Time-Dependent Demineralization of Tilapia (Oreochromis niloticus) Bones Using Hydrochloric Acid for Extracellular Matrix Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Demineralization of Tilapia Bone
2.2. Histological Staining
2.3. Infrared Spectroscopy Analysis
2.4. Thermal Degradation and Denutaration Profile
2.4.1. Differential Scanning Calorimetry (DSC)
2.4.2. Thermal Gravimetric Analysis (TGA)
2.5. Residual Calcium Determination
2.6. Kinetics of Demineralization Process
2.7. Protein Quantification
2.8. Statistical Analysis
3. Results
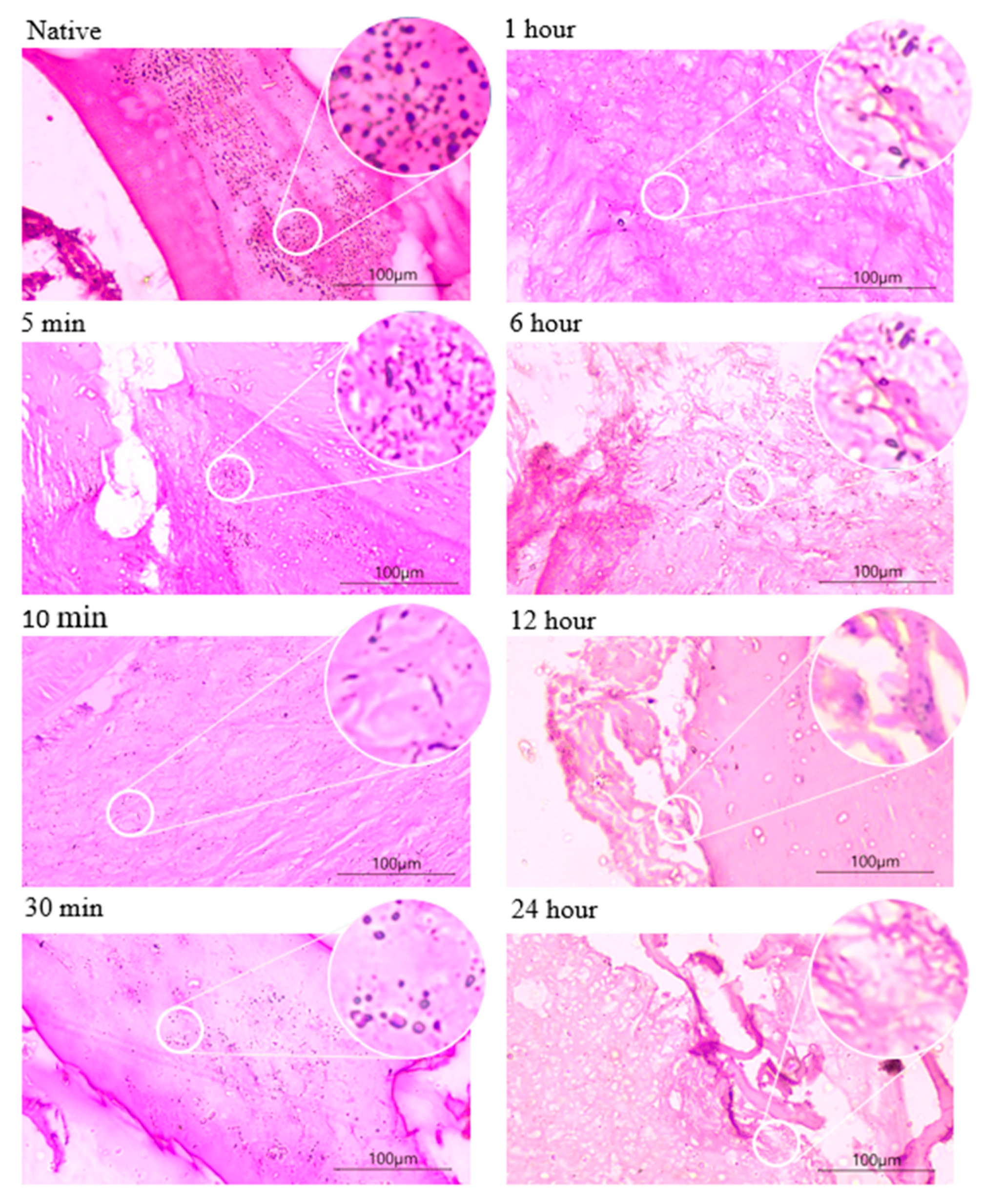
3.1. Histological Staining
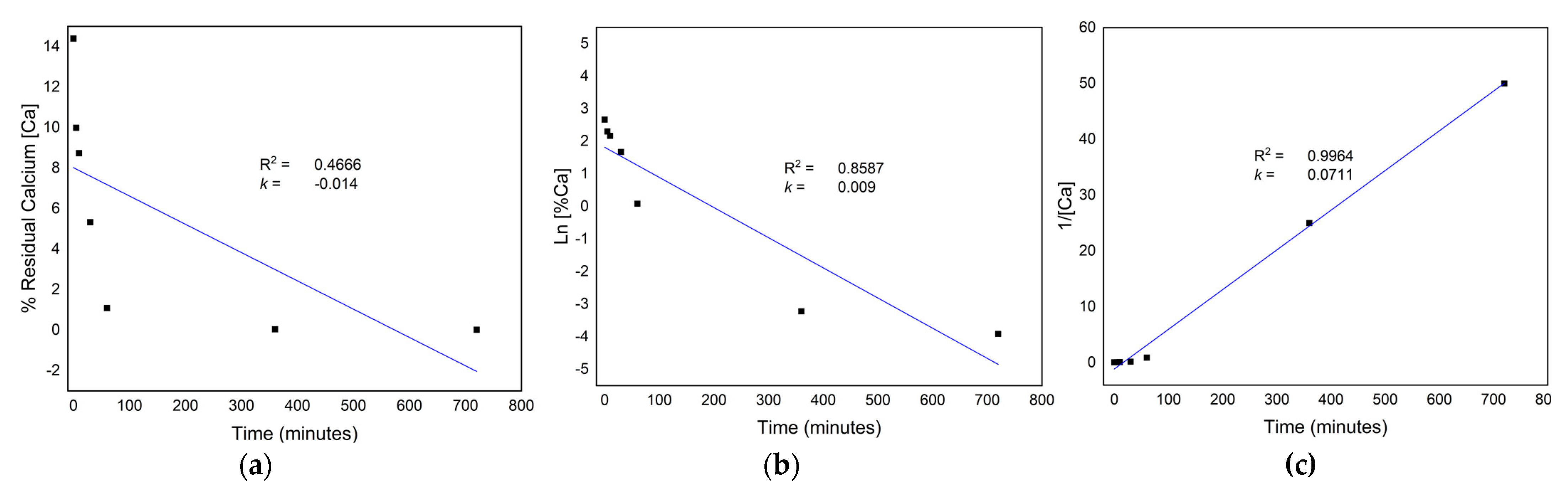
3.2. Residual Calcium
3.3. Kinetics of the Demineralization Process
3.4. Protein Quantification
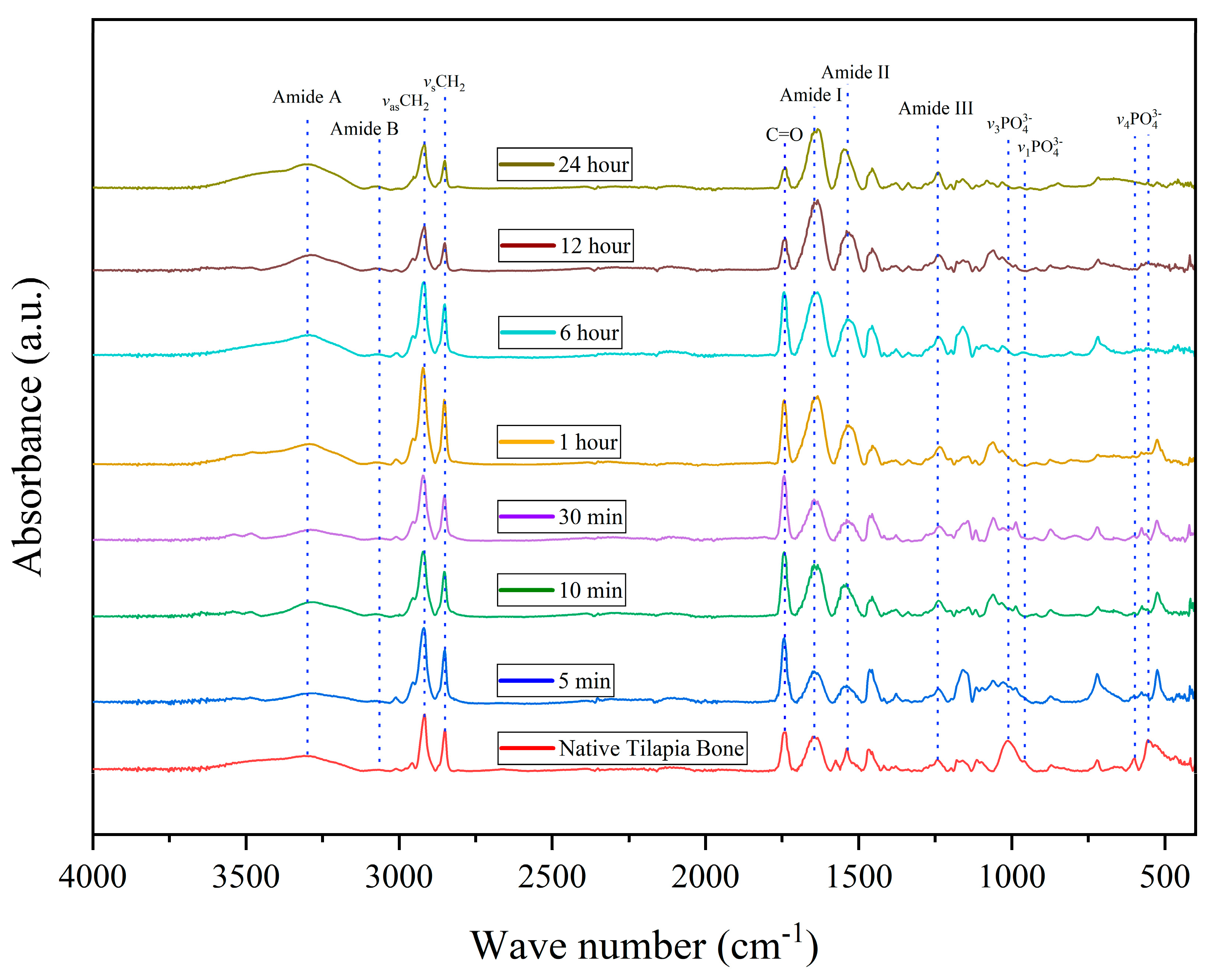
3.5. Infrared Spectroscopy Analysis
3.6. Thermal Degradation and Denaturation Profile
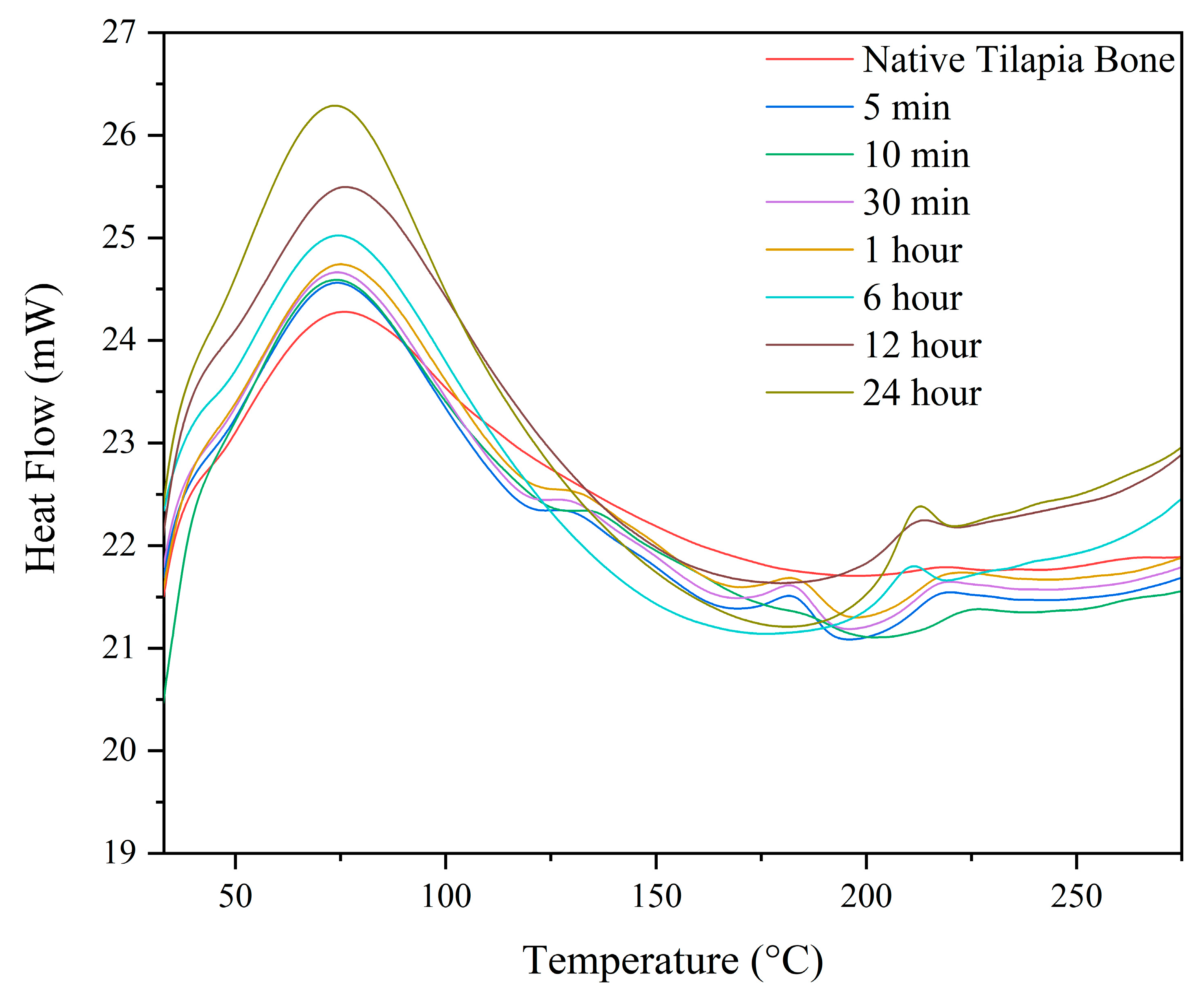
3.6.1. Differential Scanning Calorimetry (DSC)
3.6.2. Thermogravimetric Analysis (TGA)
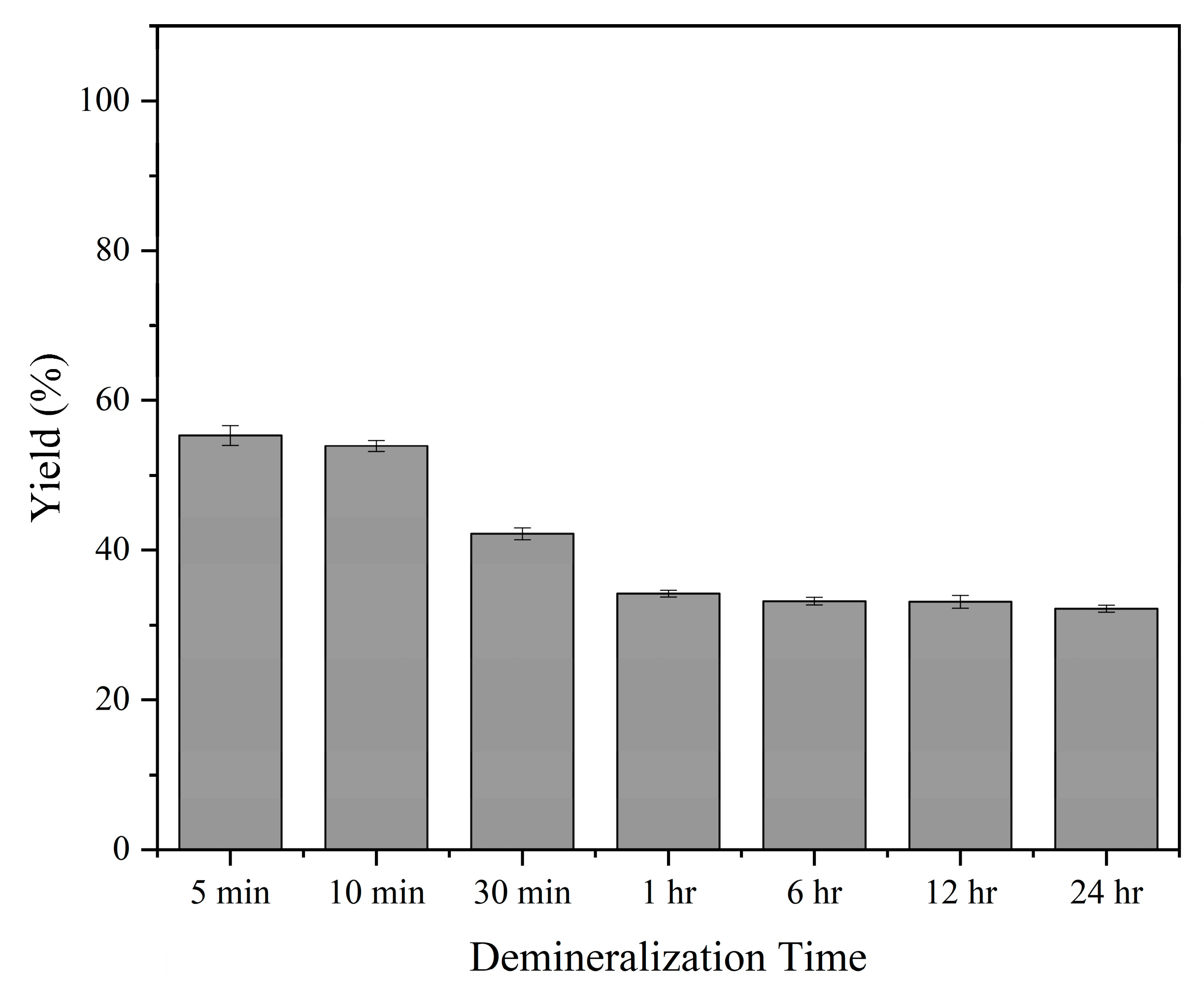
3.7. Demineralization Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philippine Tilapia Industry Roadmap (2022–2025). Available online: http://www.pcaf.da.gov.ph/index.php/cir-tilapia (accessed on 7 March 2023).
- Rajasree, R.; Aranganathan, L. Industrial Fish Processing Waste: Causes, Effects & Sustainable Solutions for Effective Management. Available online: https://www.foodinfotech.com/industrial-fish-processing-waste-causes-effects-sustainable-solutions/ (accessed on 14 April 2023).
