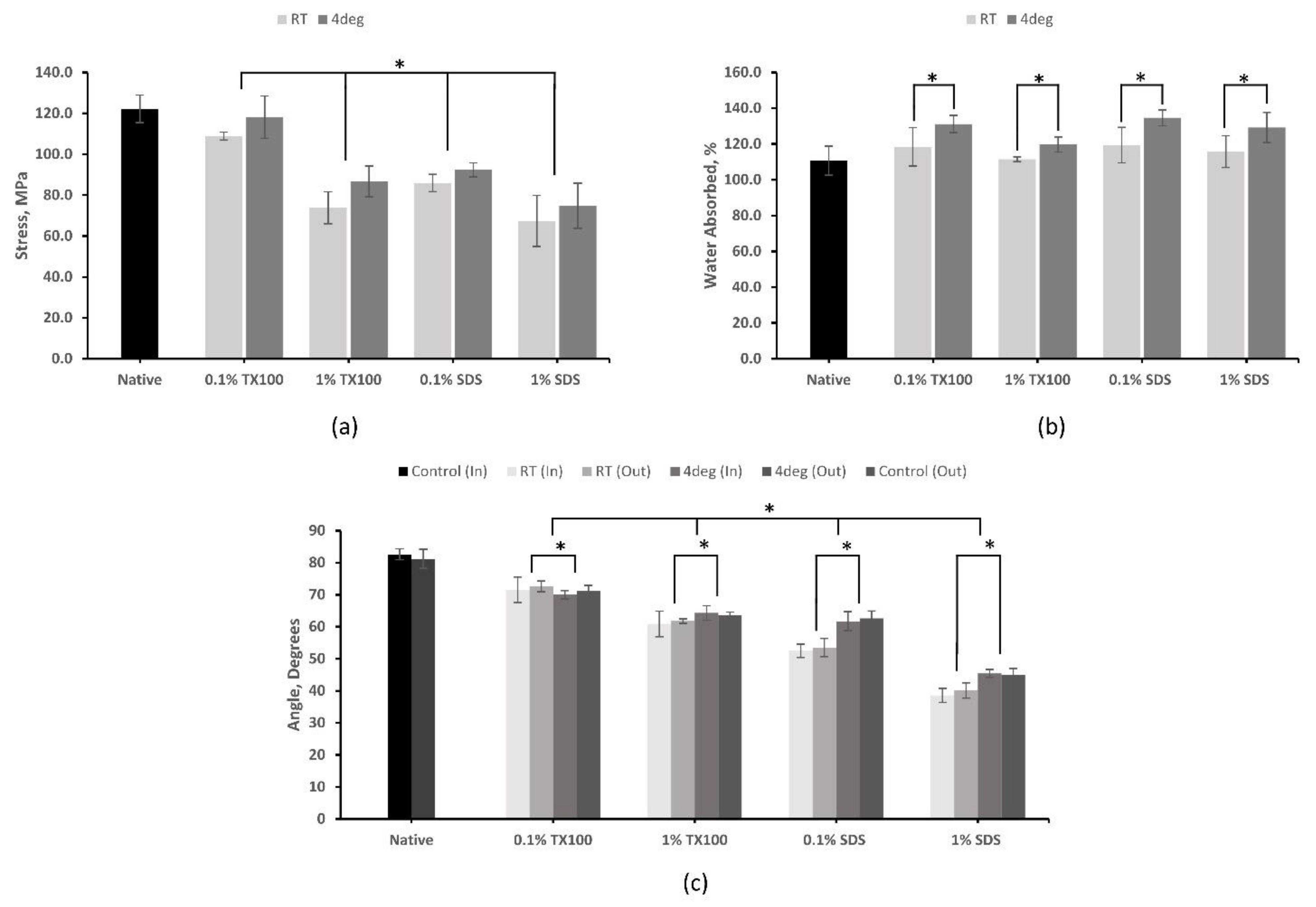
3.5.1. Tensile Strength
3.5.2. Hydrophilicity
3.5.3. Water Absorption
3.6. Thermal Degradation and Denaturation Profile
3.6.1. Thermogravimetric Analysis (TGA)
3.6.2. Differential Scanning Calorimetry (DSC)
3.7. Residual Detergent Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenneth Eduarte, B.E.; Andrey Acosta, N.; Pauline Lumanlan, J.; Magnampo, J.E.; Rose Medrano, D.; Ann Vicente, K.B.; Patrick Picar, J.C. Potential of Chanos chanos (Milkfish) Skin Gel Extract as an Alternative Approach in Treating First Degree Burn International Journal of BioScience and Applications. Int. J. Biosci. Appl. 2019, 1, 19–25. [Google Scholar]
- German, J.D.; Catabay, M.A.G. Analysis of Milkfish Supply Chain in the Philippines: A Case Study in Dagupan, Pangasinan. AIP Conf. Proc. 2018, 2045, 020047. [Google Scholar] [CrossRef]
- Investment Guide for Milk Fish; Department of Agriculture: Quezon City, Philippines, 2019.
