Long Bone Defect Filling with Bioactive Degradable 3D-Implant: Experimental Study
Abstract
:1. Introduction
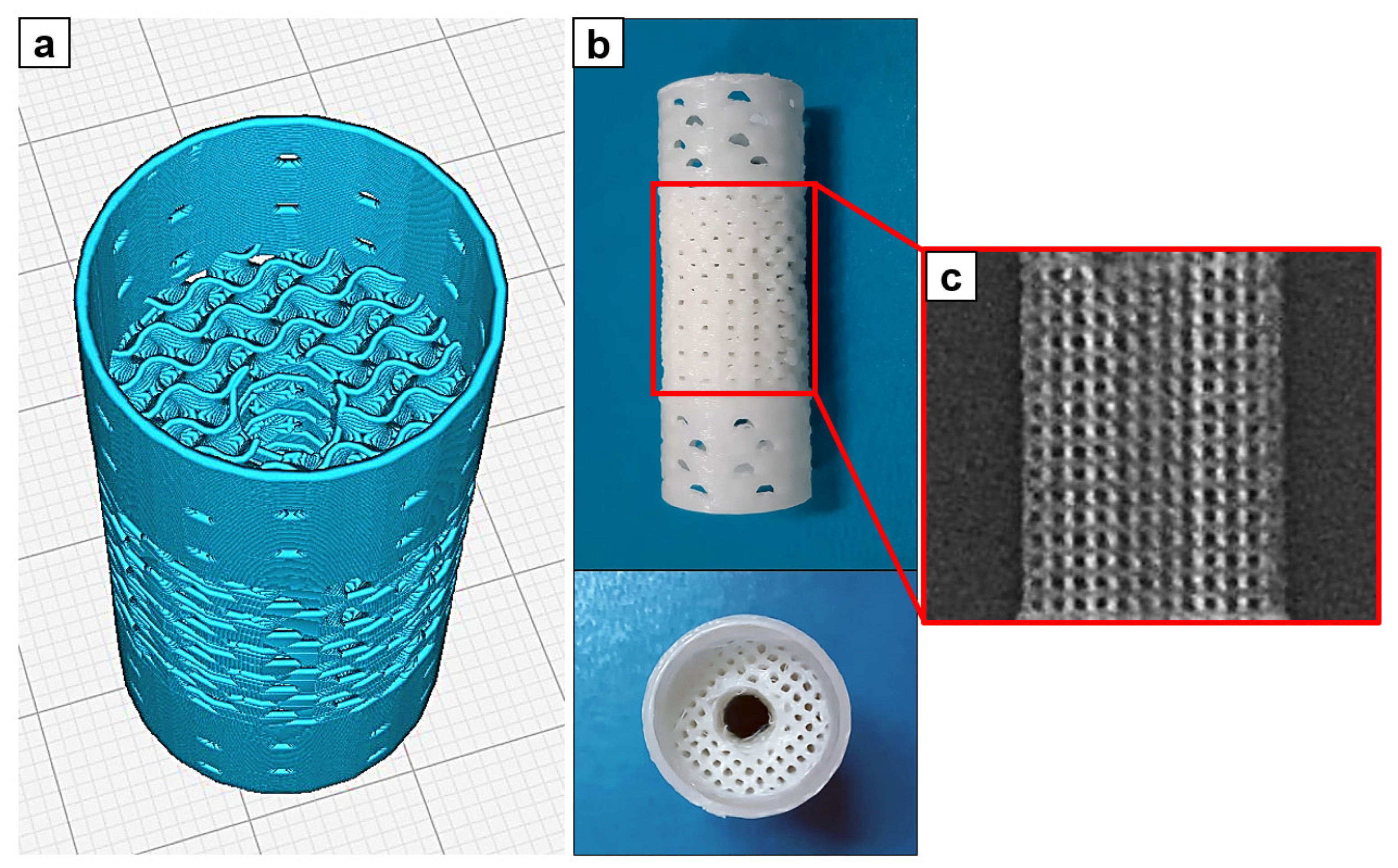
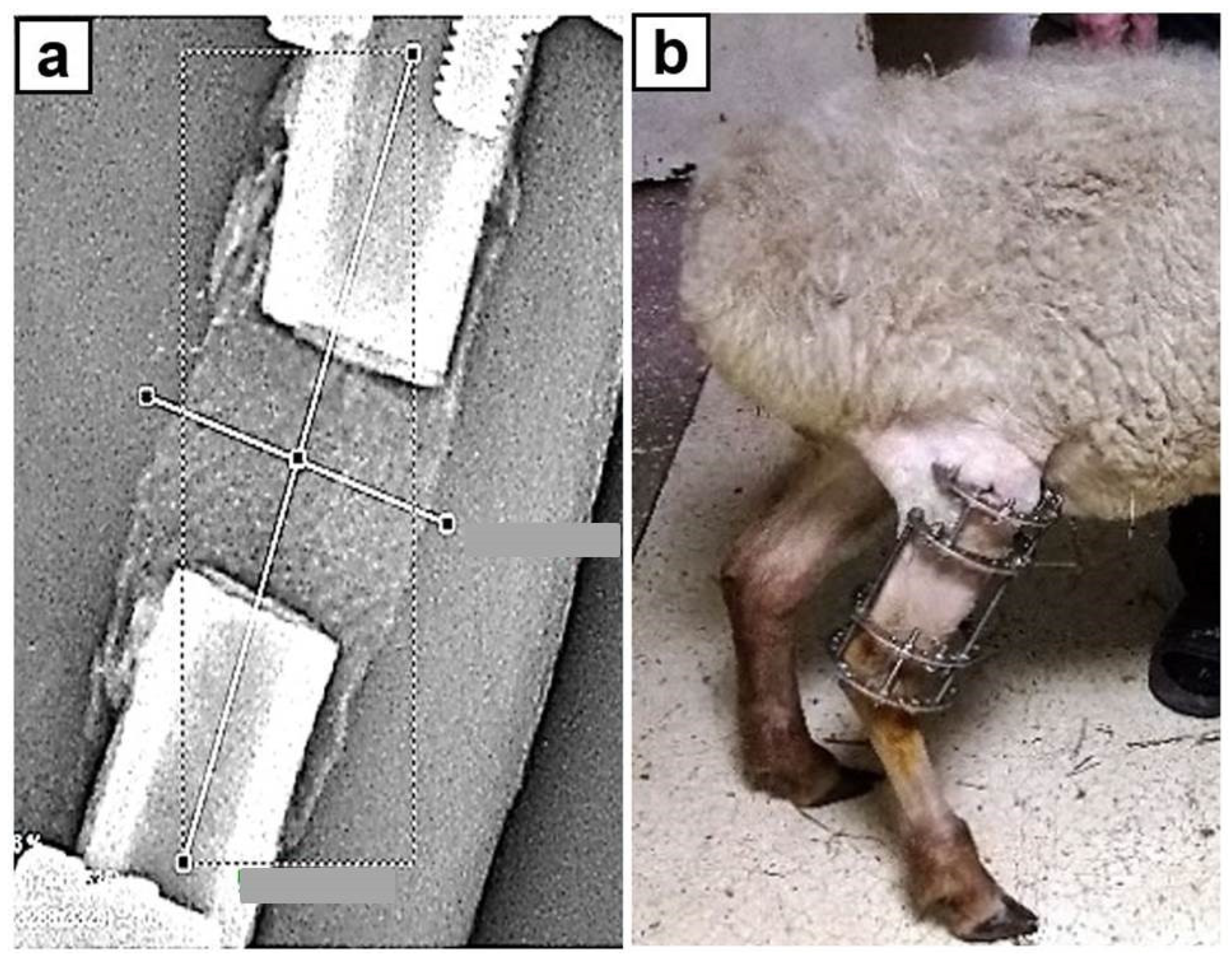
2. Materials and Methods
- 1–2 days before surgery: physical examination and standard AP and lateral radiographs of both tibiae including adjacent joints;
- D0: surgery;
- Postoperative period: standard AP and lateral radiographs were taken on the D1 and later once every week;
- Euthanasia at D30 (Euthanasia was performed by an intravenous injection of sodium thiopental 5%, 45 mg/kg).
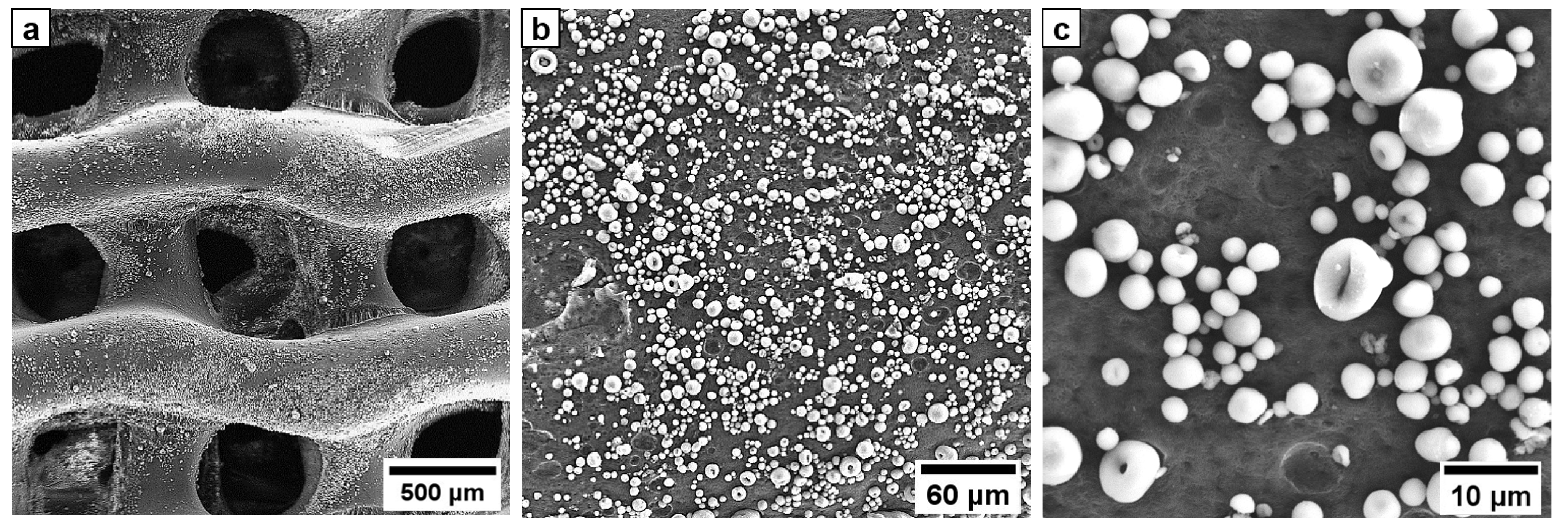
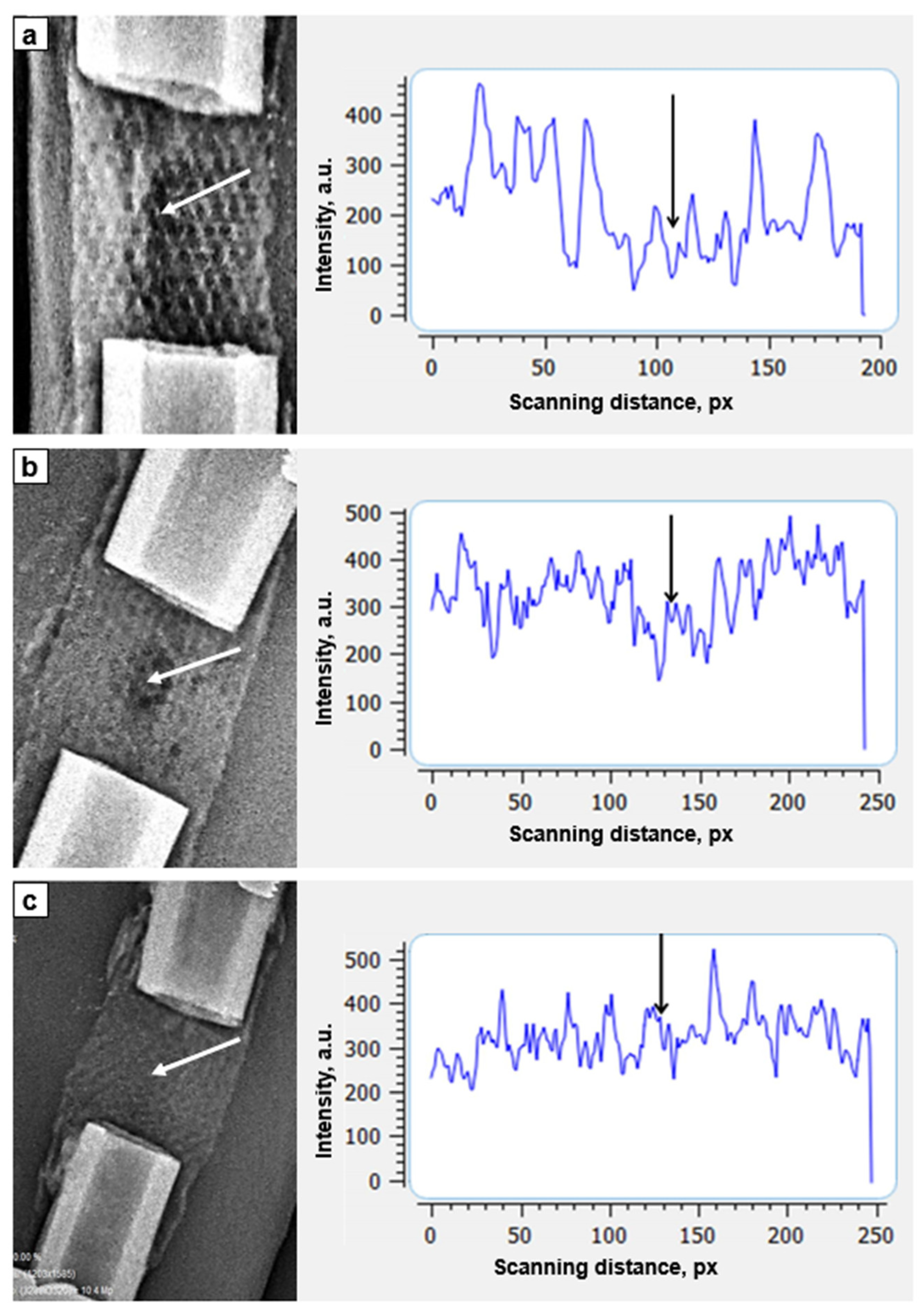
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Rosasy, M.A.; Ayoub, M.A. Traumatic Composite Bone and Soft Tissue Loss of the Leg: Region-Specific Classification and Treatment Algorithm. Injury 2020, 51, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.; Zhang, J.; Zhou, A.; Krkovic, M. Management of post-traumatic femoral defects with a monorail external fixator over an intramedullary nail. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Liu, Y.; Alimujiang, A.; Guo, J.; Chen, D.; Wang, Y.; Yusufu, A.; Ma, C. Nine-year-long complex humeral nonunion salvaged by distraction osteogenesis technique: A case report and review of the literature. BMC Surg. 2022, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, B.; El-Mowafi, H.; El-Moghazy, N. Distraction osteogenesis in management of composite bone and soft tissue defects. Int. Orthop. 2010, 34, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Rozbruch, R.S.; Weitzman, A.M.; Watson, T.J.; Freudigman, P.; Katz, H.V.; Ilizarov, S. Simultaneous treatment of tibial bone and soft tissue defects with the Ilizarov method. J. Orthop. Trauma 2006, 20, 197–205. [Google Scholar] [CrossRef]
- Smith, W.R.; Elbatrawy, Y.A.; Andreassen, G.S.; Philips, G.C.; Guerreschi, F.; Lovisetti, L.; Catagni, M.A. Treatment of traumatic forearm bone loss with Ilizarov ring fixation and bone transport. Int. Orthop. 2007, 31, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Zhang, Y.; Lipa, K.; Qing, L.; Wu, P.; Tang, J. Ilizarov Bone Transfer for Treatment of Large Tibial Bone Defects: Clinical Results and Management of Complications. J. Pers. Med. 2022, 12, 1774. [Google Scholar] [CrossRef]
- Liodakis, E.; Kenawey, M.; Krettek, C.; Wiebking, U.; Hankemeier, S. Comparison of 39 post-traumatic tibia bone transports performed with and without the use of an intramedullary rod: The long-term outcomes. Int. Orthop. 2011, 35, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xiang, C.; Yan, C.; Chen, Q.; Chen, L.; Jiang, K.; Li, Y. Effectiveness of bone transport with a locking plate versus conventional bone transport for tibial defects. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2022, 36, 983–988. (In Chinese) [Google Scholar]
