Failure without Tears: Two-Step Attachment in a Climbing Cactus
Abstract
1. Introduction
1.1. Adaptive Strategies Using Multi-Attachment Mechanisms
1.2. Failure and Safety
1.3. Climbing Cactus Model
1.4. Questions and Hypotheses
2. Materials and Methods
2.1. Collection and Environment
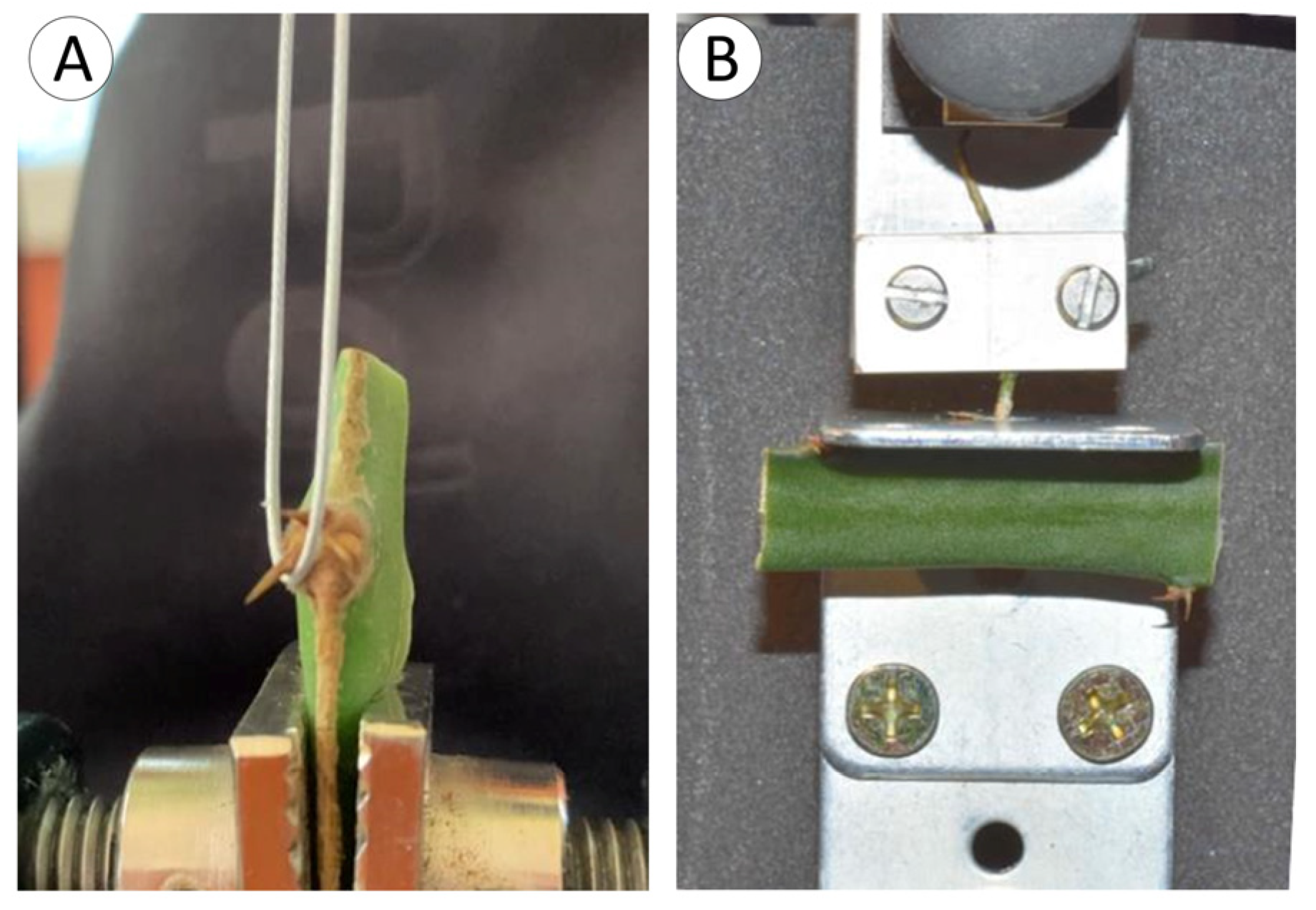
2.2. Spine Tests
2.3. Root Tests
2.4. Functional Anatomy
