Myocardial Perfusion and Coronary Physiology Assessment of Microvascular Dysfunction in Patients Undergoing Transcatheter Aortic Valve Implantation—Rationale and Design
Abstract
1. Introduction
2. Materials and Methods
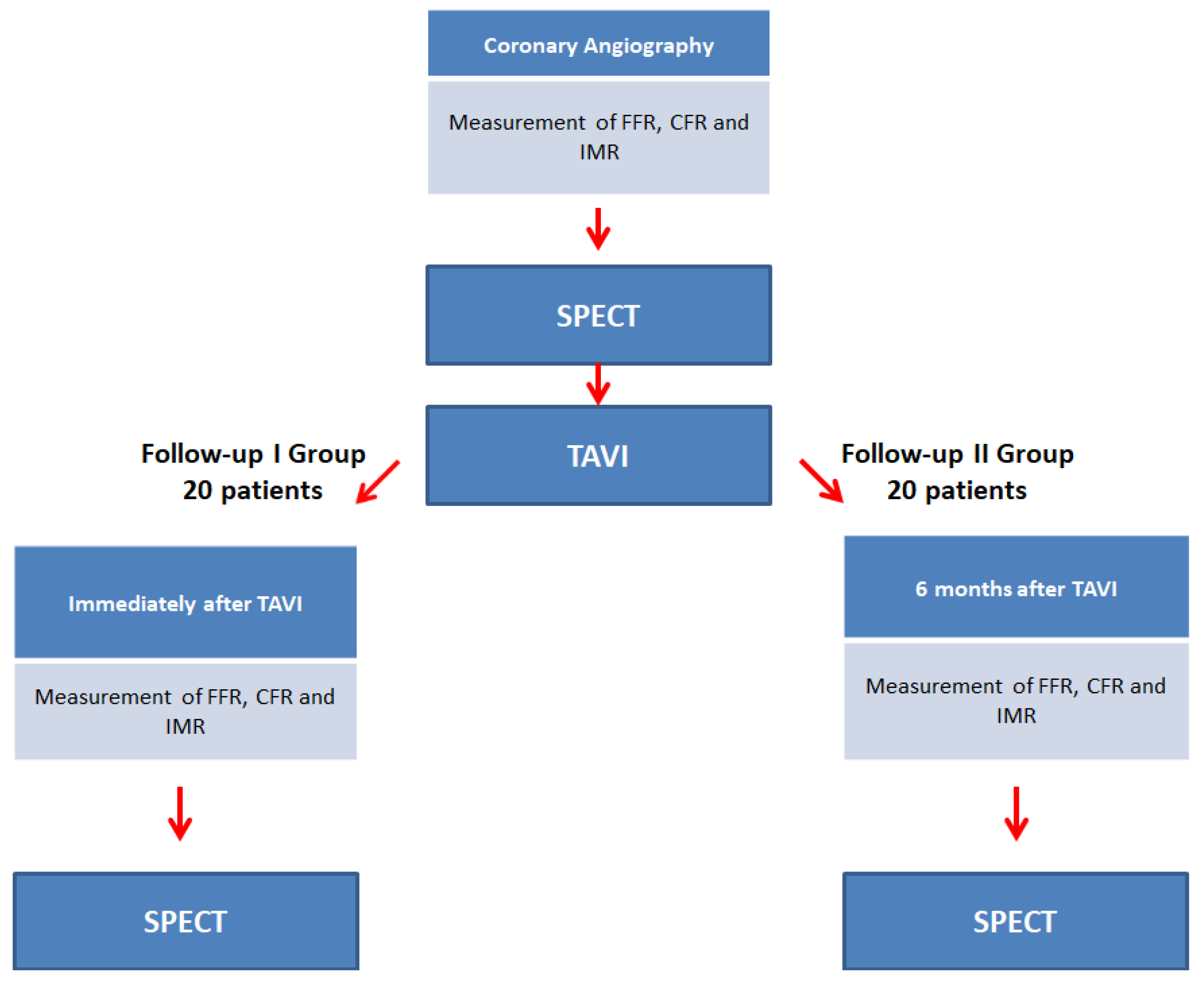
2.1. Study Design
2.2. Study Objectives
2.3. Population
2.4. Sample Size
2.5. Patient Recruitment
2.6. SPECT Protocol
2.7. FFR, CFR, IMR Protocol
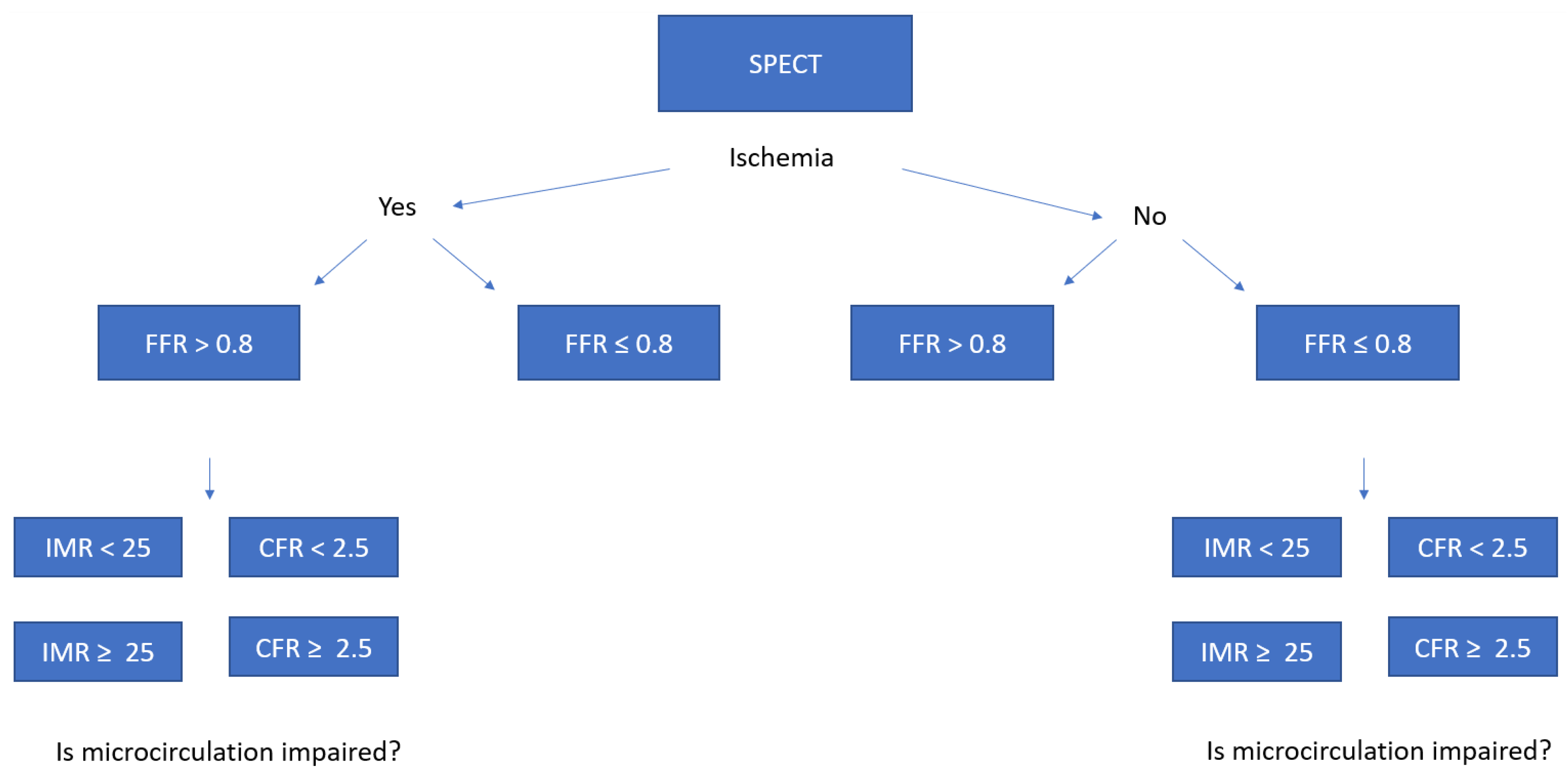
2.8. Calculation of FFR, IMR, and CFR
2.8.1. Fractional Flow Reserve
2.8.2. Index of Microvascular Resistance
2.8.3. Coronary Flow Reserve
2.8.4. Calculation of Myocardial Blood Flow by SPECT
2.9. Statistical Analysis
3. Discussion
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iung, B.; Vahanian, A. Degenerative calcific aortic stenosis: A natural history. Heart 2012, 98 (Suppl. 4), iv7–iv13. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.S.; Ige, M.; Tuzcu, E.M.; Ellis, S.G.; Stewart, W.J.; Svensson, L.G.; Lytle, B.W.; Kapadia, S.R. Severe aortic stenosis and coronary artery disease--implications for management in the transcatheter aortic valve replacement era: A comprehensive review. J. Am. Coll. Cardiol. 2013, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Lipscomb, K. Effects of coronary stenoses on coronary flow reserve and resistance. Am. J. Cardiol. 1974, 34, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, M.; Scarsini, R.; Venturi, G.; Pesarini, G.; Pighi, M.; Gratta, A.; Gottin, L.; Barbierato, M.; Caprioglio, F.; Piccoli, A.; et al. Physiological Versus Angiographic Guidance for Myocardial Revascularization in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2019, 8, e012618. [Google Scholar] [CrossRef] [PubMed]
- Pesarini, G.; Scarsini, R.; Zivelonghi, C.; Piccoli, A.; Gambaro, A.; Gottin, L.; Rossi, A.; Ferrero, V.; Vassanelli, C.; Ribichini, F. Functional Assessment of Coronary Artery Disease in Patients Undergoing Transcatheter Aortic Valve Implantation: Influence of Pressure Overload on the Evaluation of Lesions Severity. Circ. Cardiovasc. Interv. 2016, 9, e004088. [Google Scholar] [CrossRef]
- Ahmad, Y.; Götberg, M.; Cook, C.; Howard, J.P.; Malik, I.; Mikhail, G.; Frame, A.; Petraco, R.; Rajkumar, C.; Demir, O.; et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc. Interv. 2018, 11, 2019–2031. [Google Scholar] [CrossRef]