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; De Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Wibawa, F.S.; Retnoningrum, S.D.; Suhartono, T.M. Acid Soluble Collagen from Skin of Common Carp (Cyprinus carpio L), Red Snapper (Lutjanus Sp.) And Milkfish (Chanos chanos). World Appl. Sci. J. 2015, 33, 990–995. [Google Scholar] [CrossRef]
- Patel, M.; Lantis, J.C., II. Fish Skin Acellular Dermal Matrix: Potential in the Treatment of Chronic Wounds. Chronic Wound Care Manag. Res. 2019, 6, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Kamalvand, M.; Biazar, E.; Daliri-Joupari, M.; Montazer, F.; Rezaei-Tavirani, M.; Heidari-Keshel, S. Design of a Decellularized Fish Skin as a Biological Scaffold for Skin Tissue Regeneration. Tissue Cell 2021, 71, 101509. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mishra, N.C.; Dhasmana, A. Decellularization Methods for Scaffold Fabrication. In Methods in Molecular Biology; Turksen, K., Ed.; Springer: New York, NY, USA, 2018; Volume 1577, pp. 1–10. ISBN 978-1-4939-7656-0. [Google Scholar]
- Bual, R.P.; Ijima, H. Intact Extracellular Matrix Component Promotes Maintenance of Liver-Specific Functions and Larger Aggregates Formation of Primary Rat Hepatocytes. Regen. Ther. 2019, 11, 258–268. [Google Scholar] [CrossRef]
- Fischer, I.; Westphal, M.; Rossbach, B.; Bethke, N.; Hariharan, K.; Ullah, I.; Reinke, P.; Kurtz, A.; Stachelscheid, H. Comparative Characterization of Decellularized Renal Scaffolds for Tissue Engineering. Biomed. Mater. 2017, 12, 45005. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, N.; Sciences, B.; Singh, H.; Gangwar, A. Effects of Crosslinking Treatments on the Physical Properties of Acellular Fish Swim Bladder. Trends Biomater. Artif. Organs 2013, 27, 93–101. [Google Scholar] [CrossRef]
- Miranda, C.M.F.C.; Leonel, L.C.P.C.; Cañada, R.R.; Maria, D.A.; Miglino, M.A.; Del Sol, M.; Lobo, S.E. Effects of Chemical and Physical Methods on Decellularization of Murine Skeletal Muscles. An. Acad. Bras. Cienc. 2021, 93, e20190942. [Google Scholar] [CrossRef]
- Zvarova, B.; Uhl, F.E.; Uriarte, J.J.; Borg, Z.D.; Coffey, A.L.; Bonenfant, N.R.; Weiss, D.J.; Wagner, D.E. Residual Detergent Detection Method for Nondestructive Cytocompatibility Evaluation of Decellularized Whole Lung Scaffolds. Tissue Eng.—Part C Methods 2016, 22, 418–428. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rezakhani, L.; Soleimannejad, M.; Sharifi, E.; Anjomshoa, M.; Alizadeh, A. Evaluation of Vacuum Washing in the Removal of SDS from Decellularized Bovine Pericardium: Method and Device Description. Heliyon 2019, 5, e02253. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.L.; Su, J.; Yan, M.; Meng, F.; Glaser, S.S.; Alpini, G.D.; Green, R.M.; Sosa-Pineda, B.; Shah, R.N. Complex Bile Duct Network Formation within Liver Decellularized Extracellular Matrix Hydrogels. Sci. Rep. 2018, 8, 12220. [Google Scholar] [CrossRef] [Green Version]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The Impact of Detergents on the Tissue Decellularization Process: A ToF-SIMS Study. Acta Biomater. 2017, 50, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.C.; Garzón, I.; Ionescu, A.M.; Carriel, V.; Cardona, J.d.l.C.; González-Andrades, M.; Pérez, M.d.M.; Alaminos, M.; Campos, A. Evaluation of Small Intestine Grafts Decellularization Methods for Corneal Tissue Engineering. PLoS ONE 2013, 8, e66538. [Google Scholar] [CrossRef] [Green Version]
- Ijima, H.; Nakamura, S.; Bual, R.P.; Yoshida, K. Liver-Specific Extracellular Matrix Hydrogel Promotes Liver-Specific Functions of Hepatocytes in Vitro and Survival of Transplanted Hepatocytes in Vivo. J. Biosci. Bioeng. 2019, in press. [CrossRef]
- Shirafkan, A. Wettability and Hydrophilicity of Rigid and Soft Contact Lens Surfaces. Ph.D. Thesis, City University London, London, UK, 1997. [Google Scholar]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Camuffo, D. Chapter 19—Measuring Time of Wetness and Moisture in Materials. In Microclimate for Cultural Heritage, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 459–482. ISBN 978-0-444-64106-9. [Google Scholar]