- Kumar, A.; Nune, K.C.; Misra, R.D.K. Design and biological functionality of a novel hybrid Ti-6Al-4V/hydrogel system for reconstruction of bone defects. J. Tissue Eng. Regen. Med. 2018, 12, 1133–1144. [Google Scholar] [CrossRef]
- Wang, Z.; Han, L.; Zhou, Y.; Cai, J.; Sun, S.; Ma, J.; Wang, W.; Li, X.; Ma, L. The combination of a 3D-Printed porous Ti-6Al-4V alloy scaffold and stem cell sheet technology for the construction of biomimetic engineered bone at an ectopic site. Mater. Today Bio. 2022, 16, 100433. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, X.; Zhou, Y.; Ji, X.; Cheng, S.; Bian, D.; Fan, L.; Zhou, L.; Ning, C.; Zhang, Y. Biomimetic Ti-6Al-4V alloy/gelatin methacrylate hybrid scaffold with enhanced osteogenic and angiogenic capabilities for large bone defect restoration. Bioact. Mater. 2021, 6, 3437–3448. [Google Scholar] [CrossRef]
- Popkov, A.; Kononovich, N.; Gorbach, E.; Popkov, D. Osteointegration technology in long bone defect reconstruction: Experimental study. Acta Bioeng. Biomech. 2020, 22, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, U.; Beiser, S.; Kuhn, S.; Stark, T.; Hoess, A.; Cristina-Schmitz, H.; Vasilikos, I.; Metzger, M.C.; Rothweiler, R. A fully ingrowing implant for cranial reconstruction: Results in critical size defects in sheep using 3D-printed titanium scaffold. Biomater. Adv. 2022, 136, 212754. [Google Scholar] [CrossRef]
- Liu, B.; Hou, G.; Yang, Z.; Li, X.; Zheng, Y.; Wen, P.; Liu, Z.; Zhou, F.; Tian, Y. Repair of critical diaphyseal defects of lower limbs by 3D printed porous Ti6Al4V scaffolds without additional bone grafting: A prospective clinical study. J. Mater. Sci. Mater. Med. 2022, 33, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Alonzo, M.; Primo, F.A.; Kumar, S.A.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances and scaffolds for treatment of bone defects. Curr. Opin. Biomed Eng. 2021, 17, 100248. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Fu, Z.; Cui, J.; Zhao, B.; Shen, S.G.; Lin, K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J. Orthop. Translat. 2021, 28, 118–130. [Google Scholar] [CrossRef]
- Bal, Z.; Korkusuz, F.; Ishiguro, H.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Kodama, J.; Tateiwa, D.; Ukon, Y.; Nakagawa, S.; et al. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021, 21, 865–873. [Google Scholar] [CrossRef]