- Zelis, J.M.; Tonino, P.A.L.; Johnson, N.P. Why Can Fractional Flow Reserve Decrease After Transcatheter Aortic Valve Implantation? J. Am. Heart Assoc. 2020, 9, e04905. [Google Scholar] [CrossRef]
- Camuglia, A.C.; Syed, J.; Garg, P.; Kiaii, B.; Chu, M.W.; Jones, P.M.; Bainbridge, D.; Teefy, P.J. Invasively Assessed Coronary Flow Dynamics Improve Following Relief of Aortic Stenosis With Transcatheter Aortic Valve Implantation. J. Am. Coll. Cardiol. 2014, 63, 1808–1809. [Google Scholar] [CrossRef][Green Version]
- Vendrik, J.; Ahmad, Y.; Eftekhari, A.; Howard, J.P.; Wijntjens, G.W.M.; Stegehuis, V.E.; Cook, C.; Terkelsen, C.J.; Christiansen, E.H.; Koch, K.T.; et al. Long-Term Effects of Transcatheter Aortic Valve Implantation on Coronary Hemodynamics in Patients With Concomitant Coronary Artery Disease and Severe Aortic Stenosis. J. Am. Heart Assoc. 2020, 9, e015133. [Google Scholar] [CrossRef]
- Scarsini, R.; Pesarini, G.; Zivelonghi, C.; Piccoli, A.; Ferrero, V.; Lunardi, M.; Gottin, L.; Zanetti, C.; Faggian, G.; Ribichini, F. Physiologic evaluation of coronary lesions using instantaneous wave-free ratio (iFR) in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention. J. Eur. Collab. Work. Gr. Interv. Cardiol. Eur. Soc. Cardiol. 2018, 13, 1512–1519. [Google Scholar] [CrossRef]
- Lotfi, A.; Davies, J.E.; Fearon, W.F.; Grines, C.L.; Kern, M.J.; Klein, L.W. Focused update of expert consensus statement: Use of invasive assessments of coronary physiology and structure: A position statement of the society of cardiac angiography and interventions. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2018, 92, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. Corrigendum to: 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.J.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. J. Cardiovasc. Magn. Reson. 2002, 4, 203–210. [Google Scholar] [CrossRef]
- Pijls, N.H.; van Son, J.A.; Kirkeeide, R.L.; De Bruyne, B.; Gould, K.L. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 1993, 87, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Baudhuin, T.; Melin, J.A.; Pijls, N.H.; Sys, S.U.; Bol, A.; Paulus, W.J.; Heyndrickx, G.R.; Wijns, W. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation 1994, 89, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Yong, A.S.; Layland, J.; Fearon, W.F.; Ho, M.; Shah, M.G.; Daniels, D.; Whitbourn, R.; Macisaac, A.; Kritharides, L.; Wilson, A.; et al. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc. Interv. 2013, 6, 53–58. [Google Scholar] [CrossRef]
