Investigating Foot Morphology in Rock Climbing Mammals: Inspiration for Biomimetic Climbing Shoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photographing of Samples
2.2. Image Analysis
2.3. Data Analysis
3. Results
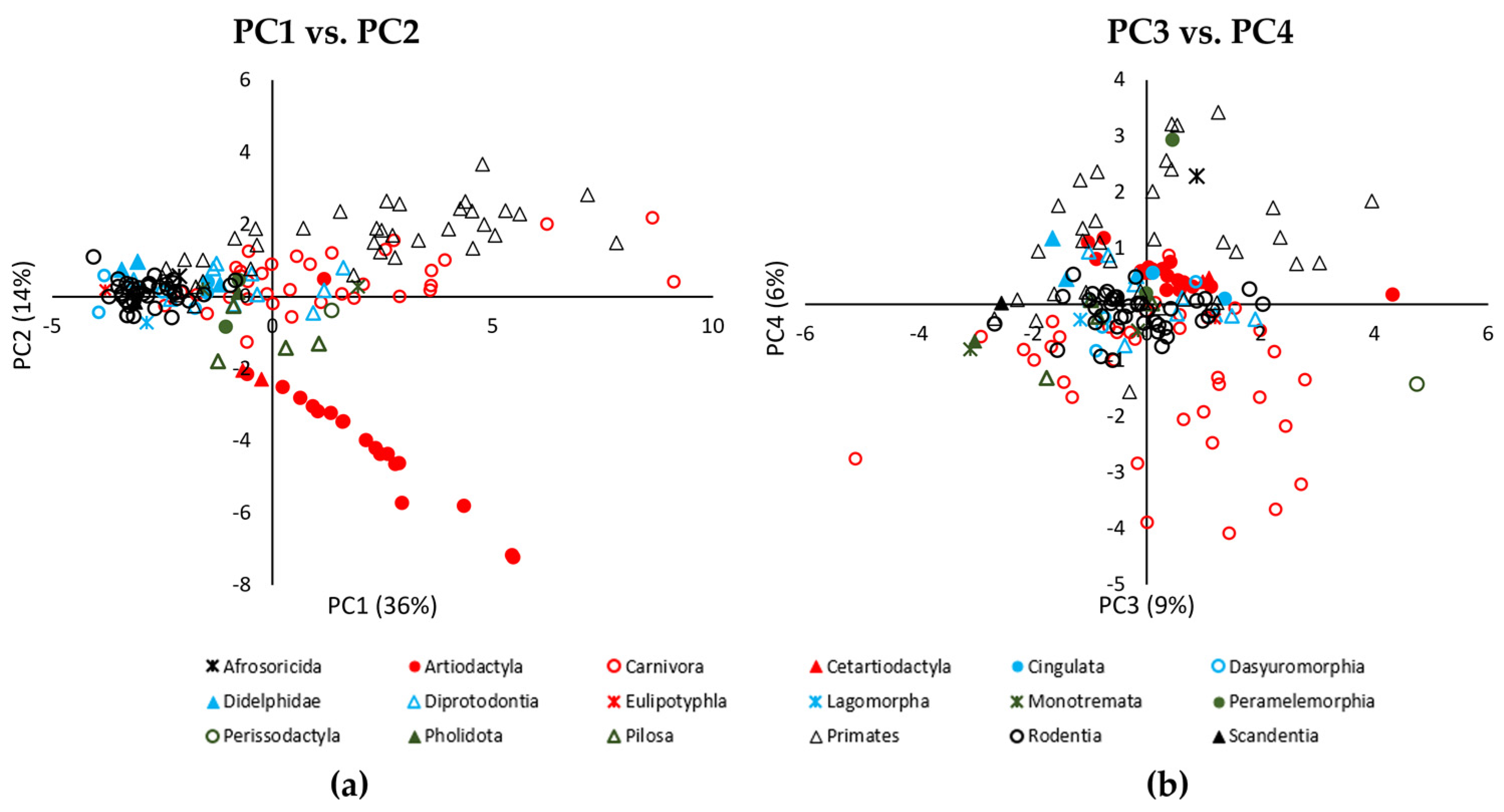
3.1. Principal Component Analysis of Quantitative Metrics
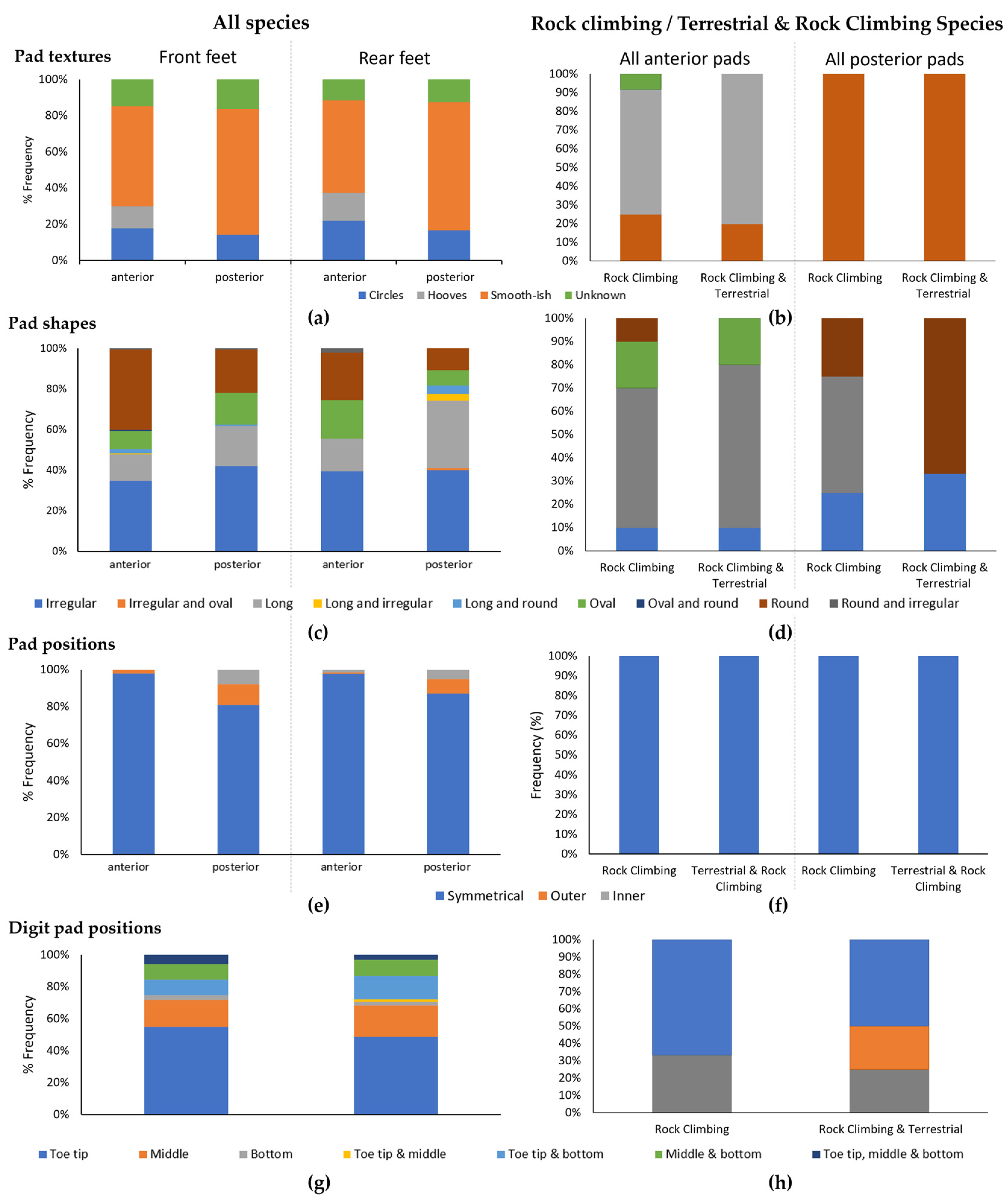
3.2. Pad Positions, Shapes and Textures
4. Discussion
Implications for Bio-Inspired Climbing Shoe Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López Forniés, I.; Berges Muro, L. A top-down biomimetic design process for product concept generation. Int. J. Des. Nat. Ecodyn. 2012, 7, 27–48. [Google Scholar] [CrossRef] [Green Version]
- Angeleska, E.; Sidorenko, S. Bio-inspired back support system for backpacks. FME Trans. 2021, 49, 327–334. [Google Scholar] [CrossRef]
- Zdravkova, A.; Mircheski, I.; Sidorenko, S. Bio-inspired approach for innovative design of knee protectors for recreational sports. FME Trans. 2020, 48, 849–854. [Google Scholar] [CrossRef]
- Chamberlain, M.; Miller, J.; Dowd, T.; Rhim, J.S.; Heflin, D.; Akturk, I.; Coffing, J.; Fassnacht, M.; Mansson, J.-A. Development of a bicycle crank arm demonstrator via Industry 4.0 principles for sustainable and cost-effective manufacturing. Sport. Eng. 2023, 26, 2. [Google Scholar] [CrossRef]
- Singh, A.V.; Rahman, A.; Kumar, N.S.; Aditi, A.; Galluzzi, M.; Bovio, S.; Barozzi, S.; Montani, E.; Parazzoli, D. Bio-inspired approaches to design smart fabrics. Mater. Des. 2012, 36, 829–839. [Google Scholar] [CrossRef]
- Soltani, A.; Noroozi, R.; Bodaghi, M.; Zolfagharian, A.; Hedayati, R. 3D printing on-water sports boards with bio-inspired core designs. Polymers 2020, 12, 250. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, B.S.; Velasco-Hogan, A.; Gómez-del Río, T.M.; Meyers, A.; Jasiuk, I. A review of impact resistant biological and bioinspired materials and structures. J. Mater. Res. Technol. 2020, 9, 15705–15738. [Google Scholar] [CrossRef]
