Abstract
The limited regenerative capacity of the human body, in conjunction with a shortage of healthy autologous tissue, has created an urgent need for alternative grafting materials. A potential solution is a tissue-engineered graft, a construct which supports and integrates with host tissue. One of the key challenges in fabricating a tissue-engineered graft is achieving mechanical compatibility with the graft site; a disparity in these properties can shape the behaviour of the surrounding native tissue, contributing to the likelihood of graft failure. The purpose of this review is to examine the means by which researchers have altered the mechanical properties of tissue-engineered constructs via hybrid material usage, multi-layer scaffold designs, and surface modifications. A subset of these studies which has investigated the function of their constructs in vivo is also presented, followed by an examination of various tissue-engineered designs which have been clinically translated.
1. Introduction
The means of restoring mechanical functionality to damaged biological tissues has proven to be a longstanding challenge to medical practitioners. The complexity of the task is largely due to the intricacy of the native tissue, where biomechanical properties are largely dictated by the extracellular matrix (ECM) [1,2]. This structure consists of a multifaceted network of various constituents such as water, polysaccharides, and proteins such as collagen and elastin [3]. When under load, the ECM relies primarily on these proteins working in conjunction with one another for support [4], with the collagen providing mechanical resistance to deformation under increased load conditions, and elastin being responsible for maintaining the elasticity of the tissue and resistance under relatively low loading [5]. Mechanical integrity and elasticity are not only critical for explicit functions such as joint articulation and other musculoskeletal interactions; they are just as significant for various parasympathetic processes such as peristalsis, respiration, and vasodilation [6,7,8]. The degradation of tissue biomechanics, whether via natural processes such as ageing, or via exposure to circumstances leading to internal or external injury of soft and hard tissues, can precede issues ranging from loss of mobility and discomfort, to critical conditions such as pulmonary fibrosis and supravalvular aortic stenosis [9,10,11].
The complexity of repairing extensively damaged tissues is such that contemporary regenerative medicinal practices are limited in treatment options [12]. The transplantation of fresh tissue is seen as the optimal solution, as the new material is theoretically able to fulfil the physiological and mechanical requirements of the original tissue [13]. However, both allografts and autologous grafts bear several limitations. In the case of allografting, graft-versus-host disease, transplant rejection, bleeding, and infection are constant risk factors [14], whereas autologous grafting is not always an option, due to either previous harvesting of the donor site or systemic disease rendering the tissue unsuitable [15]. An alternative therapeutic in promoting the repair of damaged tissues, stem cell treatment, is largely in its infancy as a research area, and faces several significant hurdles, namely, a high risk of immune response, scalability, and overcoming the negative public perception of such a treatment [16,17]. An alternative means of restoring the mechanical properties of biological tissue is therefore necessary in order to address the ever-increasing demand for biological tissue transplantation.
Tissue engineering is a rapidly expanding field which aims to design constructs which supplement or replace damaged biological tissue. The fabrication of a tissue-engineered graft can be undertaken in a variety of ways, including phase separation, bioprinting, gas foaming and electrospinning techniques [18,19,20,21,22,23]. From these methods, a dense interconnected network of material may be formed, which is termed a scaffold [24]. The purpose of a scaffold is to provide a chemically and mechanically appropriate environment to facilitate cellular growth, which may then be implanted into a given patient [25,26,27]. The ideal tissue-engineered scaffold would allow the development and restoration of fully functional tissue at the graft site, maintaining structural integrity under physiological stress, and ultimately be removed by the body’s natural processes after fulfilling its function [28,29,30].
A key challenge within this area is ensuring the greatest possible resemblance between the mechanical response of the tissue-engineered graft and the host’s native tissue. This is a crucial aspect of a successful design, as biological tissues are load-bearing by nature, regardless of function, from the strongest regions of cortical bone with mechanical strength in the gigapascal range [31] to the most delicate of neural fibres throughout the nervous system whose strength generally lies within the low kilopascal scale [32]. The requirement to maintain the physical integrity of the scaffold while also retaining biocompatibility has given rise to so-called ‘biomaterials’, which the majority of tissue-engineered scaffolds are composed of and which fall into several broad camps: natural polymers, synthetic polymers, ceramics, decellularized matrices, hydrogels, and metals [33,34,35,36,37,38]. Natural polymers can include agarose, alginates, and chitosan while synthetic polymers include polycaprolactone, poly(l-lactic acid), and Poly(ethylene glycol) diacrylate. Ceramics can comprise aluminium oxide, bioglass, and hydroxyapatite, while decellurised matrices can be derived from almost any bodily feature, such as bones and organs. Hydrogels are typically composed of naturally derived constituents such as collagen, gelatine and hyaluronic acid, and metals used for scaffolds can include magnesium, tantalum, and titanium [26,36,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. The broad range of characteristics of these materials allows for a vast range of tissue types to be mechanically accounted for; an overview of the typical strength of these material groups in comparison to the stiffness of organic tissues is given in Figure 1 [32,54,55,56,57,58,59,60,61,62,63,64,65,66,67].
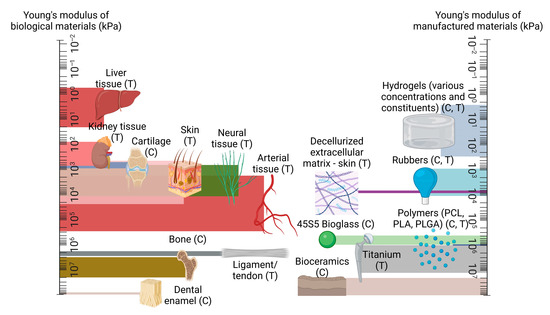
Figure 1.
A comparison of mechanical strength between native tissue types, in comparison to manufactured materials, presented on logarithmic scales, with compression and tension testing denoted with (C) and (T) as appropriate. Created using Biorender.com, 15 May 2023.
One can observe from Figure 1 that particular material types are more suited for certain regions than others; it is clear, for example, that hydrogel alone is an unsuitable replacement for bone tissue. While the consequences of a graft which features an insufficient load-bearing capacity may appear self-evident, the response of surrounding native tissue is often less explicit. For example, a disparity in elastic properties between a tissue-engineered vascular graft and the surrounding native vessel tissue may cause the graft to ineffectively constrict and dilate in tandem with the surrounding vasculature, leading to an increase in smooth muscle cell proliferation, subsequent thrombus formation, and occlusion of the vessel [68,69]. Additionally, when engineered tissue is employed elsewhere in the body, such as for bone or the anterior cruciate ligament, the surrounding tissue will undergo remodelling in accordance with Wolff’s Law and its corollary, Davis’ Law, which state that both osseous and soft tissue, respectively, will structurally adapt themselves in accordance with the mechanical stresses which they encounter [70,71]. This translates to an overcompensation of the native tissue in the case of an insufficiently robust implant, while a construct which exceeds the mechanical requirements of the native tissue can lead to the degradation of the surrounding architecture, with both scenarios compromising the functionality of the original biological tissue [72,73]. It is therefore crucial that the mechanical properties of engineered tissue are characterised in such a way as to best compliment the surrounding biomechanical environment of the native tissue. This does not necessarily require achieving perfect parity between the stiffness of the existing tissue and a given implant; indeed, several of the studies considered in this review suggest that sufficiently mechanically resilient biocompatible materials are perhaps more suitable for the promotion of tissue regeneration than those who endeavour to match the native tissue’s properties in a mechanical context alone. Nevertheless, ensuring a high degree of equivalence between an engineered and a given native tissue’s mechanical properties is a functional requirement of a successful engineered graft.
To address this challenge, tissue engineering researchers are investigating a broad range of solutions to optimise the mechanical response of tissue-engineered grafts, while also maintaining the biocompatibility of the design. The current studies in this field include the use of multiple materials in conjunction with one another, such as polycaprolactone and collagen, bi- and multi-layered scaffolds which often delegate the functional requirements of the construct to each layer, and modifying existing scaffold parameters such that their performance may be tuned to better suit the conditions of the native tissue [74,75,76]. Success in these areas has led to multiple studies in vivo with such designs, and, in some cases, to full clinical translation, whereby the cases of which are examined in Section 7 of this work.
To support the development of these designs, a broad range of literature is available which describes the mechanical properties of specific native tissue types and engineered tissue scaffolds. These include bone, cartilage, liver, kidney, osteochondral tissue, muscle, and tendon, and utilise a broad range of mechanical property assessment methods, including compressive, dynamic, nanoindentation, shear, and tensile testing, with additional methods including finite element analysis (linear and non-linear) and ultrasound [58,77,78,79,80,81,82]. Using these techniques, native tissue properties such as Young’s modulus, yield stress, failure strength and strain, fracture toughness, and viscoelastic behaviour can be assessed. Review articles of this nature are invaluable as resources for the engineering of specific tissue types. However, a comparative examination of a broad variety of tissues, materials, fabrication, and testing methods would offer a unique insight into the effects of parameters and their effect on the mechanical performance of a particular design, which could potentially be applied to other tissue types. A review of this nature, to the best of the authors’ knowledge, has not been presented in the literature before.
This work provides a review of the current techniques by which researchers have successfully modified the mechanical properties of tissue-engineered grafts. The scope of this review will encapsulate all applicable construction methods which are currently employed in this regard, and all tissue types will be considered in accordance with the focus of each study. A Supplementary Table, Table S1, provides a greater deal of insight into the studies considered in this work, with a brief summary of each publication provided therein. The extent of this review is all relevant work published within the years of 2021 to 2023, and it encompasses primary articles reporting on the use of hybrid-, multi-layer-, and surface-modified-type scaffolds, as well as clinically translated designs whose use and outcomes were published within the above chronological range. Articles published prior to these dates were utilised as citations for statements and empirical data where appropriate.
2. Mechanical Properties of Biological Tissues and Their Influence
The mechanical properties of biological materials are a product of a complex array of proteins, cells, and interstitial fluid flow, whose interactions characterise the response of their respective tissue under load [83,84]. As a product of their intricacy, biological tissues exhibit complex mechanical properties such as heterogeneity, viscoelasticity, and anisotropy [83,84,85]. Accurately assessing the mechanical properties of these tissues is therefore intrinsically challenging, with research groups often attaining a variety of results for the same tissue. Table 1 illustrates this, featuring a short collection of various biological tissues, and a generally broad range of respective mechanical properties, encountered by several research groups for the same mode of analysis.

Table 1.
A table of selected tissue types and their mechanical properties.