- Fijoł, N.; Abdelhamid, H.N.; Pillai, B.; Hall, S.A.; Thomas, N.; Mathew, A.P. 3D-printed monolithic biofilters based on a polylactic acid (PLA)—hydroxyapatite (HAp) composite for heavy metal removal from an aqueous medium. RSC Adv. 2021, 11, 32408–32418. [Google Scholar] [CrossRef]
- Pérez, E. Mechanical Performance of in Vitro Degraded Polylactic Acid/Hydroxyapatite Composites. J. Mater. Sci. 2021, 56, 19915–19935. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Pereira, H.; Espregueira-Mendes, J.; Kwon, I.K.; Reis, R.L.; Oliveira, J.M. Suturable regenerated silk fibroin scaffold reinforced with 3D-printed polycaprolactone mesh: Biomechanical performance and subcutaneous implantation. J. Mater. Sci. Mater. Med. 2019, 30, 63. [Google Scholar] [CrossRef]
- Evlashin, S.; Dyakonov, P.; Tarkhov, M.; Dagesyan, S.; Rodionov, S.; Shpichka, A.; Kostenko, M.; Konev, S.; Sergeichev, I.; Timashev, P.; et al. Flexible Polycaprolactone and Polycaprolactone/Graphene Scaffolds for Tissue Engineering. Materials 2019, 12, 2991. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Lo, T.S.; Lin, Y.T.; Chien, Y.H.; Lu, C.J.; Liu, S.J. Fabrication of Drug-Eluting Polycaprolactone/poly(lactic-co-glycolic Acid) Prolapse Mats Using Solution-Extrusion 3D Printing and Coaxial Electrospinning Techniques. Polymers 2021, 13, 2295. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, S.; Mostafavi, A.; Ramesh, S.; Memic, A.; Rivero, I.V.; Rao, P.; Tamayol, A. Process-Structure-Quality Relationships of Three-Dimensional Printed Poly(Caprolactone)-Hydroxyapatite Scaffolds. Tissue Eng. Part A 2020, 26, 279–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, M.; Tonda-Turo, C.; Pierantozzi, D.; Ciardelli, G.; Mancuso, E. Towards 3D Multi-Layer Scaffolds for Periodontal Tissue Engineering Applications: Addressing Manufacturing and Architectural Challenges. Polymers 2020, 12, 2233. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.L.; Marsal, E.; Planell, J.A.; Lacroix, D. Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 2009, 30, 6142–6149. [Google Scholar] [CrossRef]
- Ravoor, J.; Thangavel, M.; Elsen, S.R. Comprehensive Review on Design and Manufacturing of Bio-scaffolds for Bone Reconstruction. ACS Appl. Bio Mater. 2021, 4, 8129–8158. [Google Scholar] [CrossRef]
- Zhao, F.; Xiong, Y.; Ito, K.; van Rietbergen, B.; Hofmann, S. Porous Geometry Guided Micro-mechanical Environment Within Scaffolds for Cell Mechanobiology Study in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 736489. [Google Scholar] [CrossRef]
- Siddique, S.H.; Hazell, P.J.; Wang, H.; Escobedo, J.P.; Ameri, A.A.H. Lessons from Nature: 3D Printed Bio-Inspired Porous Structures for Impact Energy Absorption—A Review. Addit. Manuf. 2022, 58, 103051. [Google Scholar] [CrossRef]