- Siddique, S.H.; Hazell, P.J.; Wang, H.; Escobedo, J.P.; Ameri, A.A. Lessons from nature: 3D printed bio-inspired porous structures for impact energy absorption—A review. Addit. Manuf. 2022, 58, 103051. [Google Scholar] [CrossRef]
- Islam, M.K.; Hazell, P.J.; Escobedo, J.P.; Wang, H. Biomimetic armour design strategies for additive manufacturing: A review. Mater. Des. 2021, 205, 109730. [Google Scholar] [CrossRef]
- Taraborrelli, L.; Grant, R.; Sullivan, M.; Choppin, S.; Spurr, J.; Haake, S.; Allen, T. Materials Have Driven the Historical Development of the Tennis Racket. Appl. Sci. 2019, 9, 4352. [Google Scholar] [CrossRef]
- Grant, R.A.; Taraborrelli, L.; Allen, T. Morphometrics for sports mechanics: Showcasing tennis racket shape diversity. PLOS ONE 2022, 17, e0263120. [Google Scholar] [CrossRef] [PubMed]
- Crouch, T.N.; Burton, D.; Labry, Z.A.; Blair, K.B. Riding against the wind: A review of competition cycling aerodynamics. Sports Eng. 2017, 20, 81–110. [Google Scholar] [CrossRef]
- Haake, S. Advantage Play Technologies that Changed Sporting History; Arena Sport: Tolentino, Italy, 2018. [Google Scholar]
- Haake, S.J. The impact of technology on sporting performance in Olympic sports. J. Sports Sci. 2009, 27, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Eftaxiopoulou, T.; Persad, L.; Bull, A.M. Assessment of performance parameters of a series of five ‘historical’ cricket bat designs. Proc. Inst. Mech. Eng. Part P: J. Sports Eng. Technol. 2016, 231, 57–62. [Google Scholar] [CrossRef]