The diversity in these results can not only be attributed to the structural composition of the tissues alone; factors such as hydration and age are also known to affect the mechanical response of biological tissues [104,105]. This process of tissue-specific cells responding to mechanical stimuli is termed mechanotransduction, which is a means by which organic tissue may convert external loading, such as tension or vibrational waves, to biochemical, biophysical, or molecular signals via a series of events culminating in a cellular response [106]. Figure 2 illustrates this process, beginning with a given external loading event encountered by the body’s mechanosensors. This is termed mechanocoupling, and, depending on the load type, is translated by one or more mechanosensors to adjacent cells in the ECM [107]. Via a complex series of intra-cellular interactions, these mechanical signals are converted to various signal types which instruct the relevant cell groups to behave in a particular way; for example, an increase in mechanical load due to extensive physical activity can lead to compensatory growth in relevant skeletal and muscular members [106,108].
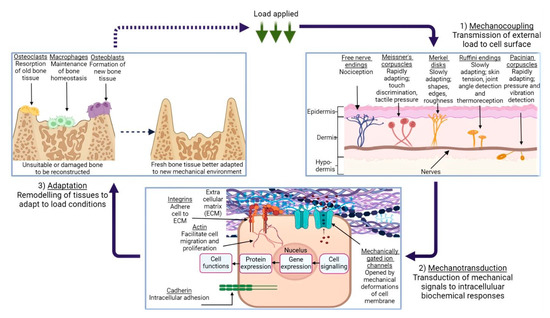
Figure 2.
An illustration of the mechanotransduction cycle, in the context of osseous tissue remodelling in response to external loading applied to a given area of skin, via the medium of a generic cuboidal cell. (1) demonstrates the various types of mechanoreceptors present in human dermis and their function, while (2) depicts the mechanotransduction pathway, followed when the ECM is subject to deformation. (3) shows the remodelling process of bone tissue in response to this loading, and the roles which the various cells play in this process. Created using BioRender.com, 24 April 2023.
It is the cellular response to various conditions which allows the human body to maintain homeostasis, while also quickly adapting to environmental circumstances; vasoconstriction and vasodilation are common examples of this [109]. However, a crucial caveat is that biological tissues have evolved to expect a certain degree of physiological loading. A reduction in the stress and strain forces imparted on, for example, the head region of the femur as a result of a hip implant, can in turn lead to the ‘stress-shielding’ effect [110]. This is where a non-native element acts as the main load-bearing facilitator during typical movement (e.g., walking or running). As a result, the incentive for cells to remodel and strengthen their respective tissues during mechanotransduction is lowered and local bone density is reduced, potentially leading to implant loosening, stress fracturing, and abnormal bone development [111,112]. It follows that other tissue-engineered designs are bound by the same factors; a mechanically under- or over-strengthened graft is not only impractical, but can cause deleterious effects on neighbouring local tissue groups as outlined above. This highlights the importance of mechanical parameters when designing a tissue-engineered graft.
3. Hybrid Materials
The non-linear biomechanical response of a tissue under load is difficult to reproduce in synthetic materials alone, as this response is characterised by complex biological structures and interactions, as described in Section 2. Current research indicates that a hybrid polymer blend, consisting of both synthetic and natural materials, allows for constructs which better represent this intricate behaviour [113]. This is due to the naturally derived constituent typically retaining its microstructural details, which can promote cell attachment and integration; this is beneficial when employing a scaffold in a biological environment [114,115]. A broad range of hybrid polymer combinations are currently undergoing extensive research, ranging from binary PCL-hydroxyapatite designs [29] to complex three- or four-part hybrid amalgamations [116,117]. Table 2 provides an overview of a selection of such work, including the materials and fabrication methods employed during each study, the intended tissue type, and which mechanical properties were assessed throughout the work. As an extension of this, Table 3 explores the Young’s moduli value ranges attained in each of the studies in question, quantifying the effects of these material combinations.

Table 2.
Summarisation of materials, fabrication methods, intended tissue type, and which mechanical properties were altered and assessed during studies which employed a hybrid polymer blend.

Table 3.
An overview of Young’s modulus value ranges attained during studies presented in Table 2.
The scope for hybrid biomaterial design is vast, which allows researchers to consider a broad range of materials. For example, several research groups have recently investigated the inclusion of multi-walled carbon nanotubes (MWCNTs), which are nanometre-diameter carbon-based tubular constructs, with the aim of utilising the nanotubes’ high tensile strength and electrical/thermal conductivity to enhance the overall biomechanical properties of their scaffold. Sang et al. dispersed MWCNTs with concentrations ranging between 1, 3, and 5% through a chitosan/polyethylene glycol scaffold, which was prepared by freeze-drying, for the purpose of neural tissue engineering [127]. The electrical conductivity of neurons is key to their function, and one of the key findings of this analysis was the rise in electrical conductivity of the scaffold in proportion to increasing MWCNT concentrations. Interestingly, the elastic modulus of the hybrid scaffold was also enhanced with an increasing concentration of MWCNTs, from 1.17 kPa to 2.09 kPa, demonstrating the load-bearing abilities of carbon nanotubes. As another example of this, Ramasamy et al. employed MWCNTs for use in a poly(l-lactic acid)–boron nitride piezoelectric nanosheet hybrid, where it was found that the combination of these materials led to a significantly stronger graft when compared to unmodified poly(l-lactic acid) nanofibers quantified by a 666% increase in tensile strength in contrast [128].
Hybrid plant-based designs are also promising in this area; a recent study by Jiawei et al. investigated the inclusion of hydroxyapatite into sugarcane stem, whose lignin had been removed (i.e., delignified) via soaking in a NaClO2/acetate buffer solution. The degree of delignification induced a proportionate increase in the sugarcane stem porosity, albeit at the cost mechanical performance under compressive load, with the greatest extent—8 h of immersion—reducing the compressive modulus of 14.6 MPa for pure sugarcane stem to 4.82 MPa. An optimal soaking time of 4 h was established, which promoted an elongated elastic response from the scaffold under load whilst maintaining structural integrity [129].
4. Multi-Layer Scaffolds
The challenge of tuning the elastic response of a scaffold in tandem with balancing its biocompatibility characteristics has led several research groups to fabricate scaffolds consisting of multiple layers. This approach allows for the functionalisation of each stratum independently and more closely represents the layered nature of most biological tissue, such as arterial walls, osseous tissue, and oesophageal tissue [130,131,132]. The potential impact of various material and fabrication combinations has allowed researchers to explore a broad range of designs consisting of materials such as silk, graphene, and titanium [133,134,135]. Table 4 provides a brief overview of the groups which have investigated the potential of bi- and multi-layered scaffold designs, while Table 5 illustrates the Young’s modulus ranges attained during these studies.

Table 4.
Summarisation of materials per layer, fabrication methods, intended tissue type, and which mechanical properties were altered and assessed during studies which employed a multi-layer scaffold.

Table 5.
An overview of Young’s modulus value ranges attained during studies presented in Table 4.
While the properties of multi-layered scaffolds are largely determined by the material selection, several groups have investigated the potential of applying novel fabrication methods in order to construct a multi-layered construct which can function within the physiological load range. Killian et al. studied the combination of calcium phosphate cement (CPC), fabricated via 3D printing, and PCL, drawn using a melting electrowriting (MEW) process for the purpose of bone tissue engineering [147]. These two materials were printed upon one another up to 10 layers in height, creating a grid-like pattern of CPC which contained multiple interspatial variations, and from which the strands of PCL fibre could intersect. Interestingly, while the addition of the PCL fibres slightly diminished the yield strength and elastic modulus of the scaffolds (which were approximately 4–10 and 40–85 MPa, respectively), the inclusion of these fibres would occasionally cause the construct to mechanically yield twice under load: once for the PCL strands, and again as the CPC failed, offering an element of redundancy to the design. Liu et al. fabricated a bilayered construct by combining electrospinning and 3D printing processes, with the intention of promoting guided bone regeneration [148]. The electrospun layer consisted of a PCL/gelatine membrane, while the 3D-printed scaffold consisted of a PCL/gelatine/HA mixture, which were combined by dissolving the electrospun membrane and adding it to the scaffold. It was found that the compressive strength of the resulting scaffold, at 13.86 MPa, closely resembled that of cancellous bone (up to ~13 MPa [149]). The similarity of these compressive stress values suggests that a greatly diminished stress-shielding effect would be observed in clinical trials, in comparison to existing bone implant devices formed from a Ti-6Al-4V alloy with a Young’s modulus of 110 GPa [150].
In addition, Thompson et al. employed a process known as embedded 3D printing to fabricate a multi-layer scaffold in an effort to replicate human vocal cord tissue [151]. This method utilises a cavity which has pre-printed ink filaments embedded within a given medium, allowing for the fabrication of a component without interference from gravity or requiring additional supports (Figure 3) [152]. For this particular study, various silicone elastomers and thinners were used to construct each layer, and then they were cured via a 700 W microwave. The elastic modulus varied depending on the layer assessed, with the superficial lamina propria (SLP) providing a 0.91 kPa tensile modulus, and the epithelium increasing this value to 39.74 kPa, which lies within the range of human vocal cords, at approximately 30 kPa [153]. The group were also successful in functionalising the construct by designing it to exhibit flow-induced vibration, representing human phonation.
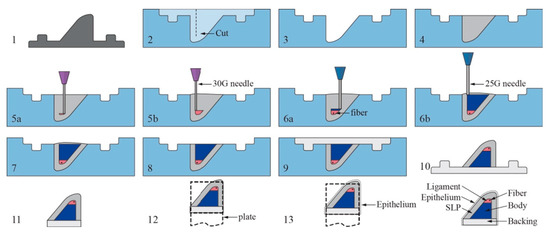
Figure 3.
An illustration of the embedded 3D printing process of the vocal cords. Steps as follows: (1) Create positive mold of SLP layer. (2) Create reservoir with cut for fiber insertion. (3) Coat reservoir with release agent. (4) Fill reservoir with SLP support matrix silicone. (5a,b) Print ligament layer within reservoir, beginning near reservoir bottom, and then insert fiber. (6a,b) Print body layer beginning at interface of ligament layer. (7–8) Remove overflow material and then cure in microwave. (9) Pour backing into remaining cavity and let model cure and cool completely. (10) Remove VF model from reservoir. (11) Trim excess backing material and gently clean model with acetone. (12) Attached model to mounting plate. (13) Pour epithelial layer and cure. Reproduced with the publisher’s permission [152].
5. Surface Modification
The mechanical properties of engineered tissue are not necessarily set in stone. By design, a substance such as a polymeric compound may have its constituents altered such that it may better serve its intended function [154,155]. In a similar manner, a tissue-engineered graft, being typically composed of one or more complex substances, may be modified in such a way that a desired property, such as increased mechanical strength, can be achieved without having to resort to less suitable or more expensive material alternatives, via processes such as annealing or salt leaching [156,157]. Recent studies have considered a broad range of surface modifications, depending on the desired functionality of the design. These range from topographical nano-modification of the scaffold [158] to grafting post-fabrication and polymer coatings [159,160]. Table 6 offers an overview of the materials, fabrication methods, and modification methods employed in recent studies involving surface modification of tissue-engineered grafts. Table 7 expands on this by showing the Young’s modulus value ranges achieved during each of the studies featured in this section.