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, D.R.; Sobreiro-Almeida, R.; Sol, P.C.; Neves, N.M. Development of non-orthogonal 3D-printed scaffolds to enhance their osteogenic performance. Biomater. Sci. 2018, 6, 1569–1579. [Google Scholar] [CrossRef]
- Hashimi, N.S.; Soman, S.S.; Govindharaj, M.; Vijayavenkataraman, S. 3D Printing of Complex Architected Metamaterial Structures by Simple Material Extrusion for Bone Tissue Engineering. Mater. Today Commun. 2022, 31, 103382. [Google Scholar] [CrossRef]
- Wang, F.; Tankus, E.B.; Santarella, F.; Rohr, N.; Sharma, N.; Märtin, S.; Michalscheck, M.; Maintz, M.; Cao, S.; Thieringer, F.M. Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers 2022, 14, 669. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhao, X. Application of TPMS structure in bone regeneration. Eng. Regen. 2021, 2, 154–162. [Google Scholar] [CrossRef]
- Zhu, H.; Li, M.; Huang, X.; Qi, D.; Nogueira, L.P.; Yuan, X.; Liu, W.; Lei, Z.; Jiang, J.; Dai, H.; et al. 3D printed tricalcium phosphate-bioglass scaffold with gyroid structure enhance bone ingrowth in challenging bone defect treatment. Appl. Mater. Today 2021, 25, 101166. [Google Scholar] [CrossRef]
- Van Hede, D.; Liang, B.; Anania, S.; Barzegari, M.; Verlee, B.; Nolens, G.; Pirson, J.; Geris, L.; Lambert, F. 3D-Printed Synthetic Hydroxyapatite Scaffold with In Silico Optimized Macrostructure Enhances Bone Formation In Vivo. Adv. Funct. Mater. 2022, 32, 2105002. [Google Scholar] [CrossRef]
- Merloz, P.; Maurel, N.; Marchard, D.; Lavaste, F.; Barnole, J.; Faure, C.; Butel, J. Three-dimensional rigidity of the Ilizarov external fixator (original and modified) implanted at the femur. Experimental study and clinical deductions. Rev. Chir. Orthop. Reparatrice L’appareil Mot. 1991, 77, 65–76. [Google Scholar]
- Glatt, V.; Samchukov, M.; Cherkashin, A.; Iobst, C. Reverse Dynamization Accelerates Bone-Healing in a Large-Animal Osteotomy Model. J. Bone Jt. Surg. Am. 2021, 103, 257–263. [Google Scholar] [CrossRef]
- Goreninskii, S.; Stankevich, K.; Bolbasov, E.; Danilenko, N.; Filimonov, V.; Tverdokhlebov, S. Comparison of the Influence of “Solvent/Non-Solvent” Treatment for the Attachment of Signal Molecules on the Structure of Electrospun PCL and PLLA Biodegradable Scaffolds. MATEC Web Conf. 2016, 79, 01025. [Google Scholar] [CrossRef] [Green Version]
- Goreninskii, S.I.; Stankevich, K.S.; Nemoykina, A.L.; Bolbasov, E.N.; Tverdokhlebov, S.I.; Filimonov, V.D. A first method for preparation of biodegradable fibrous scaffolds containing iodine on the fibre surfaces. Bull. Mater. Sci. 2018, 41, 100. [Google Scholar] [CrossRef] [Green Version]