- Morales, A.T.; Fajardo, J.A.T.; González-García, H. High-Speed Swimsuits and Their Historical Development in Competitive Swimming. Front. Psychol. 2019, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Malizia, F.; Blocken, B. Cyclist aerodynamics through time: Better, faster, stronger. J. Wind. Eng. Ind. Aerodyn. 2021, 214, 200. [Google Scholar] [CrossRef]
- Malizia, F.; Blocken, B. Bicycle aerodynamics: History, state-of-the-art and future perspectives. J. Wind. Eng. Ind. Aerodyn. 2020, 200, 104134. [Google Scholar] [CrossRef]
- Hobara, H. Running-specific prostheses: The history, mechanics, and controversy. J. Soc. Biomech. 2014, 38, 105–110. [Google Scholar] [CrossRef] [Green Version]
- McPhee, J. A review of dynamic models and measurements in golf. Sports Eng. 2022, 25, 1–19. [Google Scholar] [CrossRef]
- Livesey, A. Bicycle Engineering and Technology; Taylor and Francis: London, UK, 2020. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.; Kim, D.; Yoo, J.; Jung, J.; Park, K. Analysis of plantar pressure during climbing for the development of sports climbing shoes. Footwear Sci. 2017, 9, S111–S113. [Google Scholar] [CrossRef]
- van der Putten, E.P.; Snijders, C.J. Shoe design for prevention of injuries in sport climbing. Appl. Ergon. 2001, 32, 379–387. [Google Scholar] [CrossRef] [PubMed]
- McHenry, R.; Arnold, G.; Wang, W.; Abboud, R. Footwear in rock climbing: Current practice. Foot 2015, 25, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Cartmill, M. Climbing; Harvard University Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Menon, C.; Sitti, M. A biomimetic climbing robot based on the gecko. J. Bionic Eng. 2006, 3, 115–125. [Google Scholar] [CrossRef]
- Unver, O.; Uneri, A.; Aydemir, A.; Sitti, M. Geckobot: A gecko inspired climbing robot using elastomer adhesives. In Proceedings of the IEEE International Conference on Robotics and Automation, Orlando, FL, USA, 15–19 May 2006; Volume 2006. [Google Scholar] [CrossRef]