Table 6.
Summarisation of materials, modification methods, intended tissue type, and which mechanical properties were altered and assessed during studies which utilised surface modification techniques.

Table 7.
An overview of Young’s modulus value ranges attained during studies presented in Table 6.
Surface modification also has the capacity to facilitate the functionalisation of more unlikely material candidates for this research into more suitable substances. An example of this is offered by Mahendiran et al., who examined the potential of cellulose scaffolds derived from Borassus flabellifer for the purpose of bone tissue regeneration [166]. The scaffolds were derived from the plant’s immature endosperm which was then, after washing and oxidising the scaffolds, modified by two organosilanes; amino-terminated aminopropyltriethoxysilane (APTES) and methyl-terminated octadecyltrichlorosilane (OTS). The subsequent scaffolds formed a foamy architecture, and, in comparison to the unmodified constructs employed as a control during the study, both the APTES and OTS treatments enhanced the compressive modulus of the material, from approximately 0.3 MPa to 0.9 MPa and 1.2 MPa, respectively. Crucially, both treatment methods also bore osteoinductive properties, demonstrating the potential of such a design in a clinical context.
Nanotopographical roughness is a feature which may also be employed to modify the properties of a given scaffold. The influence of surface roughness on cell behaviour is well documented, with several groups reporting relative increases in cell proliferation, adhesion, and desired protein expression when seeded on irregular scaffold surface morphologies [167,168,169]. One method of creating roughness on engineered scaffolds is alkaline hydrolysis, which was employed by Meng et al. to alter an existing PLLA design for the purposes of bone tissue engineering [170]. For this particular study, the scaffolds, formed via MEW, were immersed in alkali solutions consisting of concentrations of 0.25 M and 0.5 M sodium hydroxide (NaOH) in ethanol (1:1 ratio) for 1, 2, 3, and 4 h at a time. While the effect of this process was perhaps most notable in terms of cell count, which, after 15 days, was largely increased in comparison to the control scaffold, perhaps the most surprising result was the improved tensile modulus of the 0.5 M NaOH scaffold. After 1, 2, and 3 h periods of immersion, a maximum modulus of 5 GPa was achieved, in comparison to 2.5 GPa of the control scaffold. It was theorised that the alkaline treatment process increased the crystallinity of the individual PLLA fibres, thus providing additional tensile strength for the scaffold overall.
6. In Vitro Limitations and Animal Research
The mechanical and biological performance of a tissue-engineered scaffold can be analysed in a number of ways: mechanically, via the methods described in Section 1 such as compressive, shear, and dynamic testing [61,171,172], while biological viability of the scaffold can be studied in vitro via cell seeding of primary or clonal cell lines, from which parameters such as cell adhesion, differentiation, and viability can be derived [173,174]. While these methods may suggest how such a design would perform in a clinical context, there are several clear limitations to such an evaluation, which exist primarily due to the complexity of physiological in vivo conditions. Mechanically, the human body is in a constant state of flux; internal and external loads are met with biomechanical responses as a product of the mechanotransducive process outlined in Section 2. As an illustration of these mechanical stresses, Figure 4 features an arterial section cutaway diagram, where the biological tissue is subjected to physiological stress conditions as a result of hemodynamic flow.
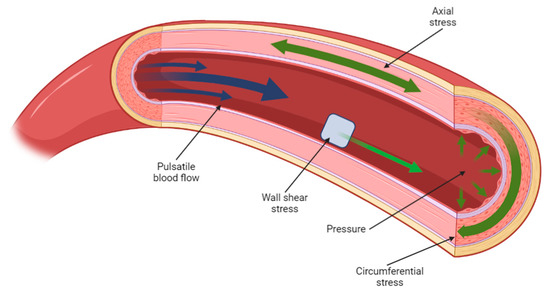
Figure 4.
A cross-sectional diagram of arterial flow, illustrating the various load conditions present at any one time in the cardiovascular system. Created using BioRender.com, 6 March 2023.
A construct designed to operate in such an environment, such as a vascular graft, would be expected to function under these load conditions; mechanical testing is therefore key, prior to the further development of such a design. However, classical mechanical assessment methods such as those outlined earlier in this section, while perhaps indicative of a particular design’s fundamental mechanical properties, are often incapable of evaluating the graft’s performance under true physiological conditions, as these are typically non-linear, heterogeneous loads which are nearly impossible to replicate via contemporary testing equipment [175].
The complex nature of these forces has led scientists to explore several avenues of research in an effort to better comprehend, and design for, their impact on tissue-engineered designs. Recent advances in finite element analysis (FEA) and computational fluid dynamics (CFD) analyses have allowed researchers to model tissues such as pulmonary arteries [176], airways [177], ventricles [178], and eye lenses [179], which carries benefits ranging from a greater understanding of the morphological and physiological characteristics of these tissue types, to assisting surgeons during complex surgical procedures [180,181]. A brief overview of additional studies such as these is given in Table 8.

Table 8.
Synopsis of studies which utilised FEA analyses to model various tissues, including model type and whether the model was based on experimental or simulation data.
Despite the promising outlook of this research, a true estimation of the in vivo performance of a tissue-engineered scaffold is still beyond the grasp of contemporary computational or in vitro methods.
The next logical step, therefore, is to assess the viability of tissue-engineered implants in vivo. This will allow researchers to understand how viable their scaffold’s design is once implanted into biological tissue, and will also validate the use of particular materials and construction methods within living organisms. Table 9 subcategorises the previously described studies thus far into those which assessed the performance of their designs in an in vivo setting, and provides an overview of the study parameters, as well as a brief summary of each respective group’s findings.

Table 9.
Summary of materials and intended tissue type, as well as animal species study length, whether the proposed implant fully integrated into the animal, and a brief synopsis of all studies considered thus far, where animal testing was employed.
The assessment of tissue-engineered designs in vivo can also offer insight into cell behaviour when uncommon scaffold morphologies are implanted within the animal. Feng et al. examined the effects of implanting a conch-like scaffold, featuring a helical inner structure, into the upper femoral region of New Zealand rabbits, with the aim of assessing such a design to promote guided bone regeneration [190]. Formed via 3D printing with β-tricalcium phosphate, various diameters, pitches, and pore sizes of the scaffold were examined, with a maximum compressive modulus of 1.75 GPa achieved with a 9.8 mm scaffold diameter, 1 mm pore diameter, and 2.4 mm pitch. It was found that the distinctive design of the scaffold encouraged a capillary action effect when placed within a cell media solution; this phenomenon was fully demonstrated when the scaffold was assessed in vivo, as the cells rapidly proliferated up the helical section, illustrating the directional osteoinductive benefits of such a scaffold morphology.
Despite developments in this area, in vivo testing in animals, especially in the current exploratory phase of tissue engineering research, suffers from a widely variable animal-to-human translational success rate [191]. This is primarily due to differing physiology between animals and humans, which also extends to genetics and epigenetics, as well as the low reproducibility of such experiments and lack of use of prospective systematic reviews [191,192]. As a potential solution to this, a rapidly emerging technology termed organ-on-chip removes the in vivo aspect of organic tissue development by providing a biomimetic substrate upon which living tissue can develop. This tissue can progress to function as an organoid, with models such as liver- and kidney-on-chip providing valuable insights as to how such tissue may be fully vascularised and function prior to use in vivo [193,194]. A brief overview of which organ-on-chip models are currently in use is provided in Table 10.

Table 10.
An overview of which organ-on-chip models are currently in use, illustrating from this subset which of these studies have considered the mechanical properties of the tissue in question.
Organ-on-chip technology bears significant promise, as it may provide a more agile and less ethically challenging means of assessing tissue-engineered constructs. However, one can observe from Table 10 that consideration of the mechanical properties of the engineered tissue examined in these designs is often not assessed in these designs.
7. Clinical Translation, Challenges, and Future Outlook
The use of synthetic designs to supplant existing damaged native tissue is a long-established practice, with the earliest total hip arthroplasty, made with ivory, being recorded in Germany in 1891 [210]. Scientific advancement since then has led to several crucial developments in the field of tissue engineering, such as the creation of various polymers, the advents of 3D printing and electrospinning, and a broader understanding of the biomechanical underpinnings of the human body. However, as illustrated in this article thus far, tissue engineering is still a developing field. Further progression in key areas such as the mechanical characteristics, biocompatibility, and fabrication methods involved in proposed designs are required before the use of tissue-engineered constructs in clinical settings is likely to become commonplace.
A further hurdle is that tissue-engineered designs are subject to various regulatory frameworks depending on the jurisdiction in which the design is to be marketed and sold within. While several jurisdictions in the European Union, United States of America, Canada, Australia, Japan, and South Korea have various classifications for tissue-based products, many others do not [211]; difficulties in introducing or adapting legislation for such complex products will likely involve protracted legal processes, potentially limiting the use of tissue-engineered designs within these regions for a significant period.
Regardless, progress in this field is clear, with several novel tissue-engineered implants having recently been successfully trialled. Table 11 provides an overview of these trials, including the material type, fabrication method, intended tissue type or region, the procedure method and study length, total study size, and a brief summary of the results of each trial.

Table 11.
Summary of clinical trials with results published within the last two years, including material type, fabrication method and intended tissue type, procedure undergone by patient(s), length of subsequent study, post-operative outcome of patient(s), and a brief summary of each study.
A clear trend from Table 11 is that, by and large, 3D printing techniques have been the dominant fabrication method for the fabrication of clinically translated implants thus far. The logic for this is relatively self-evident: human soft tissue and cardiovascular and musculoskeletal systems are unique as a result of age, health, and genetics; injuries or pathologies pertaining to these systems increase this distinctiveness further, often requiring an equally bespoke treatment method that a technique such as additive manufacturing is readily able to provide. Additionally, the short lead times associated with 3D printing as a result of rapid prototyping, manufacturing, and delivery times are a boon to often time-sensitive clinical circumstances [224].
Whilst the continuous utilisation of 3D printing technology as the basis for the clinical translation of tissue-engineered designs remains to be seen, alternative fabrication methods such as electrospun grafts, hydrogel systems, and bioprinting continue to be developed in parallel, bringing tissue-engineered constructs ever closer to clinical and commercial viability.
8. Conclusions
The limitations of contemporary surgical and therapeutic techniques to restore the original functionality of damaged organic tissue have driven the development of various tissue-engineered platforms, which aim to support and encourage the regeneration of healthy autologous tissue. One of the key challenges in this area is establishing a high degree of similarity in the mechanical properties between the engineered scaffold and the surrounding biological tissue. This is due to the typical inability of manufactured materials to replicate the complex biomechanics of organic tissue. This capability, however, is crucial for a successful tissue-engineered design.