- Afreen, M.; Ucak, I. Fish Processing Wastes Used as Feed Ingredient for Animal Feed and Aquaculture Feed. J. Surv. Fish. Sci. 2020, 6, 55–64. [Google Scholar] [CrossRef]
- Bücker, F.; Marder, M.; Peiter, M.R.; Lehn, D.N.; Esquerdo, V.M.; Antonio de Almeida Pinto, L.; Konrad, O. Fish Waste: An Efficient Alternative to Biogas and Methane Production in an Anaerobic Mono-Digestion System. Renew. Energy 2020, 147, 798–805. [Google Scholar] [CrossRef]
- Akter, S.; Rahman, M.A.; Naher, J.; Majumder, M.W.R.; Alam, A.N. Fish Glue from Tilapia Scale and Skin and Its Physical and Chemical Characters. Int. J. Fish Aquat. Sci. 2017, 5, 255–257. [Google Scholar]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of Marine By-Products for the Recovery of Value-Added Products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Feng, X.; Wenxue, Z.; Yuanyuan, Q.; Huaibin, K. Optimization of Demineralization on Cyprinus Carpio Haematopterus Scale by Response Surface Methodology. J. Food Sci. Technol. 2015, 52, 1684–1690. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and Characterization of Fish Scale Collagen of Higher Thermal Stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef]
- Ijima, H.; Nakamura, S.; Bual, R.; Shirakigawa, N.; Tanoue, S. Physical Properties of the Extracellular Matrix of Decellularized Porcine Liver. Gels 2018, 4, 39. [Google Scholar] [CrossRef]
- Bual, R.P.; Ijima, H. Intact Extracellular Matrix Component Promotes Maintenance of Liver-Specific Functions and Larger Aggregates Formation of Primary Rat Hepatocytes. Regen. Ther. 2019, 11, 258–268. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers 2021, 13, 1905. [Google Scholar] [CrossRef]