- Goreninskii, S.I.; Guliaev, R.O.; Stankevich, K.S.; Danilenko, N.V.; Bolbasov, E.N.; Golovkin, A.S.; Mishanin, A.I.; Filimonov, V.D.; Tverdokhlebov, S.I. “Solvent/non-solvent” treatment as a method for non-covalent immobilization of gelatin on the surface of poly(l-lactic acid) electrospun scaffolds. Colloids Surf. B Biointerfaces 2019, 177, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. S4), 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carotenuto, F.; Politi, S.; Ul Haq, A.; De Matteis, F.; Tamburri, E.; Terranova, M.L.; Teodori, L.; Pasquo, A.; Di Nardo, P. From Soft to Hard Biomimetic Materials: Tuning Micro/Nano-Architecture of Scaffolds for Tissue Regeneration. Micromachines 2022, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Pires, T.; Gouveia, B.P.; Castro, A.P.; Fernandes, P.R. On the permeability of TPMS scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 110, 103932. [Google Scholar] [CrossRef]
- Yang, S.D.; Lee, H.G.; Kim, J. A phase-field approach for minimizing the area of triply periodic surfaces with volume constraint. Comput. Phys. Commun. 2010, 181, 1037–1046. [Google Scholar] [CrossRef]
- Tripathi, Y.; Shukla, M.; Bhatt, A.D. Implicit-Function-Based Design and Additive Manufacturing of Triply Periodic Minimal Surfaces Scaffolds for Bone Tissue Engineering. J. Mater. Eng. Perform. 2019, 28, 7445–7451. [Google Scholar] [CrossRef]
- Liu, Y.; Yushan, M.; Liu, Z.; Liu, J.; Ma, C.; Yusufu, A. Complications of bone transport technique using the Ilizarov method in the lower extremity: A retrospective analysis of 282 consecutive cases over 10 years. BMC Musculoskelet. Disord. 2020, 21, 354. [Google Scholar] [CrossRef]
- Krappinger, D.; Irenberger, A.; Zegg, M.; Huber, B. Treatment of large posttraumatic tibial bone defects using the Ilizarov method: A subjective outcome assessment. Arch. Orthop. Trauma Surg. 2013, 133, 789–795. [Google Scholar] [CrossRef]
- Hamiti, Y.; Yushan, M.; Lu, C.; Yusufu, A. Reconstruction of massive tibial defect caused by osteomyelitis using induced membrane followed by trifocal bone transport technique: A retrospective study and our experience. BMC Surg. 2021, 21, 419. [Google Scholar] [CrossRef]
- Borzunov, D.Y.; Kolchin, S.N.; Mokhovikov, D.S.; Malkova, T.A. Ilizarov bone transport combined with the Masquelet technique for bone defects of various etiologies (preliminary results). World J. Orthop. 2022, 13, 278–288. [Google Scholar] [CrossRef]