In response to this issue, current tissue engineering research is examining a broad spectrum of potential designs aimed at enhancing the mechanical and biological compatibility of tissue-engineered constructs. From the studies described in this work, it is clear that relatively well-documented fabrication methods such as electrospinning and 3D printing can produce designs with, in some cases, a high degree of mechanical equivalence to native tissues. Techniques such as bioprinting and MEW, while promising, will likely require further validation before widespread use is achieved. In terms of materials, the usage of particular polymers such as PCL and PLA, ceramics such as HA and β-TCP, and hydrogels consisting of gelatine and alginate have seen widespread use, while the effects of more novel materials such as CNTs and quantum dots are yet to be fully examined in a tissue engineering context.
A notable observation from these studies is that accounting for the complex range of mechanical properties present in biological tissues is generally beyond the remit of a single-material-based design. Rather, a multifaceted approach consisting of multiple material types, layers, or surface treatment methods working in tandem is likely to yield the most successful designs. In terms of translation to clinical use, the time-sensitive nature of surgical treatment suggests that 3D printing, as a fabrication method, is a strong contender for the successful realisation of these designs. Regardless of approach, it is clear that with further development, tissue engineering is set to act as a transformative treatment method.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomimetics8020205/s1: Table S1: A synopsis of each study described within this article, including the authors and publication year, material and tissue type, and testing and mechanical property information, as well as a brief summary, where appropriate, of each research paper reported thus far [29,64,113,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,152,156,157,159,160,161,162,163,164,165,166,170,190].
Author Contributions
Conceptualization, A.J. and A.C.; methodology, A.J. and A.C.; software, A.J.; validation, A.J. and A.C.; formal analysis, A.J.; investigation, A.J.; resources, A.J.; data curation, A.J.; writing—original draft preparation, A.J.; writing—review and editing, A.J. and A.C.; visualization, A.J.; supervision, A.C.; project administration, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Engineering and Physical Sciences Research Council (EPSRC), grant number EP/W524384/1.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarasa-Renedo, A.; Chiquet, M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports 2005, 15, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, M.; Cox, T.R. Extracellular Matrix (ECM). In Encyclopedia of Molecular Pharmacology; Springer: Cham, Switzerland, 2022; pp. 643–650. [Google Scholar]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23 (Suppl. 1), S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.-J.; Turcotte, R.; Lin, C.P.; Zhang, Y. Arterial Extracellular Matrix: A Mechanobiological Study of the Contributions and Interactions of Elastin and Collagen. Biophys. J. 2014, 106, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Sinnott, M.D.; Cleary, P.W.; Harrison, S.M. Peristaltic transport of a particulate suspension in the small intestine. Appl. Math. Model. 2017, 44, 143–159. [Google Scholar] [CrossRef]
- Mecham, R. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef]
- Faury, G.; Ristori, M.; Verdetti, J.; Jacob, M.; Robert, L. Effect of Elastin Peptides on Vascular Tone. J. Vasc. Res. 1995, 32, 112–119. [Google Scholar] [CrossRef]
- Godinho, M.S.C.; Thorpe, C.T.; Greenwald, S.E.; Screen, H.R.C. Elastin is Localised to the Interfascicular Matrix of Energy Storing Tendons and Becomes Increasingly Disorganised with Ageing. Sci. Rep. 2017, 7, 9713. [Google Scholar] [CrossRef]
- De Brouwer, B.; Drent, M.; van den Ouweland, J.M.; Wijnen, P.A.; van Moorsel, C.H.; Bekers, O.; Grutters, J.C.; White, E.S.; Janssen, R. Increased circulating desmosine and age-dependent elastinolysis in idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 45. [Google Scholar] [CrossRef]
- Merla, G.; Brunetti-Pierri, N.; Piccolo, P.; Micale, L.; Loviglio, M.N. Supravalvular aortic stenosis: Elastin arteriopathy. Circ. Cardiovasc. Genet. 2012, 5, 692–696. [Google Scholar] [CrossRef]
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef] [PubMed]
- Rouchi, A.H.; Mahdavi-Mazdeh, M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int. J. Organ Transplant. Med. 2015, 6, 93–98. [Google Scholar]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Tonk, G.; Yadav, P.K.; Agarwal, S.; Jamoh, K. Donor site morbidity in autologous bone grafting—A comparison between different techniques of anterior iliac crest bone harvesting: A prospective study. J. Orthop. Trauma Rehabil. 2022, 29, 22104917221092163. [Google Scholar] [CrossRef]
- Batten, P.; Rosenthal, N.; Yacoub, M.H. Immune response to stem cells and strategies to induce tolerance. Philos. Trans. R. Soc. B: Biol. Sci. 2007, 362, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.; Nixon, I.J.; Emmerson, E.; Callanan, A. From hormone replacement therapy to regenerative scaffolds: A review of current and novel primary hypothyroidism therapeutics. Front. Endocrinol. 2022, 13, 2392. [Google Scholar] [CrossRef]
- Handley, E.; Callanan, A. Modulation of Tissue Microenvironment following Myocardial Infarction. Adv. NanoBiomed Res. 2022, 2, 2200005. [Google Scholar] [CrossRef]
- Sturtivant, A.; Callanan, A. The use of antifreeze proteins to modify pore structure in directionally frozen alginate sponges for cartilage tissue engineering. Biomed. Phys. Eng. Express 2020, 6, 055016. [Google Scholar] [CrossRef]
- Sofokleous, P.; Chin, M.H.; Day, R. Phase-separation technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds—Materials, Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 101–126. [Google Scholar]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef]
- Costantini, M.; Barbetta, A. Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Woodhead Publishing: Sawston, UK, 2018; pp. 127–149. [Google Scholar]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue Engineered Scaffolds in Regenerative Medicine. World J. Plast. Surg. 2014, 3, 3–7. [Google Scholar] [PubMed]
- Qiu, Y.; Chen, X.; Hou, Y.; Hou, Y.; Tian, S.; Chen, Y.; Yu, L.; Nie, M.; Liu, X. Characterization of different biodegradable scaffolds in tissue engineering. Mol. Med. Rep. 2019, 19, 4043–4056. [Google Scholar] [CrossRef] [PubMed]
- Rezania, N.; Asadi-Eydivand, M.; Abolfathi, N.; Bonakdar, S.; Mehrjoo, M.; Solati-Hashjin, M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J. Mater. Sci. Mater. Med. 2022, 33, 31. [Google Scholar] [CrossRef]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. Npj Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kim, Y.-C.; Min, J.-C.; Park, H.-J.; Lee, E.-J.; Shim, J.-H.; Choi, J.-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740. [Google Scholar] [CrossRef]
- Borschel, G.H.; Kia, K.F.; Kuzon, W.M.; Dennis, R.G. Mechanical properties of acellular peripheral nerve. J. Surg. Res. 2003, 114, 133–139. [Google Scholar] [CrossRef]
- Tomlins, P. 1—Material Types for Tissue Scaffolds, in Characterisation and Design of Tissue Scaffolds; Tomlins, P., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 1–21. [Google Scholar]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2021, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv. Healthc. Mater. 2023, 12, 2202766. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2020, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, D.; Wu, D.; Cui, Y.; Ren, G.; Wang, Y.; Wang, J.; Peng, C. Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 755. [Google Scholar] [CrossRef]
- Gao, Y.; Callanan, A. Influence of surface topography on PCL electrospun scaffolds for liver tissue engineering. J. Mater. Chem. B 2021, 9, 8081–8093. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, T.; Shao, M.; Lyu, F. Recent advances in PLLA-based biomaterial scaffolds for neural tissue engineering: Fabrication, modification, and applications. Front. Bioeng. Biotechnol. 2022, 10, 1011783. [Google Scholar] [CrossRef]
- Kotturi, H.; Abuabed, A.; Zafar, H.; Sawyer, E.; Pallipparambil, B.; Jamadagni, H.; Khandaker, M. Evaluation of Polyethylene Glycol Diacrylate-Polycaprolactone Scaffolds for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 39. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 61378. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Amirazad, H.; Dadashpour, M.; Zarghami, N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 2022, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Sarrigiannidis, S.O.; Rey, J.; Dobre, O.; González-García, C.; Dalby, M.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar]
- Gao, H.; Yang, J.; Jin, X.; Qu, X.; Zhang, F.; Zhang, D.; Chen, H.; Wei, H.; Zhang, S.; Jia, W.; et al. Porous tantalum scaffolds: Fabrication, structure, properties, and orthopedic applications. Mater. Des. 2021, 210, 110095. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Sprague, A.L.; Awokuse, D.; Pohlig, R.T.; Cortes, D.H.; Silbernagel, K.G. Relationship between mechanical properties (shear modulus and viscosity), age, and sex in uninjured Achilles tendons. Transl. Sports Med. 2020, 3, 321–327. [Google Scholar] [CrossRef]