- Sroga, G.E.; Karim, L.; Colón, W.; Vashishth, D. Biochemical Characterization of Major Bone-Matrix Proteins Using Nanoscale-Size Bone Samples and Proteomics Methodology. Mol. Cell. Proteom. 2011, 10, 1–12. [Google Scholar] [CrossRef]
- Vashishth, D. The Role of the Collagen Matrix in Skeletal Fragility. Curr. Osteoporos. Rep. 2007, 5, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Shakir, K.A.; Walsh, M.K. Functional Properties of Collagen Extracted from Catfish (Silurus triostegus) Waste. Foods 2022, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Pranoto, Y.; Lee, C.M.; Park, H.J. Characterizations of Fish Gelatin Films Added with Gellan and κ-Carrageenan. LWT 2007, 40, 766–774. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, M.A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Baht, G.S.; Hunter, G.K.; Goldberg, H.A. Bone Sialoprotein-Collagen Interaction Promotes Hydroxyapatite Nucleation. Matrix Biol. 2008, 27, 600–608. [Google Scholar] [CrossRef]
- Pang, S.; Su, F.Y.; Green, A.; Salim, J.; McKittrick, J.; Jasiuk, I. Comparison of Different Protocols for Demineralization of Cortical Bone. Sci. Rep. 2021, 11, 7012. [Google Scholar] [CrossRef]
- Figueiredo, M.; Cunha, S.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Influence of Hydrochloric Acid Concentration on the Demineralization of Cortical Bone. Chem. Eng. Res. Des. 2011, 89, 116–124. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Ali, S.N.; Chitturi, D.; Jacob, M.; Woodell-May, J.E. BMP Depletion Occurs during Prolonged Acid Demineralization of Bone: Characterization and Implications for Graft Preparation. Cell Tissue Bank. 2011, 12, 81–88. [Google Scholar] [CrossRef]