2.5. Data Handling and Statistics
3. Results
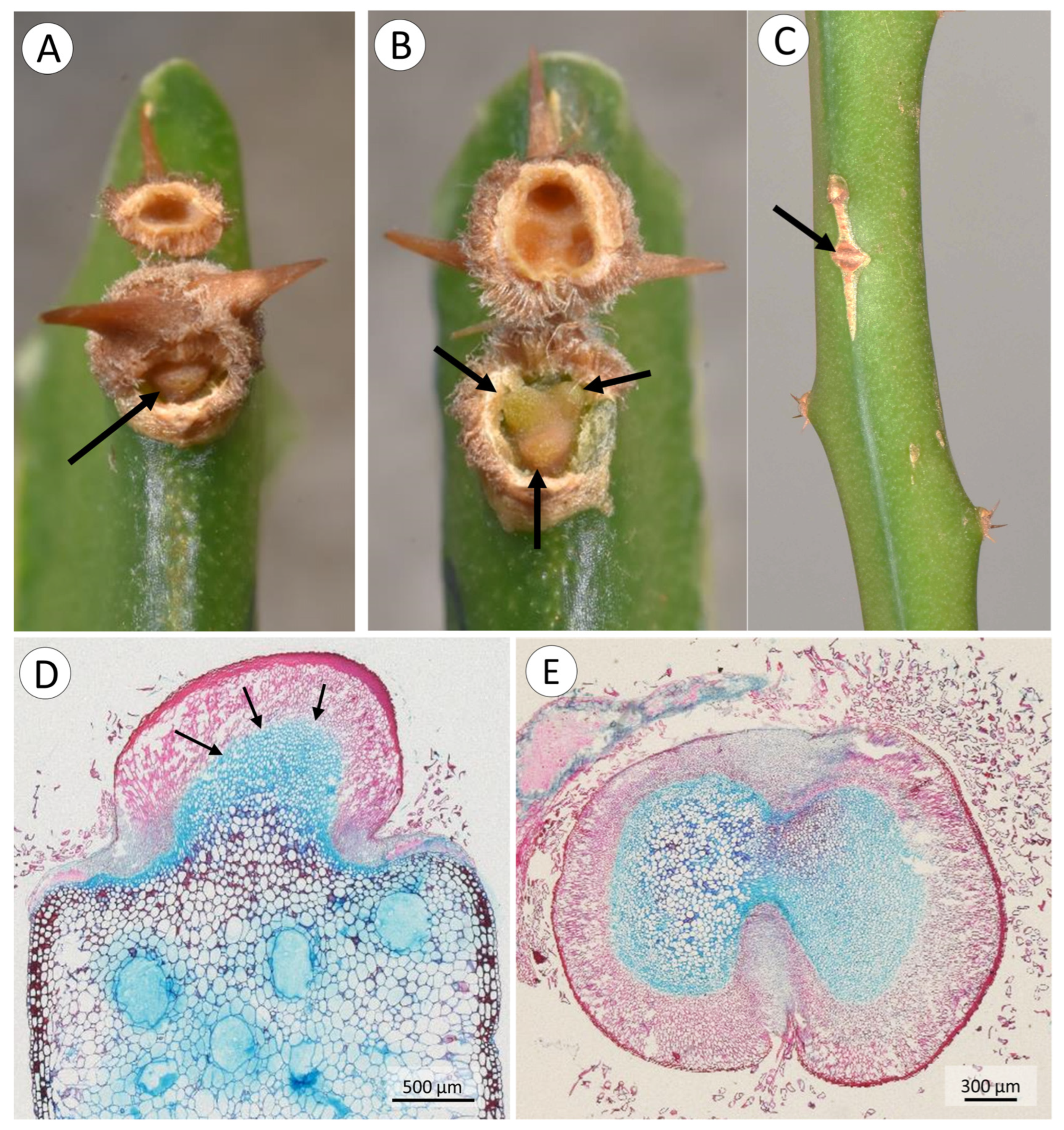
3.1. Morphology, and Deployment of Spines and Roots
| Stems | Length (cm) | Diameter (mm) | Fresh Mass (g) |
|---|---|---|---|
| n = 25 | 61.58 ± 27.02 (11.50–131.50) | 13.32 ± 3.85 (6.70–21.28) | 66.72 ± 35.44 (5.10–175.10) |
| Roots | Length (cm) | Diameter (mm) | Fresh mass (g) |