- Abad, S.A.; Sornkarn, N.; Nanayakkara, T. The role of morphological computation of the goat hoof in slip reduction. IEEE Int. Conf. Intell. Robot. Syst. 2016, 2016, 5599–5605. [Google Scholar] [CrossRef] [Green Version]
- Burris, J.N.; Lenaghan, S.C.; Stewart, C.N. Climbing plants: Attachment adaptations and bioinspired innovations. Plant Cell Rep. 2018, 37, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Labonte, D.; Federle, W. Scaling and biomechanics of surface attachment in climbing animals. Philos. Trans. R. Soc. B: Biol. Sci. 2015, 370, 20140027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbera, A.M.; Delaunay, M.G.; Dougill, G.; Grant, R.A. Paw Morphology in the Domestic Guinea Pig (Cavia porcellus) and Brown Rat (Rattus norvegicus). Anat. Rec. 2019, 302, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M. A comparison of the gross morphology and micro-anatomy of the foot pads in two fossorial and two climbing rodents (Mammalia). J. Zool. 1998, 244, 287–294. [Google Scholar] [CrossRef]
- Dobson, G.E. 1. On peculiar Structures in the Feet of certain Species of Mammals which enable them to walk on smooth perpendicular surfaces. Proc. Zool. Soc. Lond. 1876, 44, 526–535. [Google Scholar] [CrossRef]
- Lewis, O.J. The evolutionary emergence and refinement of the mammalian pattern of foot architecture. J. Anat. 1983, 137, 21–45. [Google Scholar]
- Gebo, D.L.; Simons, E.L. Morphology and locomotor adaptations of the foot in early Oligocene anthropoids. Am. J. Phys. Anthropol. 1987, 74, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-X.; Yuan, C.-X.; Meng, Q.-J.; Ji, Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 2011, 476, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.; Espinosa, R.R.; Parr, C.T.; Jones, G.; Hammond, T.; Dewey, T. The Animal Diversity. Available online: https://animaldiversity.org (accessed on 25 October 2022).