- Bual, R.; Labares, M.; Valle, K.D.D.; Pague, J.; Bantilan, Z.C.; Ducao, P.G.; Alimasag, J.; Acibar, C. Characterization of Decellularized Extracellular Matrix from Milkfish (Chanos chanos) Skin. Biomimetics 2022, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Ijima, H.; Nakamura, S.; Bual, R.P.; Yoshida, K. Liver-Specific Extracellular Matrix Hydrogel Promotes Liver-Specific Functions of Hepatocytes in Vitro and Survival of Transplanted Hepatocytes in Vivo. J. Biosci. Bioeng. 2019, 128, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Liu, H.; Huang, K. Structural Characteristics of Extracted Collagen from Tilapia (Oreochromis mossambicus) Bone: Effects of Ethylenediaminetetraacetic Acid Solution and Hydrochloric Acid Treatment. Int. J. Food Prop. 2016, 19, 63–75. [Google Scholar] [CrossRef]
- Olatunji, O.; Denloye, A. Temperature-Dependent Extraction Kinetics of Hydrolyzed Collagen from Scales of Croaker Fish Using Thermal Extraction. Food Sci. Nutr. 2017, 5, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Maktoof, A.A.; Elherarlla, R.J.; Ethaib, S. Identifying the Nutritional Composition of Fish Waste, Bones, Scales, and Fins. IOP Conf. Ser. Mater. Sci. Eng. 2020, 871, 12013. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y.; Yang, X.; Zhao, Y.; Chi, C. Gelatin and Antioxidant Peptides from Gelatin Hydrolysate of Skipjack Tuna (Katsuwonus pelamis). Mar. Drugs 2019, 17, 565. [Google Scholar] [CrossRef]