| n = 75 | na | 1.10 ± 0.28 (0.30–1.73) | na |
| Spines | Length (mm) | Diameter (mm) | Fresh mass (g) |
| n = 38 | 2.09 ± 0.47 (0.71–2.97) | na | na |
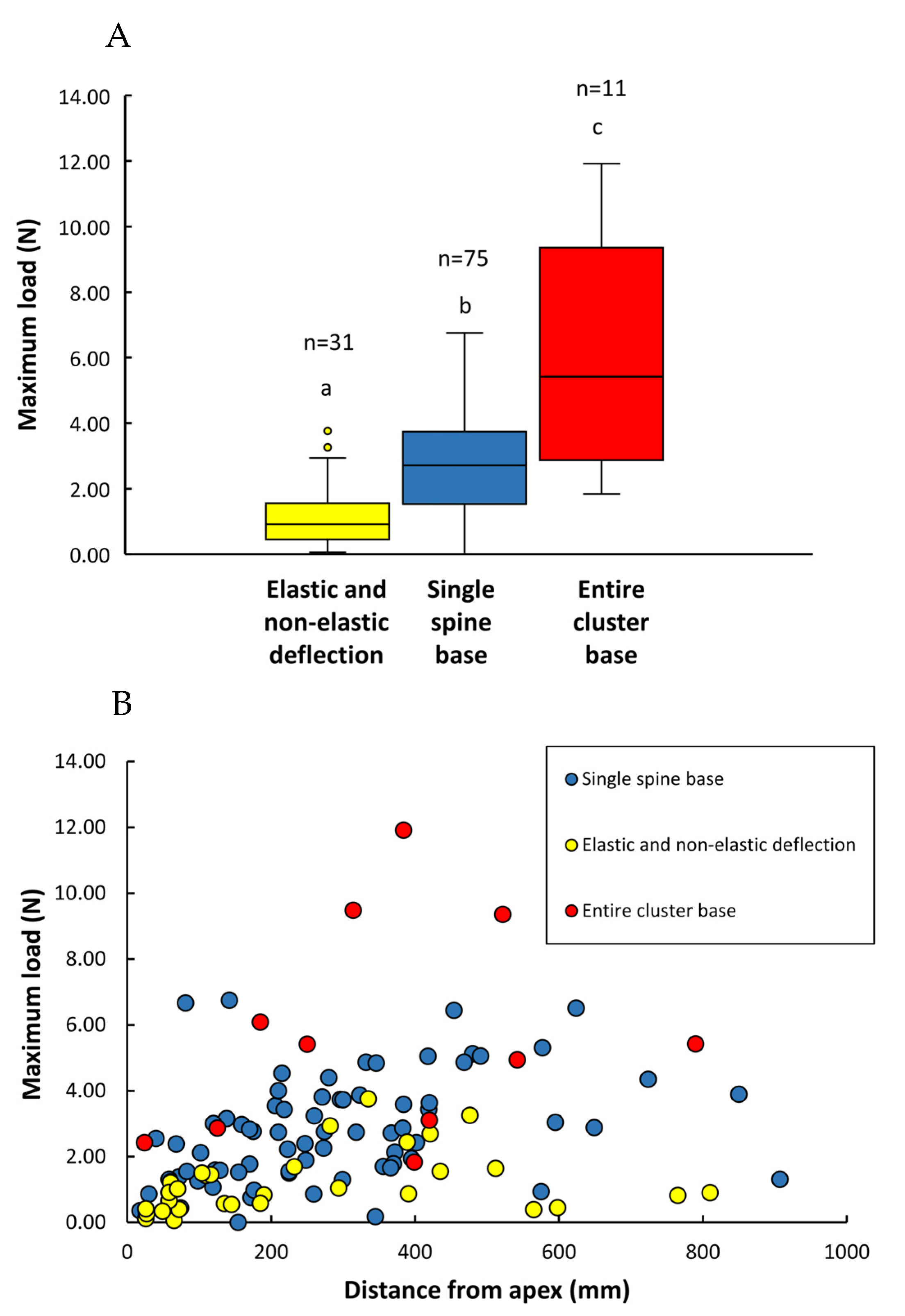
3.2. Spine Strength
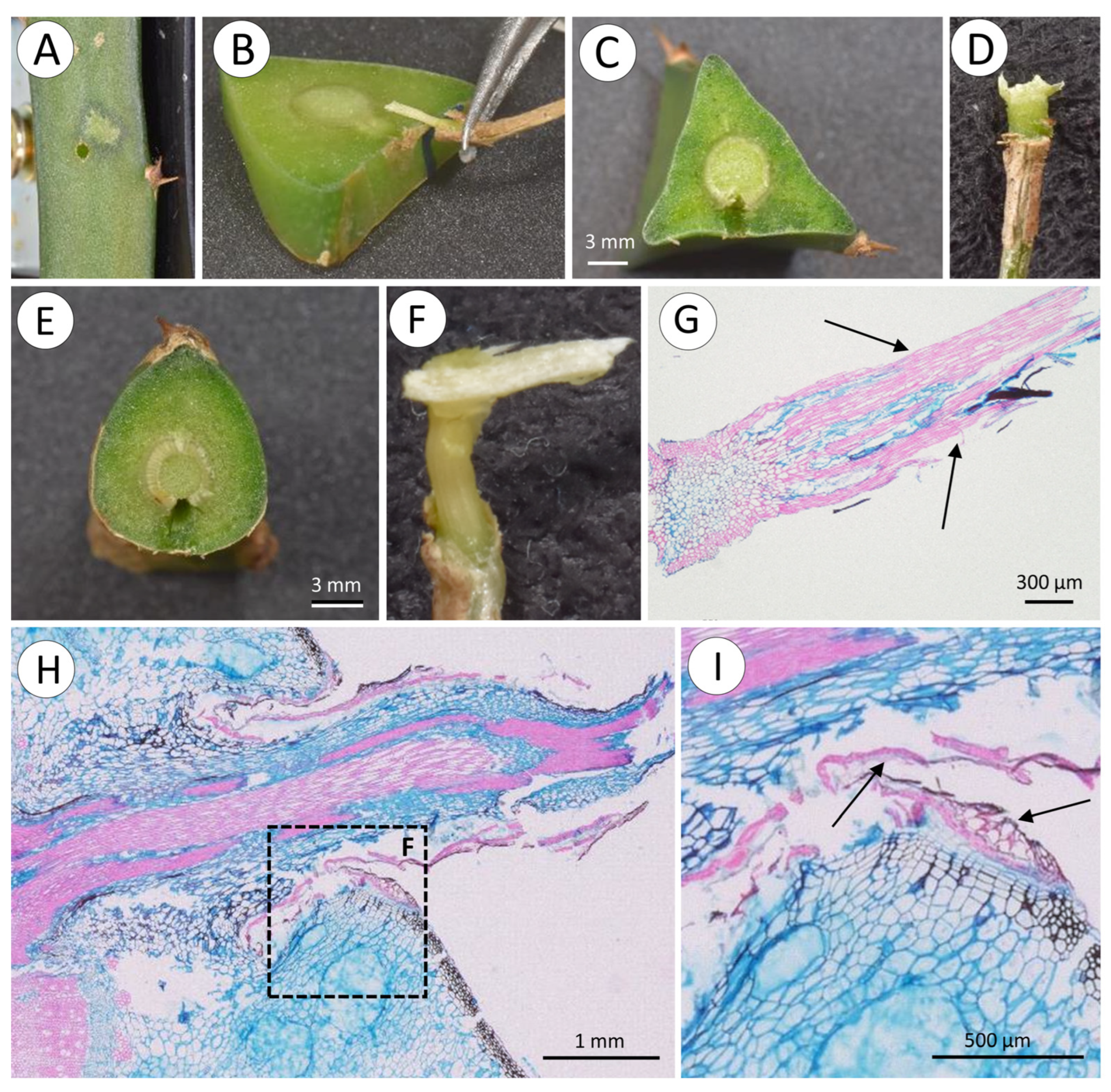
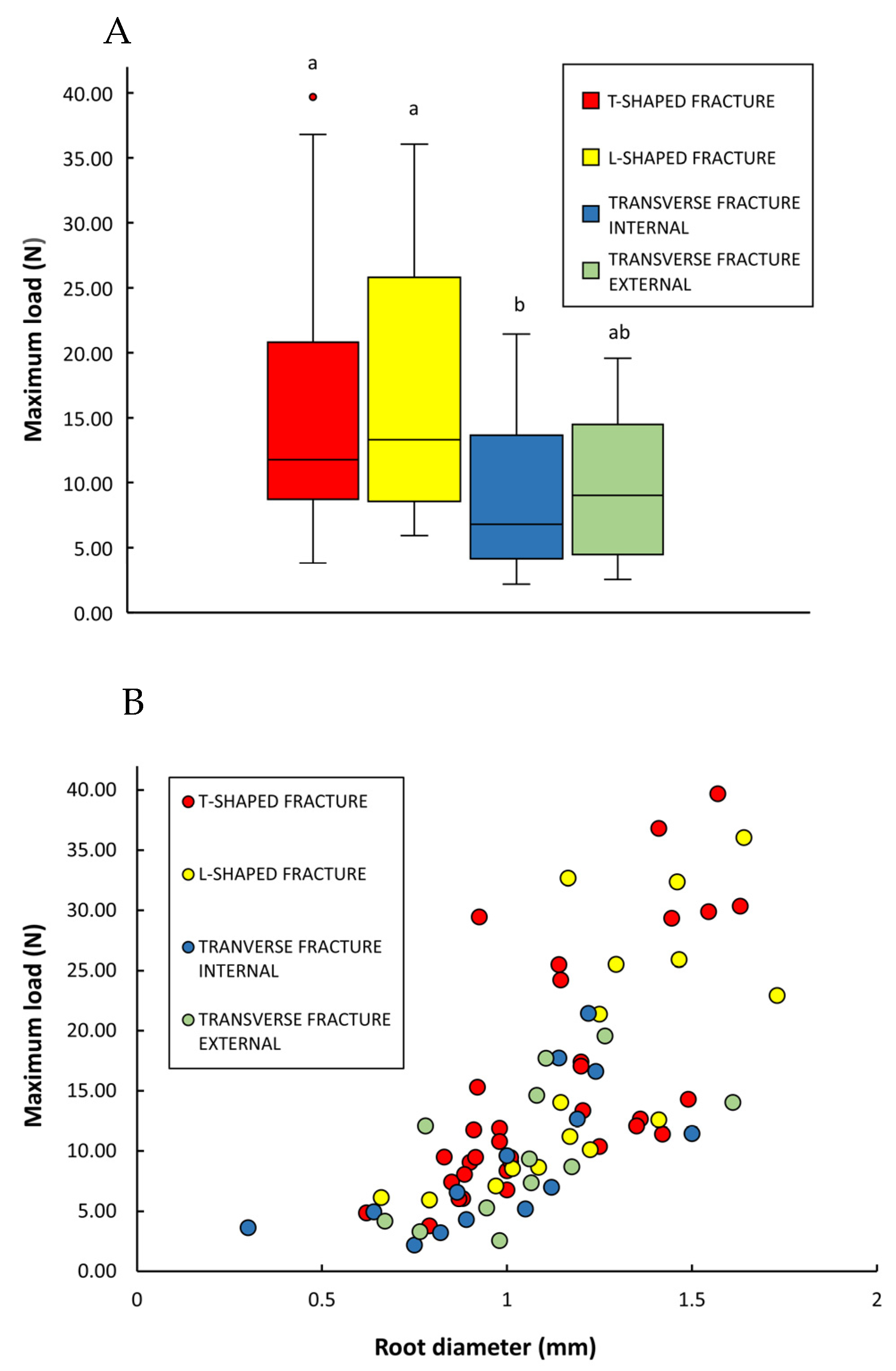
3.3. Root Strength
- Transverse fracture of the root external to the cactus stem; this involved simple transverse fracture of the root outside of the cactus stem but not at the position of the clamps gripping the root during the test.
- Transverse fracture of the root trace or root base within the soft cortex of the cactus stem (internal); this involved simple transverse failure of the root inside the stem but without any sheared flanges of tissue from the wood cylinder (Figure 9B).
- “T-shaped” fracture at the wood cylinder (Figure 9C,D); this involved separation of the root from the internal wood cylinder with flanges of sheared xylem tissue derived from the xylem cylinder above and below the point of departure of the original root trace. During the test, the flanges of sheared xylem tissue are dragged through the stem and exit the stem with the rest of the root.