- LaCroix, A.S.; Duenwald-Kuehl, S.E.; Lakes, R.S.; Vanderby, R. Relationship between tendon stiffness and failure: A metaanalysis. J. Appl. Physiol. 2013, 115, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Maganaris, C.N.; Paul, J.P. In vivo human tendon mechanical properties. J. Physiol. 1999, 521 Pt 1, 307–313. [Google Scholar] [CrossRef]
- Urban, M.W.; Rule, A.D.; Atwell, T.D.; Chen, S. Novel Uses of Ultrasound to Assess Kidney Mechanical Properties. Kidney360 2021, 2, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Shojaei, A. Measurement of the Mechanical Properties of the Human Kidney. IRBM 2017, 38, 292–297. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Wang, M.; Dahl, J.; Frinkley, K.; Nightingale, K. Quantifying Hepatic Shear Modulus In Vivo Using Acoustic Radiation Force. Ultrasound Med. Biol. 2008, 34, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Santago, A.C.; Stitzel, J.D.; Sparks, J.L.; Duma, S.M. Biomechanical response of human liver in tensile loading. Ann. Adv. Automot. Med. 2010, 54, 15–26. [Google Scholar]
- Yeh, W.-C.; Li, P.-C.; Jeng, Y.-M.; Hsu, H.-C.; Kuo, P.-L.; Li, M.-L.; Yang, P.-M.; Lee, P.H. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med. Biol. 2002, 28, 467–474. [Google Scholar] [CrossRef]
- Arikawa, H. Dynamic Shear Modulus in Torsion of Human Dentin and Enamel. Dent. Mater. J. 1989, 8, 223–235,287. [Google Scholar] [CrossRef]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef]
- Aghajanian, A.H.; Bigham, A.; Sanati, A.; Kefayat, A.; Salamat, M.R.; Sattary, M.; Rafienia, M. A 3D macroporous and magnetic Mg2SiO4-CuFe2O4 scaffold for bone tissue regeneration: Surface modification, in vitro and in vivo studies. Biomater. Adv. 2022, 137, 212809. [Google Scholar]
- Park, S.; Tao, J.; Sun, L.; Fan, C.-M.; Chen, Y. An Economic, Modular, and Portable Skin Viscoelasticity Measurement Device for In Situ Longitudinal Studies. Molecules 2019, 24, 907. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, R.; Attik, N.; Chevalier, C.; Salles, V.; Grosgogeat, B.; Gritsch, K.; Trunfio-Sfarghiu, A.-M. 3D Electrospun Polycaprolactone Scaffolds to Assess Human Periodontal Ligament Cells Mechanobiological Behaviour. Biomimetics 2023, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Diaz-Rodriguez, P.; Balouch, B.; Paulsen, S.; Wu, S.; Miller, J.; Hahn, M.; Cosgriff-Hernandez, E. Elucidating the role of graft compliance mismatch on intimal hyperplasia using an ex vivo organ culture model. Acta Biomater. 2019, 89, 84–94. [Google Scholar] [CrossRef]
- Cao, T.; Jiang, Z.; Zhao, H.; Zhang, K.-Q.; Meng, K. Numerical simulation to study the impact of compliance mismatch between artificial and host blood vessel on hemodynamics. Med. Nov. Technol. Devices 2022, 15, 100152. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wluka, A.E.; Wijethilake, P.; Wang, Y.; Ghasem-Zadeh, A.; Cicuttini, F.M. Wolff’s law in action: A mechanism for early knee osteoarthritis. Thromb. Haemost. 2015, 17, 207. [Google Scholar] [CrossRef]
- Ambrosi, D.; Ben Amar, M.; Cyron, C.J.; DeSimone, A.; Goriely, A.; Humphrey, J.D.; Kuhl, E. Growth and remodelling of living tissues: Perspectives, challenges and opportunities. J. R. Soc. Interface 2019, 16, 2019023. [Google Scholar] [CrossRef]
- Frost, H.M. A 2003 Update of Bone Physiology and Wolff’s Law for Clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar]
- Raffa, M.L.; Nguyen, V.H.; Hernigou, P.; Flouzat-Lachaniette, C.H.; Haiat, G. Stress shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ratio. J. Orthop. Res. 2021, 39, 1174–1183. [Google Scholar] [CrossRef]
- Munir, N.; Callanan, A. Novel phase separated polycaprolactone/collagen scaffolds for cartilage tissue engineering. Biomed. Mater. 2018, 13, 051001. [Google Scholar] [CrossRef]
- Garrison, C.M.; Singh-Varma, A.; Pastino, A.K.; Steele, J.A.; Kohn, J.; Murthy, N.S.; Schwarzbauer, J.E. A multilayered scaffold for regeneration of smooth muscle and connective tissue layers. J. Biomed. Mater. Res. Part A 2021, 109, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E.; Kamalvand, M.; Avani, F. Recent advances in surface modification of biopolymeric nanofibrous scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 493–512. [Google Scholar] [CrossRef]
- Bailey, S.; Vashishth, D. Mechanical Characterization of Bone: State of the Art in Experimental Approaches-What Types of Experiments Do People Do and How Does One Interpret the Results? Curr. Osteoporos. Rep. 2018, 16, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Fan, S.; Zhou, G.; Ma, K.; Yao, X.; Zhang, Y. Effects of dynamic mechanical stimulations on the regeneration of in vitro and in vivo cartilage tissue based on silk fibroin scaffold. Compos. Part B Eng. 2022, 235, 109764. [Google Scholar] [CrossRef]
- Yang, Y.; Li, K.; Sommer, G.; Yung, K.-L.; A Holzapfel, G. Mechanical characterization of porcine liver properties for computational simulation of indentation on cancerous tissue. Math. Med. Biol. J. IMA 2020, 37, 469–490. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Rayudu, N.M.; Subburaj, K.; Mei, K.; Dieckmeyer, M.; Kirschke, J.S.; Noël, P.B.; Baum, T. Finite Element Analysis-Based Vertebral Bone Strength Prediction Using MDCT Data: How Low Can We Go? Front. Endocrinol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Kurtaliaj, I.; Golman, M.; Abraham, A.C.; Thomopoulos, S. Biomechanical Testing of Murine Tendons. J. Vis. Exp. 2019, 152, e60280. [Google Scholar]
- Gough, A.; Stern, A.M.; Maier, J.; Lezon, T.; Shun, T.-Y.; Chennubhotla, C.; Schurdak, M.E.; Haney, S.A.; Taylor, D.L. Biologically Relevant Heterogeneity: Metrics and Practical Insights. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 213–237. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, M.; Thomas, E.; Hopyan, S.; Sun, Y. Existing and Potential Applications of Elastography for Measuring the Viscoelasticity of Biological Tissues In Vivo. Front. Phys. 2021, 9, 670571. [Google Scholar] [CrossRef]
- Mitchell, G.R.; Tojeira, A. Role of Anisotropy in Tissue Engineering. Procedia Eng. 2013, 59, 117–125. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M.; Shojaei, A.; Faghihi, S. Measurement of the uniaxial mechanical properties of healthy and atherosclerotic human coronary arteries. Mater. Sci. Eng. C 2013, 33, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Claes, E.; Atienza, J.; Guinea, G.; Rojo, F.; Bernal, J.; Revuelta, J.; Elices, M. Mechanical properties of human coronary arteries. 2010, 2010, 3792–3795. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3792–3795. [Google Scholar]
- A Vorp, D.; Schiro, B.J.; Ehrlich, M.P.; Juvonen, T.S.; Ergin, M.; Griffith, B.P. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann. Thorac. Surg. 2003, 75, 1210–1214. [Google Scholar] [CrossRef]
- Gijsen, F.J.H.; Wentzel, J.J.; Thury, A.; Mastik, F.; Schaar, J.A.; Schuurbiers, J.C.H.; Slager, C.J.; Van Der Giessen, W.J.; De Feyter, P.J.; Van Der Steen, A.F.W.; et al. Strain distribution over plaques in human coronary arteries relates to shear stress. Am. J. Physiol. Circ. Physiol. 2008, 295, H1608–H1614. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, J.; Zhao, J.; Gregersen, H.; Kassab, G.S. Shear modulus of porcine coronary artery: Contributions of media and adventitia. Am. J. Physiol. Circ. Physiol. 2003, 285, H1966–H1975. [Google Scholar] [CrossRef] [PubMed]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Chizhik, S.A.; Wierzcholski, K.; Trushko, A.; Zhytkova, M.A.; Miszczak, A. Properties of Cartilage on Micro- and Nanolevel. Adv. Tribol. 2010, 2010, 8. [Google Scholar] [CrossRef]
- Wang, S.; Bao, Y.; Guan, Y.; Zhang, C.; Liu, H.; Yang, X.; Gao, L.; Guo, T.; Chen, Q. Strain distribution of repaired articular cartilage defects by tissue engineering under compression loading. J. Orthop. Surg. Res. 2018, 13, 19. [Google Scholar] [CrossRef]
- Wong, B.L.; Bae, W.C.; Chun, J.; Gratz, K.R.; Lotz, M.; Robert, L.S. Biomechanics of Cartilage Articulation. Arthritis Rheum. 2008, 58, 2065–2074. [Google Scholar]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Ma, Z.; Qiang, Z.; Zhao, H.; Piao, H.; Ren, L. Mechanical properties of cortical bones related to temperature and orientation of Haversian canals. Mater. Res. Express 2020, 7, 015408. [Google Scholar] [CrossRef]
- Spatz, H.C.; Vincent, J.F. Young’s moduli and shear moduli in cortical bone. Proc. R. Soc. Lond. B 1996, 263, 287–294. [Google Scholar]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Adv. Dermatol. Allergol. 2013, 30, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Ottenio, M.; Tran, D.; Annaidh, A.N.; Gilchrist, M.D.; Bruyère, K. Strain rate and anisotropy effects on the tensile failure characteristics of human skin. J. Mech. Behav. Biomed. Mater. 2015, 41, 241–250. [Google Scholar] [CrossRef]
- Kwan, M.K.; Wall, E.J.; Massie, J.; Garfin, S.R. Strain, stress and stretch of peripheral nerve Rabbit experiments in vitro and in vivo. Acta Orthop. 1992, 63, 267–272. [Google Scholar] [CrossRef]
- Nicholson, K.J.; Winkelstein, B.A. Nerve and Nerve Root Biomechanics. Neural Tissue Biomech. 2010, 3, 203–229. [Google Scholar]
- Singh, A.; Magee, R. Mechanical Properties of Cervical Spinal Cord in Neonatal Piglet: In Vitro. Neurol. Neurobiol. 2020, 2, 3. [Google Scholar] [CrossRef]
- Durand, S.; Raffoul, W.; Christen, T.; Pedrazzi, N. Post-Operative Assessment of Ulnar Nerve Tension Using Shear-Wave Elastography. Neurol. Int. 2021, 13, 469–476. [Google Scholar] [CrossRef]
- Nicolle, S.; Palierne, J.-F. Dehydration effect on the mechanical behaviour of biological soft tissues: Observations on kidney tissues. J. Mech. Behav. Biomed. Mater. 2010, 3, 630–635. [Google Scholar] [CrossRef]