- CABI; EPPO. Invasive Species Compendium, Ricinus communis; Cabi: Sao Paulo, Brazil, 2021. [Google Scholar]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Upham, N.S.; Esselstyn, J.A.; Jetz, W. Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLOS Biol. 2019, 17, e3000494. [Google Scholar] [CrossRef]
- Cartmill, M. The volar skin of primates: Its frictional characteristics and their functional significance. Am. J. Phys. Anthropol. 1979, 50, 497–509. [Google Scholar] [CrossRef]
- Hale, J.; Lewis, R.; Carré, M.J. Effect of simulated tennis steps and slides on tread element friction and wear. Sports Eng. 2021, 24, 1–9. [Google Scholar] [CrossRef]

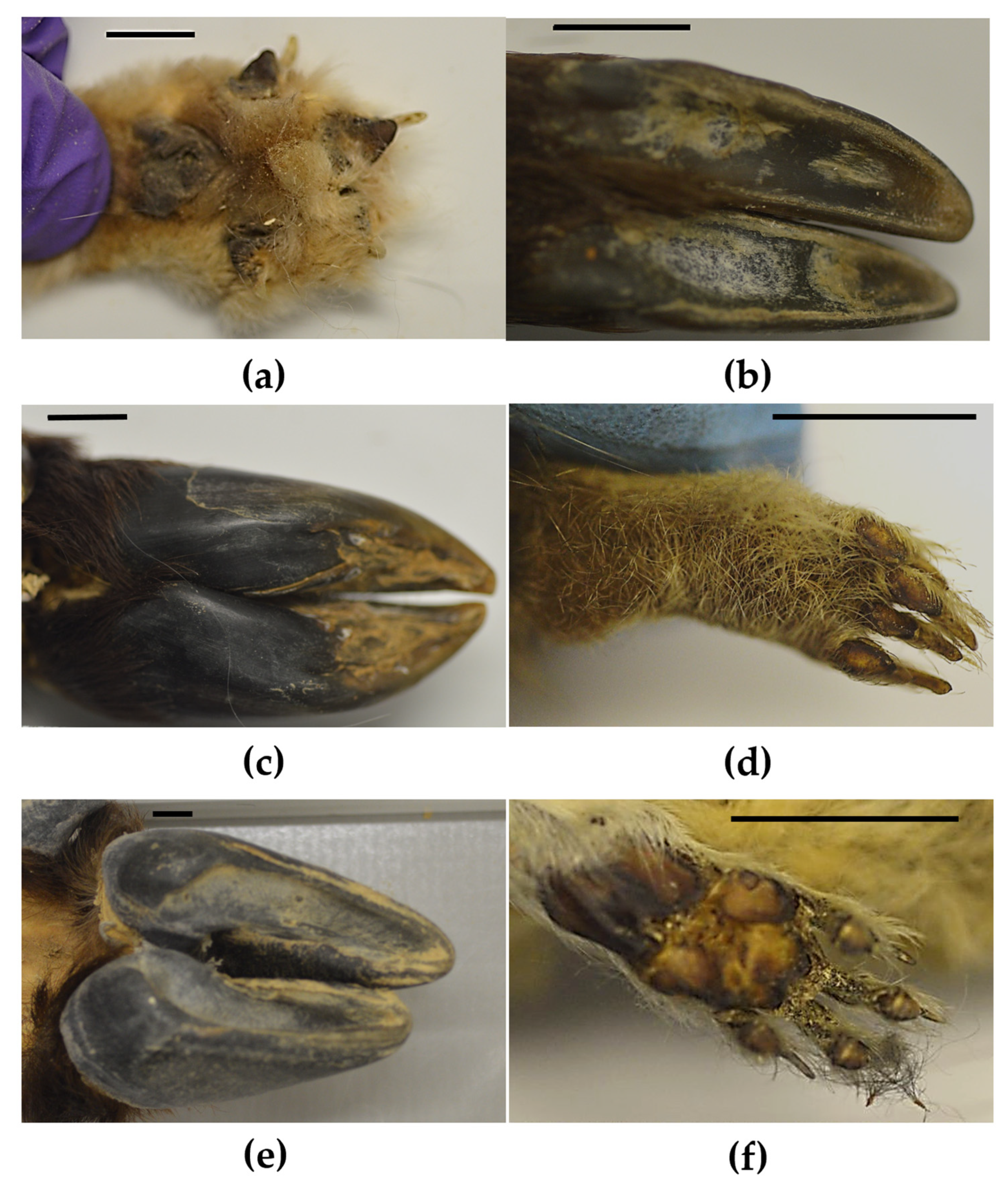

| Measurements | Unit | Description | Figure |
|---|---|---|---|
| Quantitative metrics | |||
| Proportion of digit area | - | Difference between the area of the foot with and without the digits, divided by the area of the foot with the digits.1 | Figure S1a,b |
| Foot length | mm | Length at longest point of the foot. | Figure S1d |
| Foot width | mm | Width at widest point of the foot. | Figure S1e |
| Mean digit length | mm | Mean of the tip to base length of all digits.1 | Figure S1g |
| Mean digit width | mm | Mean of the width of the widest point of all digits. | Figure S1h |
| Digit number | No. | Count of no. digits per foot. | Figure S1f |
| Digit pad number | No. | Count of total no. pads on digits. | Figure S1f |
| Total pad area on digit | Total area of digit pads. | Figure S1f | |
| Posterior pad number | No. | Count of no. posterior pads.2 | Figure S1c |
| Total posterior pad area | Total area of posterior pads. | Figure S1c | |
| Anterior pad number | No. | Count of no. anterior pads.2 | Figure S1i |
| Total anterior pad area | Total area of anterior pads. | Figure S1i | |
| Categorical metrics | |||
| Digit pad position | Scored as: toe tip, middle, bottom.3 | ||
| Anterior pad position | Scored as: inner, outer or symmetrical.2 | ||
| Posterior pad position | As above. | ||
| Posterior pad shape | Scored as: irregular, long, oval or round.3 | ||
| Anterior pad shape | As above. | ||
| Posterior pad texture | Scored as: circles, smooth-ish 4, or unknown.5 | Figure S2 | |
| Anterior pad texture | Scored as: circles, hooves 6, smooth-ish 4, or unknown.5 | Figure S2 | |
| PC1 (36%) | PC2 (14%) | PC3 (9%) | PC4 (6%) |
|---|---|---|---|