- Pavlović, B.; Cvijetić, N.; Dragačević, L.; Ivković, B.; Vujić, Z.; Kuntić, V. Direct UV Spectrophotometry and HPLC Determination of Triton X-100 in Split Virus Influenza Vaccine. J. AOAC Int. 2016, 99, 396–400. [Google Scholar] [CrossRef]
- Poornejad, N.; Schaumann, L.B.; Buckmiller, E.M.; Momtahan, N.; Gassman, J.R.; Ma, H.H.; Roeder, B.L.; Reynolds, P.R.; Cook, A.D. The Impact of Decellularization Agents on Renal Tissue Extracellular Matrix. J. Biomater. Appl. 2016, 31, 521–533. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, D.V.M. An Overview of Tissue and Whole Organ Decellularization Process. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.; Guvendiren, M.; Freeman, O.; Mao, Y.; Kohn, J. Optimization of Polymer-ECM Composite Scaffolds for Tissue Engineering: Effect of Cells and Culture Conditions on Polymeric Nanofiber Mats. J. Funct. Biomater. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed. Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Q.; Yates, K.; Tahtinen, M.; Shearier, E.; Qian, Z.; Zhao, F. Decellularization of Fibroblast Cell Sheets for Natural Extracellular Matrix Scaffold Preparation. Tissue Eng. Part C Methods 2015, 21, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, B.; Yang, Q.; Li, X.; Ma, X.; Xia, Q.; Zhang, Y.; Zhang, C.; Wu, Y.; Zhang, Y. Comparison of Decellularization Protocols for Preparing a Decellularized Porcine Annulus Fibrosus Scaffold. PLoS ONE 2014, 9, e86723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partington, L.; Mordan, N.J.; Mason, C.; Knowles, J.C.; Kim, H.-W.; Lowdell, M.W.; Birchall, M.A.; Wall, I.B. Biochemical Changes Caused by Decellularization May Compromise Mechanical Integrity of Tracheal Scaffolds. Acta Biomater. 2013, 9, 5251–5261. [Google Scholar] [CrossRef]
- Kim, S.H.; Ha, H.J.; Ko, Y.K.; Yoon, S.J.; Rhee, J.M.; Kim, M.S.; Lee, H.B.; Khang, G. Correlation of Proliferation, Morphology and Biological Responses of Fibroblasts on LDPE with Different Surface Wettability. J. Biomater. Sci. Polym. Ed. 2007, 18, 609–622. [Google Scholar] [CrossRef]

| Prewashing | Decellularization | Post washing | |
|---|---|---|---|
| Agent | PBS | 0.1% TX100, 1.0% TX100 0.1% SDS, 1.0% SDS | PBS |
| Temperature | Room temperature | 4 °C, Room temperature | Room temperature |
| Contact Time | 15 min | 48 h | 15 min × 3 |
| Conditions | H&E Staining | DNA Quantification | FTIR | Tensile Strength | Hydrophilicity | Water Absorption | DSC | TGA | Residual Detergent | |
|---|---|---|---|---|---|---|---|---|---|---|
| (Inner) | (Outer) | |||||||||
| Agent | SDS | SDS | ALL | TX100 | SDS | SDS | SDS | TX100 | SDS | TX100 |
| Conc | 0.1% | 1.0% | ALL | 0.1% | 1.0% | 1.0% | 0.1% | 1.0% | 0.1% | 0.1% |
| Temp | 4 °C | 4 °C | ALL | RT | 4 °C | RT | 4 °C | 4 °C | 4 °C | RT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bual, R.; Labares, M., Jr.; Valle, K.D.D.; Pague, J., Jr.; Bantilan, Z.C.; Ducao, P.G.; Alimasag, J.; Acibar, C. Characterization of Decellularized Extracellular Matrix from Milkfish (Chanos chanos) Skin. Biomimetics 2022, 7, 213. https://doi.org/10.3390/biomimetics7040213
Bual R, Labares M Jr., Valle KDD, Pague J Jr., Bantilan ZC, Ducao PG, Alimasag J, Acibar C. Characterization of Decellularized Extracellular Matrix from Milkfish (Chanos chanos) Skin. Biomimetics. 2022; 7(4):213. https://doi.org/10.3390/biomimetics7040213
Chicago/Turabian StyleBual, Ronald, Marionilo Labares, Jr., Kit Dominick Don Valle, Job Pague, Jr., Zesreal Cain Bantilan, Princess Grace Ducao, Johnel Alimasag, and Catherine Acibar. 2022. "Characterization of Decellularized Extracellular Matrix from Milkfish (Chanos chanos) Skin" Biomimetics 7, no. 4: 213. https://doi.org/10.3390/biomimetics7040213