- Modolon, H.B.; Inocente, J.; Bernardin, A.M.; Klegues Montedo, O.R.; Arcaro, S. Nanostructured Biological Hydroxyapatite from Tilapia Bone: A Pathway to Control Crystallite Size and Crystallinity. Ceram. Int. 2021, 47, 27685–27693. [Google Scholar] [CrossRef]
- Samouillan, V.; Delaunay, F.; Dandurand, J.; Merbahi, N.; Gardou, J.-P.; Yousfi, M.; Gandaglia, A.; Spina, M.; Lacabanne, C. The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials. J. Funct. Biomater. 2011, 2, 230–248. [Google Scholar] [CrossRef]
- Mkukuma, L.D.; Skakle, J.M.S.; Gibson, I.R.; Imrie, C.T.; Aspden, R.M.; Hukins, D.W.L. Effect of the Proportion of Organic Material in Bone on Thermal Decomposition of Bone Mineral: An Investigation of a Variety of Bones from Different Species Using Thermogravimetric Analysis Coupled to Mass Spectrometry, High-Temperature X-Ray Diffraction. Calcif. Tissue Int. 2004, 75, 321–328. [Google Scholar] [CrossRef]
- Bigi, A.; Ripamonti, A.; Cojazzi, G.; Pizzuto, G.; Roveri, N.; Koch, M.H.J. Structural Analysis of Turkey Tendon Collagen upon Removal of the Inorganic Phase. Int. J. Biol. Macromol. 1991, 13, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized Bone Matrix in Bone Repair: History and Use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C. Chapter 15—Ocular Toxicology. In Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose, 4th ed.; Shannon, M.W., Borron, S.W., Burns, M.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2007; pp. 301–315. ISBN 978-0-7216-0693-4. [Google Scholar]
