Laser-Induced Graphene Arrays-Based Three-Phase Interface Enzyme Electrode for Reliable Bioassays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of LIG-Based Three-Phase Biosensor
2.2.1. Preparation of Laser-Induced Graphene Substrate (LIG)
2.2.2. Hydrophobic Treatment of LIG Substrate
2.2.3. Electrodeposition of Pt Nanoparticles and Modification of the Enzyme Layer
2.2.4. Preparation of Reference Electrodes and Counter Electrodes
2.3. Characterization and Electrochemical Measurements
2.4. Electrochemical Testing
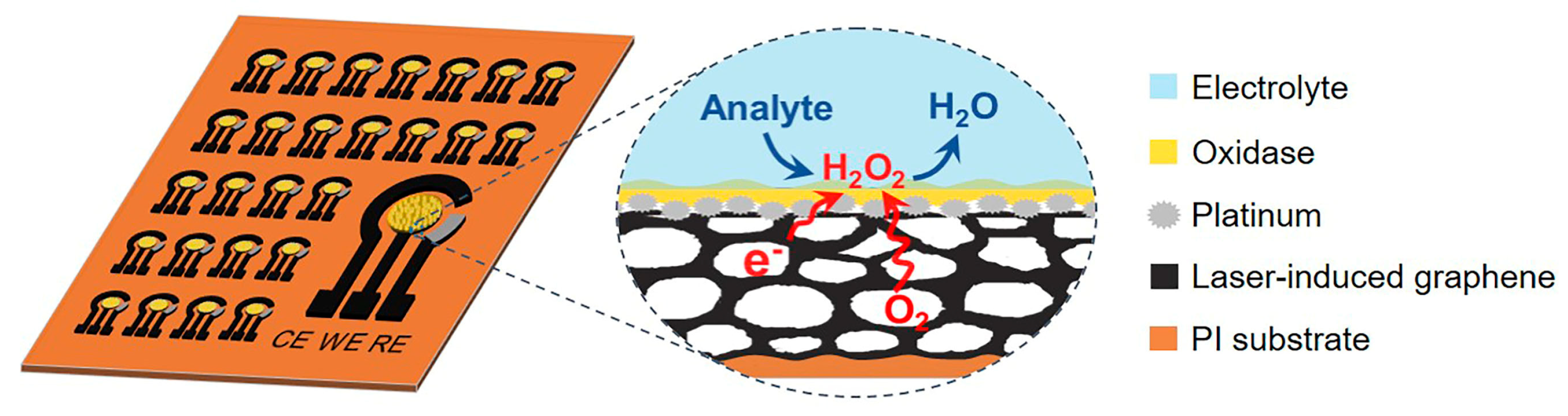
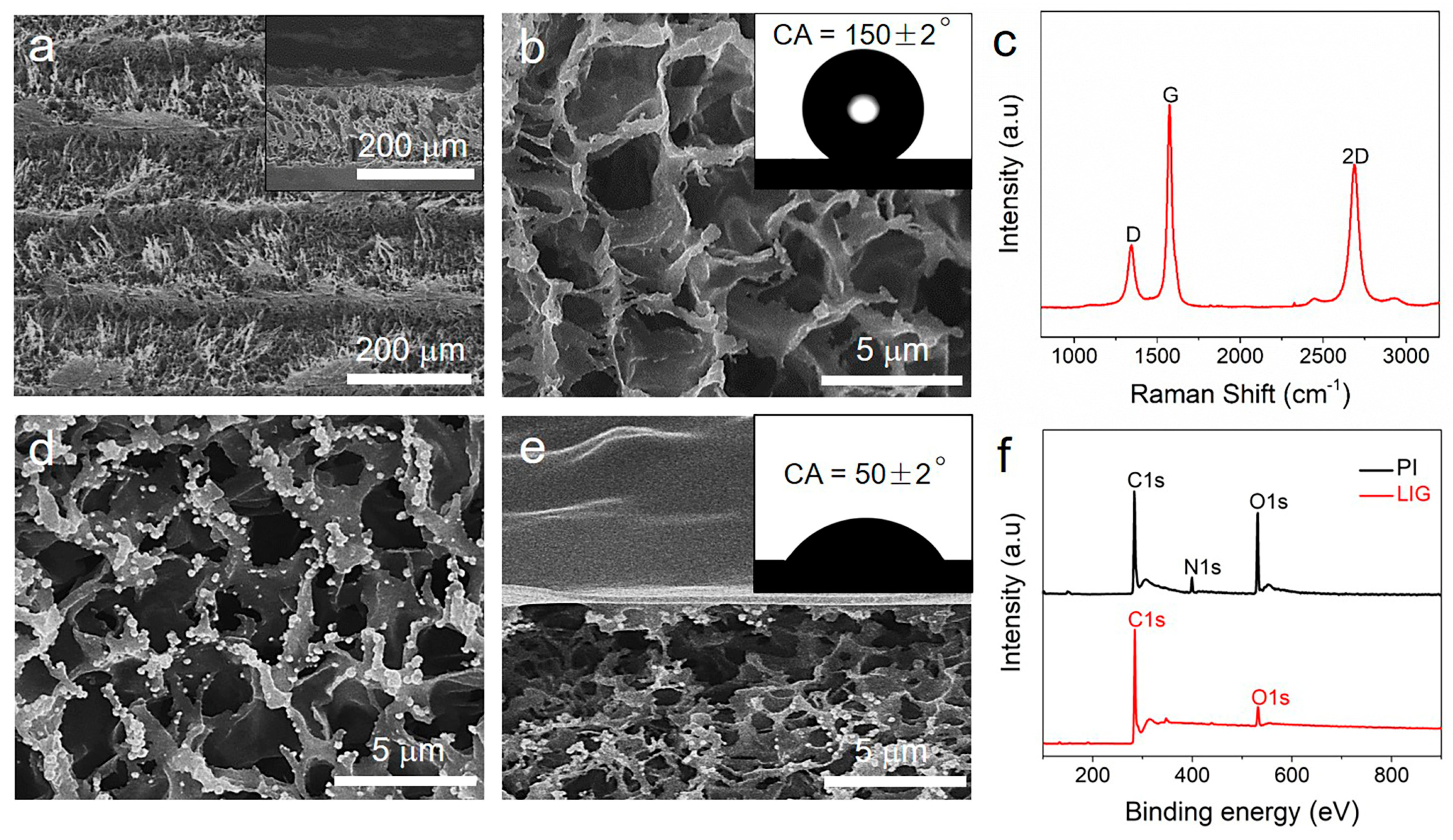
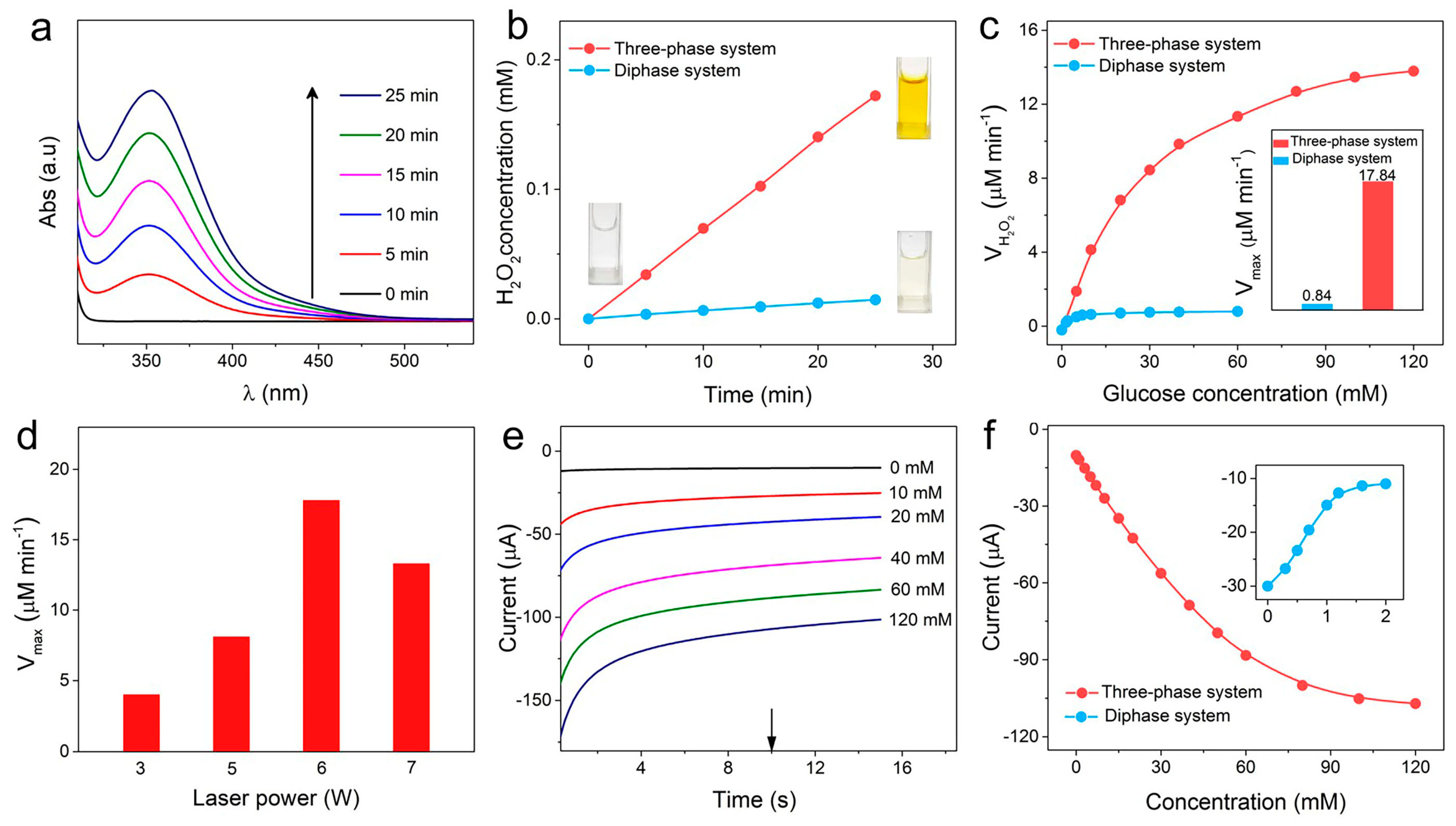
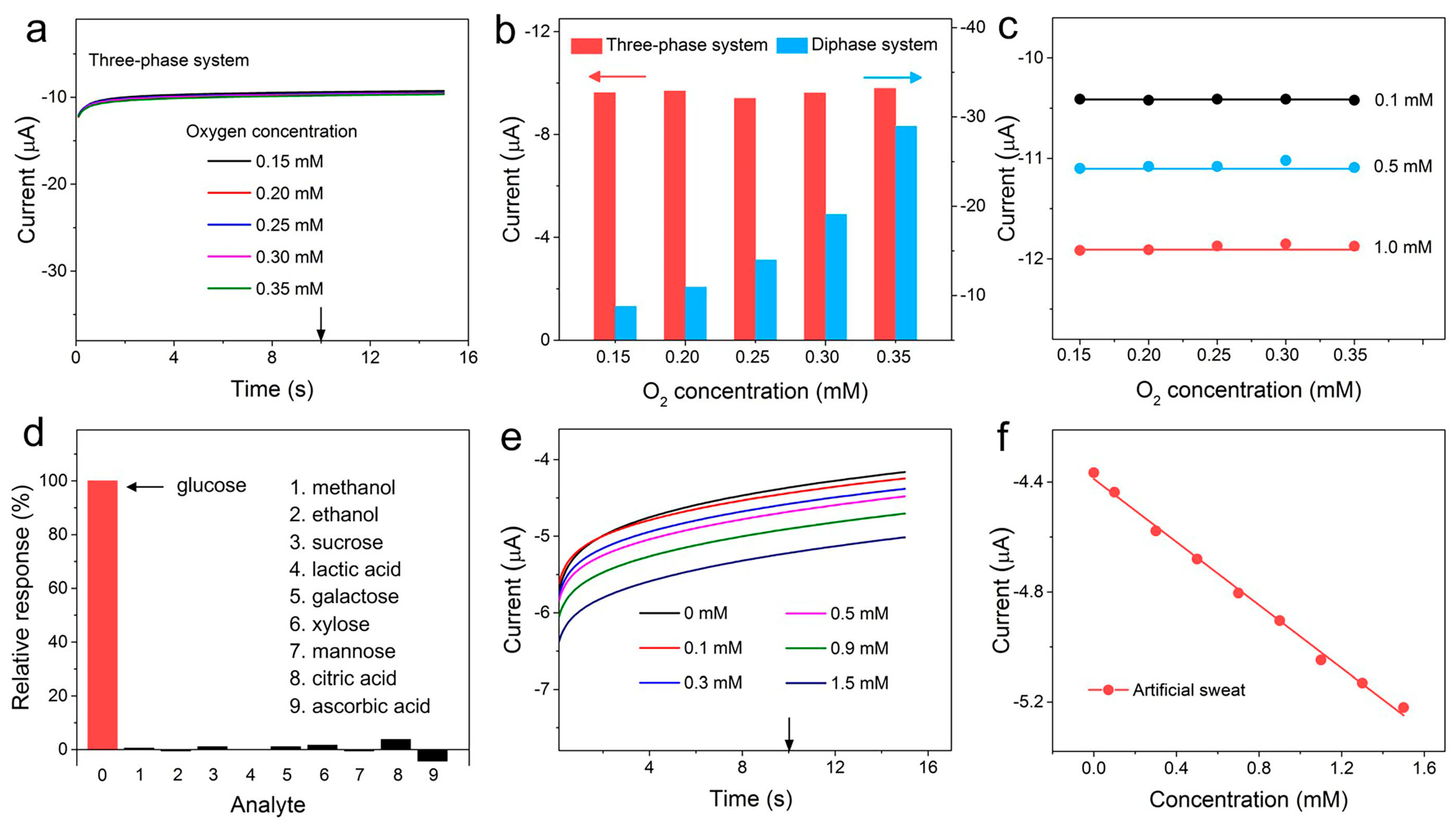
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Wang, C.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, X.; Yang, F.; Zhang, Y. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 2019, 5, eaax0649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically synthesized Prussian blue nanoparticles defeating natural enzyme peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, L.; Ma, S.; Ding, L.; Zhang, W.; Xu, Z.; Li, D.; Gao, L. Self-assembled multiple-enzyme composites for enhanced synergistic cancer starving-catalytic therapy. ACS Appl. Mater. Interfaces 2020, 12, 20191–20201. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.S.; Kim, K.; Ji, S.; Kim, Y.T.; Park, J.; Na, K.; Bae, K.H.; Kyun Kim, H.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano. Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, C.; Zhang, Q.; Ye, Y.; Cai, Y.; Li, K.; Li, Y.; Huang, X.; Wang, Y. In-situ preparation of lactate-sensing membrane for the noninvasive and wearable analysis of sweat. Biosens. Bioelectron. 2022, 210, 114303. [Google Scholar] [CrossRef]
- Si, Y.; Dong, Z.; Jiang, L. Bioinspired designs of superhydrophobic and superhydrophilic materials. ACS Cent. Sci. 2018, 4, 1102–1112. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, C.; Lan, H.; Cao, M.; Jiang, L. Improved interfacial floatability of superhydrophobic/superhydrophilic janus sheet inspired by lotus leaf. Adv. Funct. Mater. 2017, 27, 1701466. [Google Scholar]
- Liu, J.; Ye, L.; Sun, Y.; Hu, M.; Chen, F.; Wegner, S.; Mailänder, V.; Steffen, W.; Kappl, M.; Butt, H.J. Elastic superhydrophobic and photocatalytic active films used as blood repellent dressing. Adv. Mater. 2020, 32, 1908008. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Chu, Y.C.; Yen, M.H.; Lu, M.C. Enhancing condensation heat transfer on three-dimensional hybrid surfaces. Joule 2019, 3, 2806–2823. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Zhou, H.; Tian, G. Preparation and evaluation of PDMS/carbon soot particles superhydrophobic biomimetic composite coating with self-cleaning and durability. Biomimetics 2022, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Su, B.; Jiang, L. Interfacial material system exhibiting superwettability. Adv. Mater. 2014, 26, 6872–6897. [Google Scholar] [CrossRef]
- Mi, L.; Yu, J.; He, F.; Jiang, L.; Wu, Y.; Yang, L.; Han, X.; Li, Y.; Liu, A.; Wei, W.; et al. Boosting gas involved reactions at nanochannel reactor with joint gas-solid-liquid interfaces and controlled wettability. J. Am. Chem. Soc. 2017, 139, 10441–10446. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Ge, W.; Jiang, H.; Li, C. Engineering the Ni-N-C catalyst microenvironment enabling CO2 electroreduction with nearly 100% CO selectivity in acid. Adv. Mater. 2022, 34, 2201295. [Google Scholar] [CrossRef]
- Song, Z.; Xu, C.; Sheng, X.; Feng, X.; Jiang, L. Utilization of peroxide reduction reaction at air-liquid-solid joint interfaces for reliable sensing system construction. Adv. Mater. 2018, 30, 1701473. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, J.; Wang, H.; Wang, D.; Feng, X.; Jiang, L. High-performance flexible bioelectrocatalysis bioassay system based on a triphase interface. Adv. Mater. Interfaces 2020, 7, 1902172. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, J.; Wang, D.; Wang, Z.; Chen, Y.; Feng, X. Flexible triphase enzyme electrode based on hydrophobic porous PVDF membrane for high-performance bioassays. Biosens. Bioelectron. 2021, 183, 113201. [Google Scholar] [CrossRef]
- Guan, F.; Zhang, J.; Tang, H.; Chen, L.; Feng, X. An enhanced enzymatic reaction using a triphase system based on superhydrophobic mesoporous nanowire arrays. Nanoscale Horiz. 2019, 4, 231–235. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene: From discovery to translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodriguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- You, R.; Liu, Y.Q.; Hao, Y.L.; Han, D.D.; Zhang, Y.L.; You, Z. Laser fabrication of graphene-based flexible electronics. Adv. Mater. 2020, 32, 1901981. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Johnson, Z.T.; Williams, K.; Chen, B.; Sheets, R.; Jared, N.; Li, J.; Smith, E.A.; Claussen, J.C. Electrochemical sensing of neonicotinoids using laser-induced graphene. ACS Sens. 2021, 6, 3063–3071. [Google Scholar] [CrossRef]
- Avaro, A.S.; Santiago, J.G. Uncertainty quantification of Michaelis-Menten kinetic rates and its application to the analysis of CRISPR-based diagnostics. Angew Chem. Int. Ed. Engl. 2022, 61, e202209527. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Umer, M.; Lobino, M.; Thiel, D.; Nguyen, N.T.; Trinchi, A.; Shiddiky, M.J.; Gao, Y.; Li, Q. Laser induced self-N-doped porous graphene as an electrochemical biosensor for femtomolar miRNA detection. Carbon 2020, 163, 385–394. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, J.; Ding, Z.; Wang, H.; Huang, L.; Feng, X. Laser-Induced Graphene Arrays-Based Three-Phase Interface Enzyme Electrode for Reliable Bioassays. Biomimetics 2023, 8, 26. https://doi.org/10.3390/biomimetics8010026
Zhang M, Zhang J, Ding Z, Wang H, Huang L, Feng X. Laser-Induced Graphene Arrays-Based Three-Phase Interface Enzyme Electrode for Reliable Bioassays. Biomimetics. 2023; 8(1):26. https://doi.org/10.3390/biomimetics8010026
Chicago/Turabian StyleZhang, Man, Jun Zhang, Zhenyao Ding, Haili Wang, Lihui Huang, and Xinjian Feng. 2023. "Laser-Induced Graphene Arrays-Based Three-Phase Interface Enzyme Electrode for Reliable Bioassays" Biomimetics 8, no. 1: 26. https://doi.org/10.3390/biomimetics8010026
APA StyleZhang, M., Zhang, J., Ding, Z., Wang, H., Huang, L., & Feng, X. (2023). Laser-Induced Graphene Arrays-Based Three-Phase Interface Enzyme Electrode for Reliable Bioassays. Biomimetics, 8(1), 26. https://doi.org/10.3390/biomimetics8010026