| Front Foot length (0.32) | Rear foot Digit number (0.46) | Front foot Proportion of digit area (0.55) | Rear foot Digit pad number (0.52) |
| Front Foot width (0.32) | Front foot Digit number (0.44) | Rear foot Proportion of digit area | Front foot Digit pad number (0.46) |
| Rear Foot width (0.32) | Rear foot Total anterior pad area (−0.39) | (0.54) | Rear foot Posterior pad number (0.30) |
| Front foot Total anterior pad area (−0.36) |
| Comparison | Test | F | Df1, Df2 | p | Post-Hoc |
|---|---|---|---|---|---|
| PC1 (λ = 0.93, p < 0.001) | |||||
| Order | ANOVA | 9.32 | 17, 149 | <0.001 | Primates, Artiodactyla > Rodentia, Didelphimorphia, Dasyuromorphia Rodentia, Dasyuromorphia < Primates, Artiodactyla, Carnivora |
| Locomotion | ANOVA | 1.53 | 10, 156 | 0.133 | - |
| Locomotion | Phylo ANOVA | 1.36 | - | 0.800 | - |
| PC2 (λ = 0.86, p < 0.001) | |||||
| Order | ANOVA | 32.90 | 17, 149 | <0.001 | Artiodactyla < all groups apart from Cetartiodactyla Cetartiodactyla < Primates, Carnivora, Rodentia, Didelphimorphia Primates > Cetartiodactyla, Rodentia, Artiodactyla, Pilosa Rodentia > Cetartiodactyla, Artiodactyla, Pilosa |
| Locomotion | ANOVA | 8.94 | 10, 156 | <0.001 | Arboreal & Terrestrial, Arboreal > Rock Climbing, Rock Climbing & Terrestrial |
| Locomotion | Phylo ANOVA | 8.49 | - | 0.200 | - |
| PC3 (λ = 0.84, p < 0.001) | |||||
| Order | ANOVA | 2.07 | 17, 149 | 0.011 | Perissodactyla > Primates, Carnivora, Rodentia, Didelphimorphia, Monotremata, Pilosa, Pholidota, Scandentia |
| Locomotion | ANOVA | 1.11 | 10,156 | 0.360 | - |
| Locomotion | Phylo ANOVA | 0.99 | - | 1.000 | - |
| PC4 (λ = 0.77, p < 0.001) | |||||
| Order | ANOVA | 9.94 | 17, 149 | <0.001 | Carnivora < Primates, Artiodactyla, Peramelemorphia, Afrosoricida, Diprodrontia, Didelphimorphia Primates > Carnivora, Rodentia |
| Locomotion | ANOVA | 2.53 | 10, 156 | 0.008 | Arboreal > Terrestrial |
| Locomotion | Phylo ANOVA | 2.18 | - | 0.900 | - |
| Front Feet | Rear Feet | |||
|---|---|---|---|---|
| Pads | Anterior | Posterior | Anterior | Posterior |
| Locomotion | ||||
| Texture | Χ2 = 112.96, df = 32, p < 0.001 | Χ2 = 66.192, df = 24, p < 0.001 | Χ2 = 117.84, df = 32, p < 0.001 | Χ2 = 51.693, df = 24, p = 0.001 |
| Shape | Χ2 = 83.618, df = 64, p = 0.050 | Χ2 = 74.846, df = 48, p = 0.008 | Χ2 = 68.82, df = 48, p = 0.026 | Χ2 = 83.424, df = 56, p = 0.026 |