- Ariffin, A.F.; Yusof, N.; Mohd, S.; Rahman, S.A.; Ramalingam, S.; Mansor, A.; Min, N.G. Verifying Measurements of Residual Calcium Content in Demineralised Cortical Bone. Cell Tissue Bank. 2019, 20, 527–534. [Google Scholar] [CrossRef]
- Eagle, M.J.; Rooney, P.; Kearney, J.N. Development of an Improved Bone Washing and Demineralisation Process to Produce Large Demineralised Human Cancellous Bone Sponges. Cell Tissue Bank. 2015, 16, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Cutini, M.; Corno, M.; Costa, D.; Ugliengo, P. How Does Collagen Adsorb on Hydroxyapatite? Insights from Ab Initio Simulations on a Polyproline Type II Model. J. Phys. Chem. C 2019, 123, 7540–7550. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: Structure and Mechanics, an Introduction BT—Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 1–13. ISBN 978-0-387-73906-9. [Google Scholar]
- Stock, S.R. The Mineral-Collagen Interface in Bone. Calcif. Tissue Int. 2015, 97, 262–280. [Google Scholar] [CrossRef]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen Structural Hierarchy and Susceptibility to Degradation by Ultraviolet Radiation. Mater. Sci. Eng. C 2008, 28, 1420–1429. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Kolovou, P.; Papagelopoulos, P.; Theophanides, T. The Role of β-Antagonists on the Structure of Human Bone—A Spectroscopic Study. Infrared Spectrosc. Life Biomed. Sci. 2012, 25, 259–271. [Google Scholar] [CrossRef]
- Skrzyński, S.; Sionkowska, A.; Marciniak, A. DSC Study of Collagen in Disc Disease. J. Biophys. 2009, 2009, 819635. [Google Scholar] [CrossRef]