- Khaled, A.; El-Gebaly, O.; El-Rosasy, M. Masquelet-Ilizarov technique for the management of bone loss post debridement of infected tibial nonunion. Int. Orthop. 2022, 46, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Catagni, M.A.; Azzam, W.; Guerreschi, F.; Lovisetti, L.; Poli, P.; Khan, M.S.; Di Giacomo, L.M. Trifocal versus bifocal bone transport in treatment of long segmental tibial bone defects. Bone Jt. J. 2019, 101-B, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, G.; Chen, C.; Chen, Y.; Ren, G. Bone Transport for Treatment of Traumatic Composite Tibial Bone and Soft Tissue Defects: Any Specific Needs besides the Ilizarov Technique? Biomed. Res. Int. 2020, 2020, 2716547. [Google Scholar] [CrossRef] [PubMed]
- Manassero, M.; Viateau, V.; Matthys, R.; Deschepper, M.; Vallefuoco, R.; Bensidhoum, M.; Petite, H. A novel murine femoral segmental critical-sized defect model stabilized by plate osteosynthesis for bone tissue engineering purposes. Tissue Eng. Part C Methods 2013, 19, 271–280. [Google Scholar] [CrossRef]
- Lyu, L.; Yang, S.; Jing, Y.; Zhang, C.; Wang, J. Examining trabecular morphology and chemical composition of peri-scaffold osseointegrated bone. J. Orthop. Surg. Res. 2020, 15, 406. [Google Scholar] [CrossRef]
- Coriaty, N.; Pettibone, K.; Todd, N.; Rush, S.; Carter, R.; Zdenek, C. Titanium Scaffolding: An Innovative Modality for Salvage of Failed First Ray Procedures. J. Foot Ankle Surg. 2018, 57, 593–599. [Google Scholar] [CrossRef]
- Gamieldien, H.; Ferreira, N.; Birkholtz, F.F.; Hilton, T.; Campbell, N.; Laubscher, M. Filling the gap: A series of 3D-printed titanium truss cages for the management of large, lower limb bone defects in a developing country setting. Eur. J. Orthop. Surg. Traumatol. 2022, 33, 497–505. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti-based biomaterials, the ultimate choice for orthopaedic implants. A review. Prag. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Ambrosio, L. Bioactive scaffolds for bone and ligament tissue. Expert Rev. Med. Devices 2007, 4, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Noyama, Y.; Miura, T.; Ishimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone loss and reduced bone quality of the human femur after total hip arthroplasty under stress-shielding effects by titanium-based implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Habibovic, P.; Yuan, H.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Lan Levengood, S.K.; Polak, S.J.; Poellmann, M.J.; Hoelzle, D.J.; Maki, A.J.; Clark, S.G.; Wheeler, M.B.; Wagoner Johnson, A.J. The effect of BMP-2 on micro- and macroscale osteointegration of biphasic calcium phosphate scaffolds with multiscale porosity. Acta Biomater. 2010, 6, 3283–3291. [Google Scholar] [CrossRef]