| Position | Χ2 = 27.841, df = 16, p = 0.033 | Χ2 = 79.033, df = 32, p < 0.001 | Χ2 = 42.224, df = 24, p = 0.012 | Χ2 = 47.076, df = 32, p = 0.042 |
| Digit position | Χ2 = 108.85, df = 48, p < 0.001 | Χ2 = 105.44, df = 56, p < 0.001 | ||
| Order | ||||
| Texture | Χ2 = 272.4, df = 68, p < 0.001 | Χ2 = 218.68, df = 51, p < 0.001 | Χ2 = 312.47, df = 68, p < 0.001 | Χ2 = 145.79, df = 51, p < 0.001 |
| Shape | Χ2 = 247.1, df = 136, p < 0.001 | Χ2 = 213.06, df = 102, p < 0.001 | Χ2 = 207.88, df = 102, p < 0.001 | Χ2 = 197.85, df = 119, p < 0.001 |
| Position | Χ2 = 79.123, df = 34, p < 0.001 | Χ2 = 188.09, df = 68, p < 0.001 | Χ2 = 87.954, df = 51, p = 0.001 | Χ2 = 107.22, df = 68, p = 0.002 |
| Digit position | Χ2 = 367.25, df = 102, p < 0.001 | Χ2 = 525.27, df = 119, p < 0.001 | ||
| Biological Finding | Bio-Inspired Design Recommendation |
|---|---|
| Hooves or large pads common | Characterise compliance of the pads and hooves and create candidate concepts for testing, varying in compliance |
| Smooth-ish pads and hooves, but some texture present | Characterise texture of the pads and hooves and create candidate texture concepts for testing |
| Variation in pad location and shape | Test candidate pad structures with texture and compliance over a range of likely positions on the shoe |
| Order effects foot morphology | Choose representative candidate concepts based on a range of rock climbing Orders (Carnivora, Artiodactyla, Rodentia, Afrosoricida and Lagomorpha) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spurrier, S.; Allen, T.; Grant, R.A. Investigating Foot Morphology in Rock Climbing Mammals: Inspiration for Biomimetic Climbing Shoes. Biomimetics 2023, 8, 8. https://doi.org/10.3390/biomimetics8010008
Spurrier S, Allen T, Grant RA. Investigating Foot Morphology in Rock Climbing Mammals: Inspiration for Biomimetic Climbing Shoes. Biomimetics. 2023; 8(1):8. https://doi.org/10.3390/biomimetics8010008
Chicago/Turabian StyleSpurrier, Stephen, Tom Allen, and Robyn A. Grant. 2023. "Investigating Foot Morphology in Rock Climbing Mammals: Inspiration for Biomimetic Climbing Shoes" Biomimetics 8, no. 1: 8. https://doi.org/10.3390/biomimetics8010008
APA StyleSpurrier, S., Allen, T., & Grant, R. A. (2023). Investigating Foot Morphology in Rock Climbing Mammals: Inspiration for Biomimetic Climbing Shoes. Biomimetics, 8(1), 8. https://doi.org/10.3390/biomimetics8010008