- Horneman, D.A.; Ottens, M.; Hoorneman, M.; Van Der Wielen, L.A.M.; Tesson, M. Reaction and Diffusion during Demineralization of Animal Bone. AIChE J. 2004, 50, 2682–2690. [Google Scholar] [CrossRef]
- Nayak, G.; Bhuyan, S.K.; Bhuyan, R.; Sahu, A.; Kar, D.; Kuanar, A. Marine Sources as an Unexplored Bone Tissue Reconstruction Material—A Review. Egypt. J. Basic Appl. Sci. 2022, 9, 477–498. [Google Scholar] [CrossRef]
- Han, J.L.; Pan, X.D.; Chen, Q.; Huang, B.F. Health Risk Assessment of Heavy Metals in Marine Fish to the Population in Zhejiang, China. Sci. Rep. 2021, 11, 11079. [Google Scholar] [CrossRef] [PubMed]
- David, G.S.; Isangedighi, I.A. Heavy Metals Contamination in Fish: Effects on Human Health. J. Aquat. Sci. Mar. Biol. 2019, 2, 7–12. [Google Scholar]

| Duration | Calcium wt % | Protein (µg/mL) |
|---|---|---|
| Native | 14.47 ± 0.20 * | 109.0 ± 1.00 * |
| 5 min | 10.09 ± 0.13 * | 102.3 ± 1.0 * |
| 10 min | 8.72 ± 0.18 * | 98.0 ± 0.20 * |
| 30 min | 5.43 ± 0.27 * | 90.7 ± 1.20 * |
| 1 h | 1.10 ± 0.12 * | 88.7 ± 0.58 * |
| 6 h | 0.04 ± 0.02 | 51.7 ± 1.52 * |
| 12 h | 0.02 ± 0.01 | 48.7 ± 1.15 * |
| 24 h | n.d. | 44.0 ± 1.00 |
| 1st Degradation | 2nd Degradation | 3rd Degradation | ||||
|---|---|---|---|---|---|---|
| Treatment | Temperature, °C | % Weight Loss | Temperature, °C | % Weight Loss | Temperature, °C | % Weight Loss |
| Native | 30–194 | 10.02 | 194–459 | 38.38 | 459–656 | 16.6 |
| 5 min | 30–179 | 8.66 | 179–433 | 39.14 | 433–686 | 23.2 |
| 10 min | 30–181 | 9.71 | 181–441 | 38.31 | 441–687 | 36.19 |
| 30 min | 30–192 | 8.32 | 192–468 | 49.94 | 468–689 | 35.54 |
| 1 h | 30–170 | 8.68 | 170–475 | 55.67 | 475–692 | 29.65 |