- Gould, K.L.; Lipscomb, K.; Hamilton, G.W. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am. J. Cardiol. 1974, 33, 87–94. [Google Scholar] [CrossRef]
- Hoffman, J.I. Maximal coronary flow and the concept of coronary vascular reserve. Circulation 1984, 70, 153–159. [Google Scholar] [CrossRef]
- Leppo, J.A.; Meerdink, D.J. Comparison of the myocardial uptake of a technetium-labeled isonitrile analogue and thallium. Circ. Res. 1989, 65, 632–639. [Google Scholar] [CrossRef]
- Scarsini, R.; Pesarini, G.; Zivelonghi, C.; Lunardi, M.; Piccoli, A.; Zanetti, C.; Maggio, S.; Vassanelli, C.; Ribichini, F. Coronary physiology in patients with severe aortic stenosis: Comparison between fractional flow reserve and instantaneous wave-free ratio. Int. J. Cardiol. 2017, 243, 40–46. [Google Scholar] [CrossRef]
- Hongo, M.; Goto, T.; Watanabe, N.; Nakatsuka, T.; Tanaka, M.; Kinoshita, O.; Yamada, H.; Okubo, S.; Sekiguchi, M. Relation of phasic coronary flow velocity profile to clinical and hemodynamic characteristics of patients with aortic valve disease. Circulation 1993, 88, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Julius, B.K.; Spillmann, M.; Vassalli, G.; Villari, B.; Eberli, F.R.; Hess, O.M. Angina pectoris in patients with aortic stenosis and normal coronary arteries: Mechanisms and pathophysiological concepts. Circulation 1997, 95, 892–898. [Google Scholar] [CrossRef]
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.-C.; Remkes, W.S.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Achenbach, S.; Agewall, S.; Barbato, E.; Bax, J.J.; Capodanno, D.; Cuisset, T.; Deaton, C.; Dickstein, K.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, G.; Scarsini, R.; Strisciuglio, T.; De Biase, C.; Zivelonghi, C.; Franco, D.; De Bruyne, B.; Ribichini, F.; Barbato, E. Correlation between Angiographic and Physiologic Evaluation of Coronary Artery Narrowings in Patients With Aortic Valve Stenosis. Am. J. Cardiol. 2017, 120, 106–110. [Google Scholar] [CrossRef]
- Opolski, M.P.; Kim, W.-K.; Liebetrau, C.; Walther, C.; Blumenstein, J.; Gaede, L.; Kempfert, J.; Van Linden, A.; Walther, T.; Hamm, C.W.; et al. Diagnostic accuracy of computed tomography angiography for the detection of coronary artery disease in patients referred for transcatheter aortic valve implantation. Clin. Res. Cardiol. 