- Phillip, J.M.; Aifuwa, I.; Walston, J.; Wirtz, D. The Mechanobiology of Aging. Annu. Rev. Biomed. Eng. 2016, 17, 113–141. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Smith, J.; Fabry, B.; Goldmann, W.H. Mechano-Coupling and Regulation of Contractility by the Vinculin Tail Domain. Biophys. J. 2008, 94, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Scott, A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br. J. Sports Med. 2009, 43, 247–252. [Google Scholar] [CrossRef]
- Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010, 109, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Yamako, G.; Janssen, D.; Hanada, S.; Anijs, T.; Ochiai, K.; Totoribe, K.; Chosa, E.; Verdonschot, N. Improving stress shielding following total hip arthroplasty by using a femoral stem made of β type Ti-33.6Nb-4Sn with a Young’s modulus gradation. J. Biomech. 2017, 63, 135–143. [Google Scholar] [CrossRef]
- Be’Ery-Lipperman, M.; Gefen, A. A method of quantification of stress shielding in the proximal femur using hierarchical computational modeling. Comput. Methods Biomech. Biomed. Eng. 2007, 9, 35–44. [Google Scholar] [CrossRef]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2018, 13, 189–201. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, H.; Lv, J.; Fan, M.; Wang, X.; Wang, X.; Liang, Y.; Mao, L.; Zhao, Z. The Biocompatibility of Multi-Source Stem Cells and Gelatin-Carboxymethyl Chitosan-Sodium Alginate Hybrid Biomaterials. Tissue Eng. Regen. Med. 2022, 19, 491–503. [Google Scholar] [CrossRef]
- Nokhbatolfoghahaei, H.; Paknejad, Z.; Bohlouli, M.; Rad, M.R.; Aminishakib, P.; Derakhshan, S.; Amirabad, L.M.; Nadjmi, N.; Khojasteh, A. Fabrication of Decellularized Engineered Extracellular Matrix through Bioreactor-Based Environment for Bone Tissue Engineering. ACS Omega 2020, 5, 31943–31956. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hu, C.C.; Sakthivel, R.; Nabilla, S.C.; Huang, Y.W.; Yu, J.; Cheng, N.C.; Kuo, Y.J.; Chung, R.J. Preparation of gamma poly-glutamic acid/hydroxyapatite/collagen composite as the 3D-printing scaffold for bone tissue engineering. Biomater. Res. 2022, 26, 21. [Google Scholar] [CrossRef]
- Singh, B.N.; Nallakumarasamy, A.; Sinha, S.; Rastogi, A.; Mallick, S.P.; Divakar, S.; Srivastava, P. Generation of hybrid tissue engineered construct through embedding autologous chondrocyte loaded platelet rich plasma/alginate based hydrogel in porous scaffold for cartilage regeneration. Int. J. Biol. Macromol. 2022, 203, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kim, D.; Jang, C.H.; Kim, G.H. Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering. Theranostics 2022, 12, 4051–4066. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, B.; Li, H.; Feng, G.; Pan, S.; Chen, Z.; Li, B.; Song, J. Biomimetic Mineralized Hydroxyapatite Nanofiber-Incorporated Methacrylated Gelatin Hydrogel with Improved Mechanical and Osteoinductive Performances for Bone Regeneration. Int. J. Nanomed. 2022, 17, 1511–1529. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, S.; Baheiraei, N.; Salehnia, M. Fabrication and characterization of PHEMA–gelatin scaffold enriched with graphene oxide for bone tissue engineering. J. Orthop. Surg. Res. 2022, 17, 216. [Google Scholar] [CrossRef]
- Min, Q.; Tian, D.; Zhang, Y.; Wang, C.; Wan, Y.; Wu, J. Strong and Elastic Chitosan/Silk Fibroin Hydrogels Incorporated with Growth-Factor-Loaded Microspheres for Cartilage Tissue Engineering. Biomimetics 2022, 7, 41. [Google Scholar] [CrossRef]
- Dorishetty, P.; Balu, R.; Gelmi, A.; Mata, J.P.; Quigley, A.; Dutta, N.K.; Choudhury, N.R. Microporosity engineered printable silk/graphene hydrogels and their cytocompatibility evaluations. Mater. Today Adv. 2022, 14, 100233. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Xiong, X.; Cui, R.; Zhang, G.; Wang, C.; Xiao, D.; Qu, S.; Weng, J. Hybridizing gellan/alginate and thixotropic magnesium phosphate-based hydrogel scaffolds for enhanced osteochondral repair. Mater. Today Bio 2022, 14, 100261. [Google Scholar] [CrossRef]
- Baskapan, B.; Callanan, A. Electrospinning Fabrication Methods to Incorporate Laminin in Polycaprolactone for Kidney Tissue Engineering. Tissue Eng. Regen. Med. 2022, 19, 73–82. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, X.; Dai, X. Properties of Electrospun Aligned Poly(lactic acid)/Collagen Fibers With Nanoporous Surface for Peripheral Nerve Tissue Engineering. Macromol. Mater. Eng. 2022, 307, 2200256. [Google Scholar] [CrossRef]
- Salehi, R.; Mohammadzadeh, L.; Mahkam, M.; Jafarizad, A.; Rahbarghazi, R. Electrospun gelatin/methylcellulose hybrid nanofibers promoted the maturation of human cutaneous tissue progenitor cells toward keratinocyte-like cells. Cellulose 2022, 29, 7837–7848. [Google Scholar] [CrossRef]
- Li, P.; Ruan, L.; Wang, R.; Liu, T.; Song, G.; Gao, X.; Jiang, G.; Liu, X. Electrospun Scaffold of Collagen and Polycaprolactone Containing ZnO Quantum Dots for Skin Wound Regeneration. J. Bionic Eng. 2021, 18, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Cheng, R.; Cao, Y.; Yan, Y.; Shen, Z.; Zhao, Y.; Han, Y. Biocompatible chitosan/polyethylene glycol/multi-walled carbon nanotube composite scaffolds for neural tissue engineering. J. Zhejiang Univ. B 2022, 23, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.S.; Kaliannagounder, V.K.; Rahaman, A.; Park, C.H.; Kim, C.S.; Kim, B. Synergistic Effect of Reinforced Multiwalled Carbon Nanotubes and Boron Nitride Nanosheet-Based Hybrid Piezoelectric PLLA Scaffold for Efficient Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 3542–3556. [Google Scholar] [CrossRef] [PubMed]
- Jiawei, S.; Xuemeng, B.; Yahui, Z.; Jianfei, C.; Yinghong, X. Sugarcane Stem Derived Hybrid Scaffold for Bone Tissue Engineering via Top-down Approach. Compos. Interfaces 2022, 30, 323–340. [Google Scholar] [CrossRef]
- Karšaj, I.; Humphrey, J.D. A multilayered wall model of arterial growth and remodeling. Mech. Mater. 2013, 44, 110–119. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Totonelli, G.; Maghsoudlou, P.; Fishman, J.M.; Orlando, G.; Ansari, T.; Sibbons, P.; A Birchall, M.; Pierro, A.; Eaton, S.; De Coppi, P. Esophageal tissue engineering: A new approach for esophageal replacement. World J. Gastroenterol. 2012, 18, 6900–6907. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, M.; Jiang, F.; Yin, S.; Lin, S.; Yang, G.; Lu, Y.; Zhang, W.; Jiang, X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021, 6, 3976–3986. [Google Scholar] [CrossRef]
- Dargoush, S.A.; Hanaee-Ahvaz, H.; Irani, S.; Soleimani, M.; Khatami, S.M.; Sohi, A.N. A composite bilayer scaffold functionalized for osteochondral tissue regeneration in rat animal model. J. Tissue Eng. Regen. Med. 2022, 16, 559–574. [Google Scholar] [CrossRef]
- Yang, T.; Tamaddon, M.; Jiang, L.; Wang, J.; Liu, Z.; Liu, Z.; Meng, H.; Hu, Y.; Gao, J.; Yang, X.; et al. Bilayered scaffold with 3D printed stiff subchondral bony compartment to provide constant mechanical support for long-term cartilage regeneration. J. Orthop. Transl. 2021, 30, 112–121. [Google Scholar] [CrossRef]
- Tevlek, A.; Aydin, H.M. Multi-layered in vitro 3D-bone model via combination of osteogenic cell sheets with electrospun membrane interlayer. J. Biomater. Appl. 2021, 36, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Semitela, Â.; Leal Pereira, A.; Sousa, C.; Mendes, A.F.; Marques, P.A.; Completo, A. Multi-layered electrospinning and electrospraying approach: Effect of polymeric supplements on chondrocyte suspension. J. Biomater. Appl. 2021, 36, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, G.; Kim, J.H.; Kim, I.; Lee, C.; Chung, E.-J.; Noh, I. Manufacturing of self-standing multi-layered 3D-bioprinted alginate-hyaluronate constructs by controlling the cross-linking mechanisms for tissue engineering applications. Biofabrication 2022, 14, 035013. [Google Scholar] [CrossRef]
- Tamaddon, M.; Blunn, G.; Tan, R.; Yang, P.; Sun, X.; Chen, S.-M.; Luo, J.; Liu, Z.; Wang, L.; Li, D.; et al. In vivo evaluation of additively manufactured multi-layered scaffold for the repair of large osteochondral defects. Bio-Design Manuf. 2022, 5, 481–496. [Google Scholar] [CrossRef]
- Rashidi, N.; Tamaddon, M.; Liu, C.; Brand, D.D.; Czernuszka, J. A Bilayer Osteochondral Scaffold with Self-Assembled Monomeric Collagen Type-I, Type-II, and Polymerized Chondroitin Sulfate Promotes Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Adv. NanoBiomed Res. 2021, 2, 2100089. [Google Scholar] [CrossRef]
- Li, M.; Song, P.; Wang, W.; Xu, Y.; Li, J.; Wu, L.; Gui, X.; Zeng, Z.; Zhou, Z.; Liu, M.; et al. Preparation and characterization of biomimetic gradient multi-layer cell-laden scaffolds for osteochondral integrated repair. J. Mater. Chem. B 2022, 10, 4172–4188. [Google Scholar] [CrossRef]
- Nejad, Z.M.; Zamanian, A.; Saeidifar, M.; Vanaei, H.R.; Amoli, M.S. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers 2021, 13, 4442. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Fabrication and Characterization of PCL/PLGA Coaxial and Bilayer Fibrous Scaffolds for Tissue Engineering. Materials 2021, 14, 6295. [Google Scholar] [CrossRef]
- Li, M.X.; Li, L.; Zhou, S.Y.; Cao, J.H.; Liang, W.H.; Tian, Y.; Shi, X.T.; Yang, X.B.; Wu, D.Y. A biomimetic orthogonal-bilayer tubular scaffold for the co-culture of endothelial cells and smooth muscle cells. RSC Adv. 2021, 11, 31783–31790. [Google Scholar] [CrossRef]
- Do, T.M.; Yang, Y.; Deng, A. Porous Bilayer Vascular Grafts Fabricated from Electrospinning of the Recombinant Human Collagen (RHC) Peptide-Based Blend. Polymers 2021, 13, 4042. [Google Scholar] [CrossRef]