- “L-shaped” fracture at the wood cylinder; this was similar to the T-shaped fracture but only involved one flange of sheared tissue from the main wood cylinder (Figure 9E–G).
| (1) Spines: types of failure | Single spine base failure (n = 75) | Entire spine base failure (n = 11) | Elastic and non-elastic deflection (n = 28) | |||
| Max Load (N) | 2.80 ± 1.59 (0.18–6.75) | 5.72 ± 3.27 (1.84–11.92) | 1.21 ± 0.94 (0.06–3.76) | |||
| (2) Roots: types of failure | T-shaped (n = 33) | L-shaped (n = 16) | Transverse fracture (internal) (n = 14) | Transverse fracture (external) (n = 12) | ||
| Max load (N) | 15.20 ± 9.71 (3.79–39.69) | 17.57 ± 10.44 (5.93–36.05) | 9.05 ± 6.08 (2.20–21.44) | 9.90 ± 5.71 (2.57–19.57) | ||
4. Discussion
4.1. Failure without Tears
4.2. Spine Deployment
4.3. Root Deployment
4.4. Spine Failure Strategies
4.5. Root Failure Strategies
4.6. Conclusions: Potential Technical Concepts and Technical Applications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzolai, B. Plant inspired growing robots. In Soft Robotics: Trends, Applications and Challenges; Laschi, C., Rossiter, J., Lida, F., Cianchetti, M., Margheri, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume Biosystems & Biorobotics. [Google Scholar] [CrossRef]
- Mazzolai, B.; Beccai, L.; Mattoli, V. Plants as model in biomimetics and biorobotics: New perspectives. Front. Bioeng. Biotechnol. 2014, 2, 2. [Google Scholar] [CrossRef]
- Fiorello, I.; Del Dottore, E.; Tramacere, F.; Mazzolai, B. Taking inspiration from climbing plants: Methodologies and benchmarks—A review. Bioinspir. Biomim. 2020, 15, 031001. [Google Scholar] [CrossRef]
- Del Dottore, E.; Mondini, A.; Sadeghi, A.; Mazzolai, B. Characterization of the growing from the tip as robot locomotion strategy. Front. Robot. AI 2019, 6, 45. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mondini, A.; Del Dottore, E.; Mattoli, V.; Beccai, L.; Taccola, S.; Lucarotti, C.; Totaro, M.; Mazzolai, B. A plant-inspired robot with soft differential bending capabilities. Bioinspir. Biomim. 2016, 12, 015001. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Mondini, A.; Mazzolai, B. Toward self-growing soft robots inspired by plant roots and based on additive manufacturing technologies. Soft Robot. 2017, 4, 211–223. [Google Scholar] [CrossRef]
- Bastola, A.K.; Rodriguez, N.; Behl, M.; Soffiatti, P.; Rowe, N.P.; Lendlein, A. Cactus-inspired design principles for soft robotics based on 3D printed hydrogel-elastomer systems. Mater. Des. 2021, 202, 109515. [Google Scholar] [CrossRef]
- Bastola, A.K.; Soffiatti, P.; Behl, M.; Lendlein, A.; Rowe, N.P. Structural performance of a climbing cactus: Making the most of softness. J. R. Soc. Interface 2021, 18, 20210040. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.; Bastola, A.K.; Behl, M.; Soffiatti, P.; Rowe, N.P.; Lendlein, A. Approaches of combining a 3D printed elastic structure and a hydrogel to create models for plant-inspired actuators. MRS Adv. 2021, 6, 625–630. [Google Scholar] [CrossRef]
- Fiorello, I.; Meder, F.; Tricinci, O.; Filippeschi, C.; Mazzolai, B. Rose-Inspired Micro-device with Variable Stiffness for Remotely Controlled Release of Objects in Robotics. In Biomimetic and Biohybrid Systems. Living Machines 2019; Martinez-Hernandez, U., Vouloutsi, V., Mura, A., Mangan, M., Asada, M., Prescott, T.J., Verschure, P.F.M.J., Eds.; Biomimetic and Biohybrid Systems; Springer International Publishing: Cham, Switzerland, 2019; Volume 11556, pp. 122–133. [Google Scholar] [CrossRef]