- Chen, D.; Li, D.; Pan, K.; Gao, S.; Wang, B.; Sun, M.; Zhao, C.; Liu, X.; Li, N. Strength enhancement and modulus modulation in auxetic meta-biomaterials produced by selective laser melting. Acta Biomater. 2022, 153, 596–613. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Van Hede, D.; Plougonven, E.; Nolens, G.; Verlée, B.; De Pauw, M.C.; Lambert, F. In vitro and in vivo biocompatibility of calcium-phosphate scaffolds three-dimensional printed by stereolithography for bone regeneration. J. Biomed. Mater. Res. A 2020, 108, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, Y.; Li, J.; Sheng, J.; Long, S.; Li, Z.; Jiang, B.; Fu, H.; Weng, J.; Wu, J.; et al. Titanium scaffold loaded with strontium and copper double-doped hydroxyapatite can inhibit bacterial growth and enhance osteogenesis. J. Biomater. Appl. 2022, 37, 195–203. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Jiang, L.; Li, Z.; Lee, S.; Liu, C.; Wang, J.; Zhang, J. Recombinant human BMP-2 accelerates the migration of bone marrow mesenchymal stem cells via the CDC42/PAK1/LIMK1 pathway in vitro and in vivo. Biomater. Sci. 2018, 7, 362–372. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef]
- Steib, J.-P.; Bouchaib, J.; Walter, A.; Shuller, S.; Charles, P. Could an osteoinductor result in degeneration of a neurofibroma in NF 1? Eur. Spine J. 2010, 19 (Suppl. S2), S220–S225. [Google Scholar] [CrossRef] [Green Version]
- Zweymuller, K.A. Bony ongrowth on the surface of HA-coated femoral implants: An x-ray analysis. Z Orthop. Unfall. 2012, 150, 27–31. [Google Scholar] [PubMed]
- Popkov, A.V.; Gorbach, E.N.; Kononovich, N.A.; Popkov, D.A.; Tverdokhlebov, S.I.; Shesterikov, E.V. Bioactivity and osteointegration of hydroxyapatite-coated stainless steel and titanium wires used for intramedullary osteosynthesis. Strateg. Trauma Limb Reconstr. 2017, 12, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolbasov, E.N.; Popkov, A.V.; Popkov, D.A.; Gorbach, E.N.; Khlusov, I.A.; Golovkin, A.S.; Sinev, A.; Bouznik, V.M.; Tverdokhlebov, S.I.; Anissimov, Y.G. Osteoinductive composite coatings for flexible intramedullary nails. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Uklejewski, R.; Winiecki, M.; Krawczyk, P.; Tokłowicz, R. Native Osseous CaP Biomineral Coating on a Biomimetic Multi-Spiked Connecting Scaffold Prototype for Cementless Resurfacing Arthroplasty Achieved by Combined Electrochemical Deposition. Materials 2019, 12, 3994. [Google Scholar] [CrossRef] [Green Version]
- Rogala, P.; Uklejewski, R.; Winiecki, M.; Dąbrowski, M.; Gołańczyk, J.; Patalas, A. First Biomimetic Fixation for Resurfacing Arthroplasty: Investigation in Swine of a Prototype Partial Knee Endoprosthesis. Biomed. Res. Int. 2019, 2019, 6952649. [Google Scholar] [CrossRef]