- Smith, M.J.; Dempsey, S.G.; Veale, R.W.; Duston-Fursman, C.G.; Rayner, C.A.F.; Javanapong, C.; Gerneke, D.; Dowling, S.G.; A Bosque, B.; Karnik, T.; et al. Further structural characterization of ovine forestomach matrix and multi-layered extracellular matrix composites for soft tissue repair. J. Biomater. Appl. 2021, 36, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Kilian, D.; von Witzleben, M.; Lanaro, M.; Wong, C.S.; Vater, C.; Lode, A.; Allenby, M.C.; Woodruff, M.A.; Gelinsky, M. 3D Plotting of Calcium Phosphate Cement and Melt Electrowriting of Polycaprolactone Microfibers in One Scaffold: A Hybrid Additive Manufacturing Process. J. Funct. Biomater. 2022, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zou, Q.; Wang, C.; Lin, M.; Li, Y.; Zhang, R.; Li, Y. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Mater. Des. 2021, 210, 110047. [Google Scholar] [CrossRef]
- Schoenfeld, C.M.; Lautenschlager, E.P.; Meyer, P.R. Mechanical properties of human cancellous bone in the femoral head. Med. Biol. Eng. Comput. 1974, 12, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Chiba, D.; Yamada, N.; Mori, Y.; Oyama, M.; Ohtsu, S.; Kuwahara, Y.; Baba, K.; Tanaka, H.; Aizawa, T.; Hanada, S.; et al. Mid-term results of a new femoral prosthesis using Ti-Nb-Sn alloy with low Young’s modulus. BMC Musculoskelet. Disord. 2021, 22, 987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, N. A mini-review of embedded 3D printing: Supporting media and strategies. J. Mater. Chem. B 2020, 8, 10474–10486. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, T.E.; Thomson, S.L. Embedded 3D printing of multi-layer, self-oscillating vocal fold models. J. Biomech. 2021, 121, 110388. [Google Scholar] [CrossRef]
- Alipour, F.; Vigmostad, S. Measurement of Vocal Folds Elastic Properties for Continuum Modeling. J. Voice 2012, 26, 816.e21–816.e29. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Srinivas, S.; Narain, R. Chapter 5—Modification of Polymers, in Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–104. [Google Scholar]
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymers for Tissue Engineering Applications: Arginine Acts as a Sticky Protein Equivalent for Viable Cell Accommodation. ACS Omega 2018, 3, 4242–4251. [Google Scholar] [CrossRef]
- Browe, D.C.; Díaz-Payno, P.J.; Freeman, F.E.; Schipani, R.; Burdis, R.; Ahern, D.P.; Nulty, J.M.; Guler, S.; Randall, L.D.; Buckley, C.T.; et al. Bilayered extracellular matrix derived scaffolds with anisotropic pore architecture guide tissue organization during osteochondral defect repair. Acta Biomater. 2022, 143, 266–281. [Google Scholar] [CrossRef]
- DiCerbo, M.; Benmassaoud, M.M.; Vega, S.L. Porous Scaffold-Hydrogel Composites Spatially Regulate 3D Cellular Mechanosensing. Front. Med. Technol. 2022, 4, 884314. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Wang, L.; Deng, J.; Zheng, Y.; Ke, Q.; Yang, X.; Zhang, X.; Jia, W.; Huang, C. Enhanced cellular infiltration of tissue-engineered scaffolds fabricated by PLLA nanogrooved microfibers. Nano Res. 2022, 16, 1614–1625. [Google Scholar] [CrossRef]
- Sheikhi, F.; Khorram, M.; Hashemi, S.-S.; Mohammadi, A.; Peyrovedin, H. Preparation, Characterization, and Surface Modification of Polycaprolactone-Based Nanofibrous Scaffold by Grafting with Collagen for Skin Tissue Engineering. Regen. Eng. Transl. Med. 2022, 8, 545–562. [Google Scholar] [CrossRef]
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 989729. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Shu, Z.; Zhang, C.; Chen, X.; Wang, M.; Fan, L. Surface modification of silk fibroin composite bone scaffold with polydopamine coating to enhance mineralization ability and biological activity for bone tissue engineering. J. Appl. Polym. Sci. 2022, 139, e52900. [Google Scholar] [CrossRef]
- Habibizadeh, M.; Nadri, S.; Fattahi, A.; Rostamizadeh, K.; Mohammadi, P.; Andalib, S.; Hamidi, M.; Forouzideh, N. Surface modification of neurotrophin-3 loaded PCL/chitosan nanofiber/net by alginate hydrogel microlayer for enhanced biocompatibility in neural tissue engineering. J. Biomed. Mater. Res. Part A 2021, 109, 2237–2254. [Google Scholar] [CrossRef]
- Zhong, H.; Li, Z.; Zhao, T.; Chen, Y. Surface Modification of Nanofibers by Physical Adsorption of Fiber-Homologous Amphiphilic Copolymers and Nanofiber-Reinforced Hydrogels with Excellent Tissue Adhesion. ACS Biomater. Sci. Eng. 2021, 7, 4828–4837. [Google Scholar] [CrossRef]
- Wang, D.; Yu, X.; Xu, Y.; Wang, X.; Wang, H.; Zhang, Y.; Li, Q.; Turng, L.-S. Physical shish-kebab modification vs. chemical surface coating on expanded polytetrafluoroethylene vascular grafts for enhanced endothelial cell adhesion. Mater. Des. 2022, 220, 110889. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, W.; Wang, Y.; Zhang, C.; Zhang, X.; Zhang, S.; Wu, W. Recombinant DTβ4-inspired porous 3D vascular graft enhanced antithrombogenicity and recruited circulating CD93+/CD34+ cells for endothelialization. Sci. Adv. 2022, 8, eabn1958. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Janani, G.; Mandal, B.B.; Rajendran, S.; Krishnakumar, G.S. Surface Modification of Decellularized Natural Cellulose Scaffolds with Organosilanes for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 2000–2015. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Wang, T.; Feng, Z.-Q.; Leach, M.K.; Wu, J.; Jiang, Q. Nanoporous fibers of type-I collagen coated poly(l-lactic acid) for enhancing primary hepatocyte growth and function. J. Mater. Chem. B 2012, 1, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Tehran, M.A.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Boschetto, F.; Yagi, S.; Marin, E.; Adachi, T.; Chen, X.; Pezzotti, G.; Sakurai, S.; Sasaki, S.; Aoki, T.; et al. Enhancing the bioactivity of melt electrowritten PLLA scaffold by convenient, green, and effective hydrophilic surface modification. Biomater. Adv. 2022, 135, 112686. [Google Scholar] [CrossRef] [PubMed]
- Dokos, S.; LeGrice, I.J.; Smaill, B.H.; Kar, J.; Young, A. A Triaxial-Measurement Shear-Test Device for Soft Biological Tissues. J. Biomech. Eng. 2000, 122, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Ajayan, P.M.; Narayanan, T.N. Dynamic mechanical analysis in materials science: The Novice’s Tale. Oxf. Open Mater. Sci. 2020, 1, itaa001. [Google Scholar] [CrossRef]
- Villalona, G.A.; Udelsman, B.; Duncan, D.R.; McGillicuddy, E.; Sawh-Martinez, R.F.; Hibino, N.; Painter, C.; Mirensky, T.; Erickson, B.; Shinoka, T.; et al. Cell-Seeding Techniques in Vascular Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 341–350. [Google Scholar] [CrossRef]
- Keong, L.C.; Halim, A.S. In Vitro Models in Biocompatibility Assessment for Biomedical-Grade Chitosan Derivatives in Wound Management. Int. J. Mol. Sci. 2009, 10, 1300–1313. [Google Scholar] [CrossRef]
- Buffinton, C.M.; Tong, K.J.; Blaho, R.A.; Buffinton, E.M.; Ebenstein, D.M. Comparison of mechanical testing methods for biomaterials: Pipette aspiration, nanoindentation, and macroscale testing. J. Mech. Behav. Biomed. Mater. 2015, 51, 367–379. [Google Scholar] [CrossRef]
- Wałdoch, A.; Sabiniewicz, R.; Meyer-Szary, J. Interventional treatment using a 3D model of a right pulmonary artery to left atrial fistula in an infant. Adv. Interv. Cardiol. 2022, 18, 170–172. [Google Scholar] [CrossRef]
- Chen, W.; Ma, L.; Shao, J.; Bi, C.; Xie, Y.; Zhao, S. Morphological specificity analysis of an image-based 3D model of airway filling in a difficult airway. BMC Anesthesiol. 2022, 22, 336. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Prather, R.; Divo, E.; Kassab, A.; Nykanen, D.; Farias, M.; DeCampli, W.M. Computational fluid dynamics investigation of the novel hybrid comprehensive stage II operation. JTCVS Open 2021, 7, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Querzoli, G.; Badas, M.G.; Angius, F.; Telani, S.; Ripandelli, G. Computational Fluid Dynamics of Intraocular Silicone Oil Tamponade. Transl. Vis. Sci. Technol. 2021, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, J.; Osborne, N.H.; Hernandez-Garcia, L.; Figueroa, C.A. A Combined Computational Fluid Dynamics and Arterial Spin Labeling MRI Modeling Strategy to Quantify Patient-Specific Cerebral Hemodynamics in Cerebrovascular Occlusive Disease. Front. Bioeng. Biotechnol. 2021, 9, 722445. [Google Scholar] [CrossRef] [PubMed]
- Eltes, P.E.; Bartos, M.; Hajnal, B.; Pokorni, A.J.; Kiss, L.; Lacroix, D.; Varga, P.P.; Lazary, A. Development of a Computer-Aided Design and Finite Element Analysis Combined Method for Affordable Spine Surgical Navigation with 3D-Printed Customized Template. Front. Surg. 2021, 7, 583386. [Google Scholar] [CrossRef]
- Irarrázaval, S.; Ramos-Grez, J.A.; Pérez, L.I.; Besa, P.; Ibáñez, A. Finite element modeling of multiple density materials of bone specimens for biomechanical behavior evaluation. SN Appl. Sci. 2021, 3, 776. [Google Scholar] [CrossRef]
- Jahangir, S.; Mohammadi, A.; Mononen, M.E.; Hirvasniemi, J.; Suomalainen, J.-S.; Saarakkala, S.; Korhonen, R.K.; Tanska, P. Rapid X-ray-Based 3-D Finite Element Modeling of Medial Knee Joint Cartilage Biomechanics during Walking. Ann. Biomed. Eng. 2022, 50, 666–679. [Google Scholar] [CrossRef]
- Jing, M.; Cui, Z.; Fu, H.; Chen, X. Real-Time Deformation Simulation of Kidney Surgery Based on Virtual Reality. J. Shanghai Jiaotong Univ. (Sci.) 2021, 26, 290–297. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shiinoki, T.; Yuasa, Y.; Tanaka, H. Estimation of liver elasticity using the finite element method and four-dimensional computed tomography images as a biomarker of liver fibrosis. Med. Phys. 2021, 48, 1286–1298. [Google Scholar] [CrossRef]