- Fiorello, I.; Tricinci, O.; Mishra, A.K.; Tramacere, F.; Filippeschi, C.; Mazzolai, B. Artificial System Inspired by Climbing Mechanism of Galium aparine Fabricated via 3D Laser Lithography. In Biomimetic and Biohybrid Systems. Living Machines 2018; Lecture Notes in Computer Science; Vouloutsi, V., Halloy, J., Mura, A., Mangan, M., Lepora, N., Prescott, T.J., Verschure, P.F.M.J., Eds.; Biomimetic and Biohybrid Systems; Springer International Publishing: Cham, Switzerland, 2018; Volume 10928, pp. 168–178. [Google Scholar] [CrossRef]
- Fiorello, I.; Tricinci, O.; Naselli, G.A.; Mondini, A.; Filippeschi, C.; Tramacere, F.; Mishra, A.K.; Mazzolai, B. Climbing plant-inspired micropattered devices for reversible attachment. Adv. Funct. Mater. 2020, 30, 2003380. [Google Scholar] [CrossRef]
- Walker, I.D. Biologically inspired vine-like and tendril-like robots. In Proceedings of the Science and Information Conference (SAI), London, UK, 28–30 July 2015; pp. 714–720. [Google Scholar] [CrossRef]
- Wooten, M.; Frazelle, C.; Walker, I.D.; Kapadia, A.; Lee, J.H. Exploration and inspection with vine-inspired continuum robots. In Proceedings of the 2018 IEEE International Conference on Robotics and Automation (ICRA), Brisbane, Australia, 21–25 May 2018; pp. 5526–5533. [Google Scholar] [CrossRef]
- Wooten, M.; Walker, I.D. Circumnutation: From plants to robots. In From Animals to Animats 14. SAB 2016; Tuci, E., Giagkos, A., Wilson, M., Hallam, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 9825, pp. 1–11. [Google Scholar] [CrossRef]
- Rowe, N.P.; Speck, T. Biomechanics of lianas. In The Ecology of Lianas; Schnitzer, S., Bongers, F., Burnham, R., Putz, F., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 323–341. [Google Scholar] [CrossRef]
- Putz, F.E.; Mooney, H.A. The Biology of Vines; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar] [CrossRef]
- Xu, K.; Zi, P.; Ding, X. Learning from biological attachment devices: Applications of bioinspired reversible adhesive methods in robotics. Front. Mech. Eng. 2022, 17, 43. [Google Scholar] [CrossRef]
- De Mestral, G. Separable Fastening Device. U.S. Patent 3,009,235, 1961. Internat Velcro Company. Available online: https://www.freepatentsonline.com/3009235.html (accessed on 9 May 2023).
- Palecek, A.M.; Garner, A.M.; Klittich, M.R.; Stark, A.Y.; Scherger, J.D.; Bernard, C.; Niewiarowski, P.H.; Dhinojwala, A. An investigation of gecko attachment on wet and rough substrates leads to the application of surface roughness power spectral density analysis. Sci. Rep. 2022, 12, 11556. [Google Scholar] [CrossRef]
- Isnard, S.; Cobb, A.R.; Holbrook, N.M.; Zwieniecki, M.; Dumais, J. Tensioning the helix: A mechanism for force generation in twining plants. Proc. R. Soc. Ser. B 2009, 276, 2643–2650. [Google Scholar] [CrossRef]
- Soffiatti, P.; Rowe, N.P. Mechanical innovations of a climbing cactus: Functional insights for a new generation of growing robots. Front. Robot. AI 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Gallenmüller, F.; Rowe, N.P.; Speck, T. Development and growth form of the neotropical liana Croton nuntians: The effect of light and mode of attachment on the biomechanics of the stem. J. Plant Growth Regul. 2004, 23, 83–97. [Google Scholar] [CrossRef]
- Lehnebach, R.; Paul-Victor, C.; Courric, E.; Rowe, N.P. Microspines in tropical climbing plants: A small-scale fix for life in an obstacle course. J. Exp. Bot. 2022, 73, 5650–5670. [Google Scholar] [CrossRef] [PubMed]
- Melzer, B.; Steinbrecher, T.; Seidel, R.; Kraft, O.; Schwaiger, R.; Speck, T. The attachment strategy of English ivy: A complex mechanism acting on several hierarchical levels. J. R. Soc. Interface 2010, 7, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.B.; Ewers, F.W. Wound healing in stems of lianas after twisting and girdling. Bot. Gaz. 1989, 150, 251–265. [Google Scholar] [CrossRef]