2015, 104, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Rolandi, M.C.; Wiegerinck, E.M.A.; Casadonte, L.; Yong, Z.Y.; Koch, K.T.; Vis, M.; Piek, J.J.; Baan, J.; Spaan, J.A.E.; Siebes, M. Transcatheter replacement of stenotic aortic valve normalizes cardiac-coronary interaction by restoration of systolic coronary flow dynamics as assessed by wave intensity analysis. Circ. Cardiovasc. Interv. 2016, 9, e002356. [Google Scholar] [CrossRef]
- Wiegerinck, E.M.A.; Van De Hoef, T.P.; Rolandi, M.C.; Yong, Z.Y.; Van Kesteren, F.; Koch, K.T.; Vis, M.M.; De Mol, B.A.J.M.; Piek, J.J.; Baan, J. Impact of aortic valve stenosis on coronary hemodynamics and the instantaneous effect of transcatheter aortic valve implantation. Circ. Cardiovasc. Interv. 2015, 8, e002443. [Google Scholar] [CrossRef]
- Rajappan, K.; Rimoldi, O.E.; Dutka, D.P.; Ariff, B.; Pennell, D.J.; Sheridan, D.J.; Camici, P.G. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation 2002, 105, 470–476. [Google Scholar] [CrossRef]
- Lumley, M.; Williams, R.; Asrress, K.N.; Arri, S.; Briceno, N.; Ellis, H.; Rajani, R.; Siebes, M.; Piek, J.J.; Clapp, B.; et al. Coronary Physiology During Exercise and Vasodilation in the Healthy Heart and in Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2016, 68, 688–697. [Google Scholar] [CrossRef]
- Yamanaka, F.; Shishido, K.; Ochiai, T.; Moriyama, N.; Yamazaki, K.; Sugitani, A.; Tani, T.; Tobita, K.; Mizuno, S.; Tanaka, Y.; et al. Instantaneous Wave-Free Ratio for the Assessment of Intermediate Coronary Artery Stenosis in Patients With Severe Aortic Valve Stenosis: Comparison With Myocardial Perfusion Scintigraphy. JACC. Cardiovasc. Interv. 2018, 11, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Bocher, M.; Blevis, I.M.; Tsukerman, L.; Shrem, Y.; Kovalski, G.; Volokh, L. A fast cardiac gamma camera with dynamic SPECT capabilities: Design, system validation and future potential. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1887–1902. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Cantone, R.; Venturi, G.; De Maria, G.L.; Variola, A.; Braggio, P.; Lunardi, M.; Pesarini, G.; Ferdeghini, M.; Piccoli, A.; et al. Correlation between intracoronary physiology and myocardial perfusion imaging in patients with severe aortic stenosis. Int. J. Cardiol. 2019, 292, 162–165. [Google Scholar] [CrossRef]