| 6 h | 30–175 | 7.76 | 175–486 | 58.61 | 486–692 | 28.67 |
| 12 h | 30–177 | 7.81 | 177–486 | 58.56 | 486–697 | 28.67 |
| 24 h | 30–179 | 7.24 | 170–483 | 62.56 | 483–700 | 29.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisperos, M.J.; Bacosa, H.; Lumancas, G.; Arellano, F.; Aron, J.; Baclayon, L.; Bantilan, Z.C.; Labares, M., Jr.; Bual, R. Time-Dependent Demineralization of Tilapia (Oreochromis niloticus) Bones Using Hydrochloric Acid for Extracellular Matrix Extraction. Biomimetics 2023, 8, 217. https://doi.org/10.3390/biomimetics8020217
Nisperos MJ, Bacosa H, Lumancas G, Arellano F, Aron J, Baclayon L, Bantilan ZC, Labares M Jr., Bual R. Time-Dependent Demineralization of Tilapia (Oreochromis niloticus) Bones Using Hydrochloric Acid for Extracellular Matrix Extraction. Biomimetics. 2023; 8(2):217. https://doi.org/10.3390/biomimetics8020217
Chicago/Turabian StyleNisperos, Michael John, Hernando Bacosa, Gladine Lumancas, Fernan Arellano, Jemwel Aron, Lean Baclayon, Zesreal Cain Bantilan, Marionilo Labares, Jr., and Ronald Bual. 2023. "Time-Dependent Demineralization of Tilapia (Oreochromis niloticus) Bones Using Hydrochloric Acid for Extracellular Matrix Extraction" Biomimetics 8, no. 2: 217. https://doi.org/10.3390/biomimetics8020217
APA StyleNisperos, M. J., Bacosa, H., Lumancas, G., Arellano, F., Aron, J., Baclayon, L., Bantilan, Z. C., Labares, M., Jr., & Bual, R. (2023). Time-Dependent Demineralization of Tilapia (Oreochromis niloticus) Bones Using Hydrochloric Acid for Extracellular Matrix Extraction. Biomimetics, 8(2), 217. https://doi.org/10.3390/biomimetics8020217