- Laubach, M.; Kobbe, P.; Hutmacher, D.W. Biodegradable interbody cages for lumbar spine fusion: Current concepts and future directions. Biomaterials 2022, 288, 121699. [Google Scholar] [CrossRef]
- Popkov, A.V.; Popkov, D.A.; Kononovich, N.A.; Gorbach, E.N.; Tverdokhlebov, S.I.; Bolbasov, E.N.; Darvin, E.O. Biological activity of the implant for internal fixation. J. Tissue Eng. Regen. Med. 2018, 12, 2248–2255. [Google Scholar] [CrossRef]
- Valarmathi, M.T.; Davis, J.M.; Yost, M.J.; Goodwin, R.L.; Potts, J.D. A three-dimensional model of vasculogenesis. Biomaterials 2009, 30, 1098–1112. [Google Scholar] [CrossRef]
- Siow, R.C.; Mallawaarachchi, C.M.; Weissberg, P.L. Migration of adventitial myofibroblasts following vascular balloon injury: Insights from in vivo gene transfer to rat carotid arteries. Cardiovasc. Res. 2003, 59, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Barrère, F.; van der Valk, C.M.; Dalmeijer, R.A.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. J. Biomed. Mater. Res. A 2003, 66, 779–788. [Google Scholar] [CrossRef]
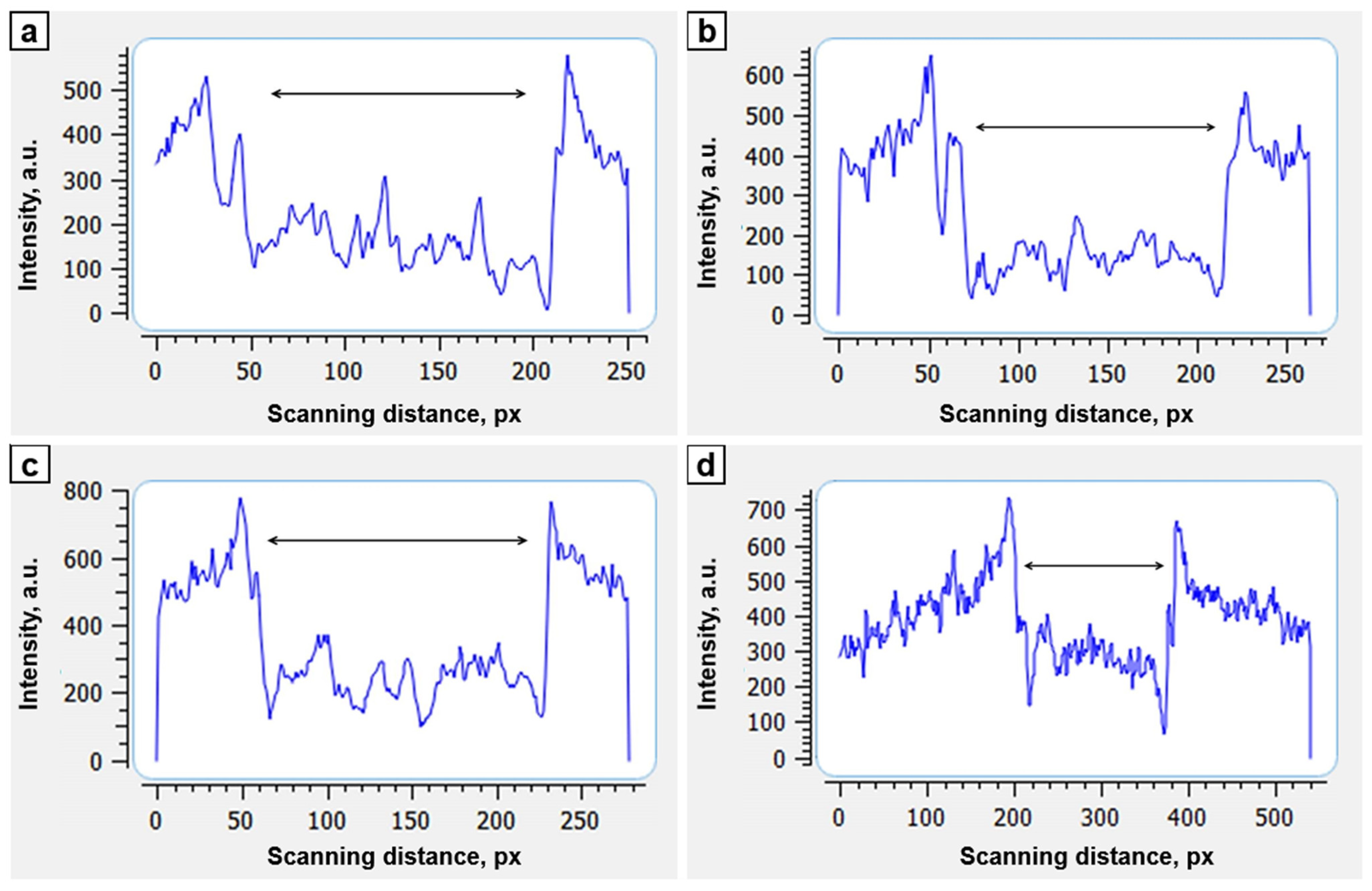

| Period of Study, Days | Average Optical Density of the Bone Defect, a.u. | Optical Density of the Shaft of the Adjacent Distal Shaft End, a.u. | Coefficient of Optical Density (Relative to the Distal Bone Fragment) |
|---|---|---|---|
| D0 (surgery) | 103 ± 10.7 | 382 ± 10.4 | 0.20 |
| 7 | 106 ± 20.4 | 349 ± 17.4 | 0.33 |
| 14 | 149 ± 21.2 | 405 ± 20.1 | 0.38 |
| 21 | 159 ± 23.1 | 307 ± 15.5 | 0.50 |
| 28 | 312 ± 15.9 | 409 ± 20.3 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popkov, A.; Kononovich, N.; Dubinenko, G.; Gorbach, E.; Shastov, A.; Tverdokhlebov, S.; Popkov, D. Long Bone Defect Filling with Bioactive Degradable 3D-Implant: Experimental Study. Biomimetics 2023, 8, 138. https://doi.org/10.3390/biomimetics8020138
Popkov A, Kononovich N, Dubinenko G, Gorbach E, Shastov A, Tverdokhlebov S, Popkov D. Long Bone Defect Filling with Bioactive Degradable 3D-Implant: Experimental Study. Biomimetics. 2023; 8(2):138. https://doi.org/10.3390/biomimetics8020138
Chicago/Turabian StylePopkov, Arnold, Natalia Kononovich, Gleb Dubinenko, Elena Gorbach, Alexander Shastov, Sergei Tverdokhlebov, and Dmitry Popkov. 2023. "Long Bone Defect Filling with Bioactive Degradable 3D-Implant: Experimental Study" Biomimetics 8, no. 2: 138. https://doi.org/10.3390/biomimetics8020138
APA StylePopkov, A., Kononovich, N., Dubinenko, G., Gorbach, E., Shastov, A., Tverdokhlebov, S., & Popkov, D. (2023). Long Bone Defect Filling with Bioactive Degradable 3D-Implant: Experimental Study. Biomimetics, 8(2), 138. https://doi.org/10.3390/biomimetics8020138