- Giubbini, R.; Bertoli, M.; Durmo, R.; Bonacina, M.; Peli, A.; Faggiano, I.; Albano, D.; Milan, E.; Stern, E.; Paghera, B.; et al. Comparison between N13NH3-PET and 99mTc-Tetrofosmin-CZT SPECT in the evaluation of absolute myocardial blood flow and flow reserve. J. Nucl. Cardiol. 2019, 28, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Nkoulou, R.; Fuchs, T.A.; Pazhenkottil, A.P.; Kuest, S.M.; Ghadri, J.R.; Stehli, J.; Fiechter, M.; Herzog, B.A.; Gaemperli, O.; Buechel, R.R.; et al. Absolute myocardial blood flow and flow reserve assessed by gated SPECT with cadmium-zinc-telluride detectors using 99mTc-tetrofosmin: Head-to-head comparison with 13N-ammonia PET. J. Nucl. Med. 2016, 57, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
| Inclusion criteria |
|---|
| Severe aortic valve stenosis qualification for TAVI by the heart team |
| Presence of an intermediate (30%–70%) coronary lesion in LAD |
| Exclusion criteria |
| Patients who are hemodynamically unstable |
| LVEF < 50% |
| Pregnancy or lactation |
| Contraindications to adenosine |
| Chronic kidney disease (eGFR < 30 mL/min/1.73 m2) |
| Coronary artery disease which requires revascularisation |
| CABG in the past |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrolinska, M.M.; Gąsior, P.; Błach, A.; Gocoł, R.; Hudziak, D.; Wojakowski, W. Myocardial Perfusion and Coronary Physiology Assessment of Microvascular Dysfunction in Patients Undergoing Transcatheter Aortic Valve Implantation—Rationale and Design. Biomimetics 2022, 7, 230. https://doi.org/10.3390/biomimetics7040230
Dobrolinska MM, Gąsior P, Błach A, Gocoł R, Hudziak D, Wojakowski W. Myocardial Perfusion and Coronary Physiology Assessment of Microvascular Dysfunction in Patients Undergoing Transcatheter Aortic Valve Implantation—Rationale and Design. Biomimetics. 2022; 7(4):230. https://doi.org/10.3390/biomimetics7040230
Chicago/Turabian StyleDobrolinska, M. M., P. Gąsior, A. Błach, R. Gocoł, D. Hudziak, and W. Wojakowski. 2022. "Myocardial Perfusion and Coronary Physiology Assessment of Microvascular Dysfunction in Patients Undergoing Transcatheter Aortic Valve Implantation—Rationale and Design" Biomimetics 7, no. 4: 230. https://doi.org/10.3390/biomimetics7040230
APA StyleDobrolinska, M. M., Gąsior, P., Błach, A., Gocoł, R., Hudziak, D., & Wojakowski, W. (2022). Myocardial Perfusion and Coronary Physiology Assessment of Microvascular Dysfunction in Patients Undergoing Transcatheter Aortic Valve Implantation—Rationale and Design. Biomimetics, 7(4), 230. https://doi.org/10.3390/biomimetics7040230