- Peshin, S.; Karakulova, Y.; Kuchumov, A.G. Finite Element Modeling of the Fingers and Wrist Flexion/Extension Effect on Median Nerve Compression. Appl. Sci. 2023, 13, 1219. [Google Scholar] [CrossRef]
- Hislop, B.D.; Heveran, C.M.; June, R.K. Development and analytical validation of a finite element model of fluid transport through osteochondral tissue. J. Biomech. 2021, 123, 110497. [Google Scholar] [CrossRef] [PubMed]
- Jobanputra, R.D.; Hayes, J.; Royyuru, S.; Masen, M.A. A numerical analysis of skin–PPE interaction to prevent facial tissue injury. Sci. Rep. 2021, 11, 16248. [Google Scholar] [CrossRef]
- Helou, B.; Bel-Brunon, A.; Dupont, C.; Ye, W.; Silvestro, C.; Rochette, M.; Lucas, A.; Kaladji, A.; Haigron, P. Patient-specific finite element simulation of peripheral artery percutaneous transluminal angioplasty to evaluate the procedure outcome without stent implantation. Int. J. Numer. Methods Biomed. Eng. 2023, 39, e3685. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Zhang, M.; Qin, C.; Zhai, D.; Wang, Y.; Zhou, Y.; Chang, J.; Zhu, Y.; Wu, C. 3D printing of conch-like scaffolds for guiding cell migration and directional bone growth. Bioact. Mater. 2022, 22, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Ritskes-Hoitinga, M. Can prospective systematic reviews of animal studies improve clinical translation? J. Transl. Med. 2020, 18, 15. [Google Scholar] [CrossRef]
- Bas-Cristóbal Menéndez, A.; Du, Z.; van den Bosch, T.P.; Othman, A.; Gaio, N.; Silvestri, C.; Quirós, W.; Lin, H.; Korevaar, S.; Merino, A.; et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci. Rep. 2022, 12, 20699. [Google Scholar] [CrossRef]
- Dhwaj, A.; Roy, N.; Jaiswar, A.; Prabhakar, A.; Verma, D. 3D-Printed Impedance Micropump for Continuous Perfusion of the Sample and Nutrient Medium Integrated with a Liver-On-Chip Prototype. ACS Omega 2022, 7, 40900–40910. [Google Scholar] [CrossRef]
- Li, P.; Cui, F.; Chen, H.; Yang, Y.; Li, G.; Mao, H.; Lyu, X. A Microfluidic Cell Co-Culture Chip for the Monitoring of Interactions between Macrophages and Fibroblasts. Biosensors 2022, 13, 70. [Google Scholar] [CrossRef]
- Xu, C.; Wang, K.; Huang, P.; Liu, D.; Guan, Y. Single-Cell Isolation Microfluidic Chip Based on Thermal Bubble Micropump Technology. Sensors 2023, 23, 3623. [Google Scholar] [CrossRef]
- Yang, J.; Hirai, Y.; Iida, K.; Ito, S.; Trumm, M.; Terada, S.; Sakai, R.; Tsuchiya, T.; Tabata, O.; Kamei, K.-I. Integrated-gut-liver-on-a-chip platform as an in vitro human model of non-alcoholic fatty liver disease. Commun. Biol. 2023, 6, 310. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Ohk, K.; Won, J.; Choi, D.-H.; Jung, Y.H.; Yang, J.H.; Jun, Y.; Kim, J.-A.; Chung, S.; Lee, S.-H. Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system. Nat. Commun. 2023, 14, 1488. [Google Scholar] [CrossRef] [PubMed]
- Özkan, A.; Stolley, D.L.; Cressman, E.N.K.; McMillin, M.; Yankeelov, T.E.; Rylander, M.N. Vascularized Hepatocellular Carcinoma on a Chip to Control Chemoresistance through Cirrhosis, Inflammation and Metabolic Activity. Small Struct. 2023, 2200403. [Google Scholar] [CrossRef]
- Das, S.L.; Sutherland, B.P.; Lejeune, E.; Eyckmans, J.; Chen, C.S. Mechanical response of cardiac microtissues to acute localized injury. Am. J. Physiol. Circ. Physiol. 2022, 323, H738–H748. [Google Scholar] [CrossRef]
- Podunavac, I.; Djocos, M.; Vejin, M.; Birgermajer, S.; Pavlovic, Z.; Kojic, S.; Petrovic, B.; Radonic, V. 3D-Printed Microfluidic Chip for Real-Time Glucose Monitoring in Liquid Analytes. Micromachines 2023, 14, 503. [Google Scholar] [CrossRef]
- Waldrop, T.I.; Graham, C.; Gard, W.; Ingle, K.; Ptacek, T.; Nguyen, N.; Lose, B.; Sethu, P.; Lee, T. Biomimetic cardiac tissue chip and murine arteriovenous fistula models for recapitulating clinically relevant cardiac remodeling under volume overload conditions. Front. Bioeng. Biotechnol. 2023, 11, 1101622. [Google Scholar] [CrossRef]
- Boot, R.C.; Roscani, A.; van Buren, L.; Maity, S.; Koenderink, G.H.; Boukany, P.E. High-throughput mechanophenotyping of multicellular spheroids using a microfluidic micropipette aspiration chip. Lab Chip 2023, 23, 1768–1778. [Google Scholar] [CrossRef]
- Ferrari, D.; Sengupta, A.; Heo, L.; Pethö, L.; Michler, J.; Geiser, T.; Perez, V.A.D.J.; Kuebler, W.M.; Zeinali, S.; Guenat, O.T. Effects of biomechanical and biochemical stimuli on angio- and vasculogenesis in a complex microvasculature-on-chip. iScience 2023, 26, 106198. [Google Scholar] [CrossRef]
- Hart, D.C.t.; Yildiz, D.; Palacio-Castañeda, V.; Li, L.; Gumuscu, B.; Brock, R.; Verdurmen, W.P.R.; van der Vlag, J.; Nijenhuis, T. Co-Culture of Glomerular Endothelial Cells and Podocytes in a Custom-Designed Glomerulus-on-a-Chip Model Improves the Filtration Barrier Integrity and Affects the Glomerular Cell Phenotype. Biosensors 2023, 13, 339. [Google Scholar] [CrossRef]
- Wu, J.; Chen, C.; Qin, C.; Li, Y.; Jiang, N.; Yuan, Q.; Duan, Y.; Liu, M.; Wei, X.; Yu, Y.; et al. Mimicking the Biological Sense of Taste In Vitro Using a Taste Organoids-on-a-Chip System. Adv. Sci. 2023, 10, e2206101. [Google Scholar] [CrossRef]
- Erbay, I.H.; Polatli, E.; Koc, A.C.; Özbilgiç, R.; Karaman, O.; Güven, S. Bioengineering Bone-on-a-Chip Model Harnessing Osteoblastic and Osteoclastic Resolution. Adv. Eng. Mater. 2023, 25, 2201063. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, K.S.; Seo, E.U.; Seo, S.; Lee, B.C.; Choi, N.; Choi, J.; Kim, H.N. Vascularized Lung Cancer Model for Evaluating the Promoted Transport of Anticancer Drugs and Immune Cells in an Engineered Tumor Microenvironment. Adv. Healthc. Mater. 2022, 11, 2102581. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, W.E.; Pawate, A.S.; Larry, T.A.; Schieferstein, J.M.; Whittenberg, J.J.; Leckband, D.E.; Kenis, P.J.A. Development of microfluidic platform that enables ‘on-chip’ imaging of cells exposed to shear stress and cyclic stretch. Microfluid. Nanofluidics 2023, 27, 11. [Google Scholar] [CrossRef]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty—Over 100 years of operative history. Orthop. Rev. 2011, 3, e16. [Google Scholar]
- Oberweis, C.V.; Marchal, J.A.; López-Ruiz, E.; Galvez-Martin, M.P. A Worldwide Overview of Regulatory Frameworks for Tissue-Based Products. Tissue Eng. Part B Rev. 2020, 26, 181–196. [Google Scholar] [CrossRef]
- Waldburger, L.; Schaller, R.; Furthmüller, C.; Schrepfer, L.; Schaefer, D.J.; Kaempfen, A. 3D-Printed Hand Splints versus Thermoplastic Splints: A Randomized Controlled Pilot Feasibility Trial. Int. J. Bioprinting 2021, 8, 474. [Google Scholar] [CrossRef]
- Laubach, M.; Suresh, S.; Herath, B.; Wille, M.-L.; Delbrück, H.; Alabdulrahman, H.; Hutmacher, D.W.; Hildebrand, F. Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects. J. Orthop. Transl. 2022, 34, 73–84. [Google Scholar] [CrossRef]
- Hee, M.; Greasley, S.; Whiting, G.; Harkin, C.; Oliver, G.; Marsden, D.; Andrews, R.; Sireau, S.; Price, R.D.; Anwar, F.; et al. 3D printed customised external cranial plate in a patient with syndrome of trephined: ‘A case report’. 3D Print Med. 2021, 7, 35. [Google Scholar]
- Sharma, U.; Dabadi, S.; Dhungel, R.R.; Shrestha, D.; Gurung, P.; Shrestha, R.; Pant, B. Customized cost-effective polymethyl-methacrylate cranioplasty implant using three-dimensional printer. Asian J. Neurosurg. 2021, 16, 150–154. [Google Scholar]
- Mainard, N.; Sharma, D.; Fron, D.; Mezel, A.; Canavese, F.; Bonnevalle, M.; Nectoux, E. Porous Ceramic Sternal Prosthesis Implantation in a 13-Year-Old Patient Presenting with Metastatic Ewing’s Sarcoma. Eur. J. Pediatr. Surg. Rep. 2022, 10, e1–e5. [Google Scholar] [CrossRef]
- Morales, D.L.; Herrington, C.; Bacha, E.A.; Morell, V.O.; Prodán, Z.; Mroczek, T.; Sivalingam, S.; Cox, M.; Bennink, G.; Asch, F.M. A Novel Restorative Pulmonary Valve Conduit: Early Outcomes of Two Clinical Trials. Front. Cardiovasc. Med. 2021, 7, 583360. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, G.; Zheng, Y.; Gao, J.; Fu, Y.; Wang, Q.; Huang, L.; Pan, X.; Ding, J. ‘Invisible’ orthodontics by polymeric ‘clear’ aligners molded on 3D-printed personalized dental models. Regen. Biomater. 2022, 9, rbac007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Ding, S.; Liang, J.; Kuang, J.; Mao, Q.; Ying, W.; Shu, Y.; Li, J.; Jiang, C. A clinical trial to compare a 3D-printed bolus with a conventional bolus with the aim of reducing cardiopulmonary exposure in postmastectomy patients with volumetric modulated arc therapy. Cancer Med. 2022, 11, 1037–1047. [Google Scholar] [CrossRef]
- Kalaskar, R.; Bhaje, P.; Balasubramanian, S.; Kalaskar, A. Effectiveness of the novel impression tray “cleftray” for infants with cleft lip and palate: A randomized controlled clinical trial. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Şenayli, A.; Çankaya, G.; Öztorun, C.I.; Oflaz, H.; Şenel, E. Clinical trials of 3D printing splints to avoid contracture development in burned children. Turk. J. Med. Sci. 2021, 51, 2543–2553. [Google Scholar] [PubMed]
- Cui, D.; Wu, B.; He, D.; Wang, Y.; Jiao, Y.; Zhang, B. 3D-Printed Cold Preservation Device in Renal Autotransplantation for the Treatment of a Patient with Renal Artery Stenosis. Front. Bioeng. Biotechnol. 2022, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Desvigne, M.N.; Bauer, K.; Holifield, K.; Day, K.; Gilmore, D.; Wardman, A.L. Case Report: Surgical Closure of Chronic Soft Tissue Defects Using Extracellular Matrix Graft Augmented Tissue Flaps. Front. Surg. 2021, 7, 559450. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 5340616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).