- Ménard, L.; McKey, D.; Mühlen, G.S.; Clair, B.; Rowe, N.P. The evolutionary fate of phenotypic plasticity and functional traits under domestication in manioc: Changes in stem biomechanics and the appearance of stem brittleness. PLoS ONE 2013, 8, e74727. [Google Scholar] [CrossRef]
- Putz, F.E.; Holbrook, N.M. Biomechanical studies of vines. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 73–97. [Google Scholar] [CrossRef]
- Goodman, A.M. Mechanical adaptation of cleavers (Galium aparine). Ann. Bot. 2005, 95, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Danninger, E.; Harder, D.; Speck, T.; Kraft, O.; Schwaiger, R. Quantifying the attachment strength of climbing plants: A new approach. Acta Biomater. 2010, 6, 1497–1504. [Google Scholar] [CrossRef]
- Isnard, S.; Rowe, N.P. The climbing habit in palms: Biomechanics of the cirrus and flagellum. Am. J. Bot. 2008, 95, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Isnard, S.; Rowe, N.P. Mechanical role of the leaf sheath in rattans. New Phytol. 2008, 177, 643–652. [Google Scholar] [CrossRef]
- Gallenmuller, F.; Feus, A.; Fiedler, K.; Speck, T. Rose prickles and asparagus spines—Different hook structures as attachment devices in climbing plants. PLoS ONE 2015, 10, e0143850. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica. 4, 9p. 2001. Available online: https://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 9 May 2023).
- Dransfield, J.; Uhl, N.W.; Asmussen, C.B.; Baker, W.J.; Harley, M.M.; Lewis, C.E. Genera palmarum; Royal Botanic Gardens Kew: London, UK, 2008; p. 732. [Google Scholar] [CrossRef]
- Sicangco, C.K.; Bavdekar, S.; Subhash, G.; Putz, F.E. Active space garnering by leaves of a rosette plant. Curr. Biol. 2022, 32, 352–353. [Google Scholar] [CrossRef]
- Mauseth, J.D. Structure-function relationships in highly modified shoots of Cactaceae. Ann. Bot. 2006, 98, 901–926. [Google Scholar] [CrossRef]
- Zhou, N.; Simonneau, F.; Thouroude, T.; Oyant, L.H.-S.; Foucher, F. Morphological studies of rose prickles provide new insights. Hortic. Res. 2021, 8, 221. [Google Scholar] [CrossRef]
- Ennos, A.R.; van Casteren, A. Transverse stresses and modes of failure in tree branches and other beams. Proc. R. Soc. 2010, 277, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Hausladen, M.M.; Zhao, B.; Kubala, M.S.; Francis, L.F.; Kowalewski, T.M.; Ellison, C.J. Synthetic growth by self-lubricated photopolymerization and extrusion inspired by plants and fungi. Proc. Natl. Acad. Sci. USA 2001, 119, e2201776119. [Google Scholar] [CrossRef] [PubMed]
- Mylo, M.D.; Krüger, F.; Speck, T.; Speck, O. Self-Repair in Cacti Branches: Comparative Analyses of Their Morphology, Anatomy, and Biomechanics. Int. J. Mol. Sci. 2020, 21, 4630. [Google Scholar] [CrossRef]
- Gould, S.J.; Vrba, E.S. Exaptation—A missing term in the science of form. Paleobiology 1982, 8, 4–15. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowe, N.P.; Cheng Clavel, L.; Soffiatti, P. Failure without Tears: Two-Step Attachment in a Climbing Cactus. Biomimetics 2023, 8, 220. https://doi.org/10.3390/biomimetics8020220
Rowe NP, Cheng Clavel L, Soffiatti P. Failure without Tears: Two-Step Attachment in a Climbing Cactus. Biomimetics. 2023; 8(2):220. https://doi.org/10.3390/biomimetics8020220
Chicago/Turabian StyleRowe, Nick P., Lily Cheng Clavel, and Patricia Soffiatti. 2023. "Failure without Tears: Two-Step Attachment in a Climbing Cactus" Biomimetics 8, no. 2: 220. https://doi.org/10.3390/biomimetics8020220
APA StyleRowe, N. P., Cheng Clavel, L., & Soffiatti, P. (2023). Failure without Tears: Two-Step Attachment in a Climbing Cactus. Biomimetics, 8(2), 220. https://doi.org/10.3390/biomimetics8020220





