Harnessing Biofabrication Strategies to Re-Surface Osteochondral Defects: Repair, Enhance, and Regenerate
Abstract
1. Introduction
2. Osteochondral Tissue Architecture
3. Osteochondral Diseases and Clinical Management
3.1. Palliative Treatments
3.2. Reparative Treatments
3.3. Regenerative Treatments
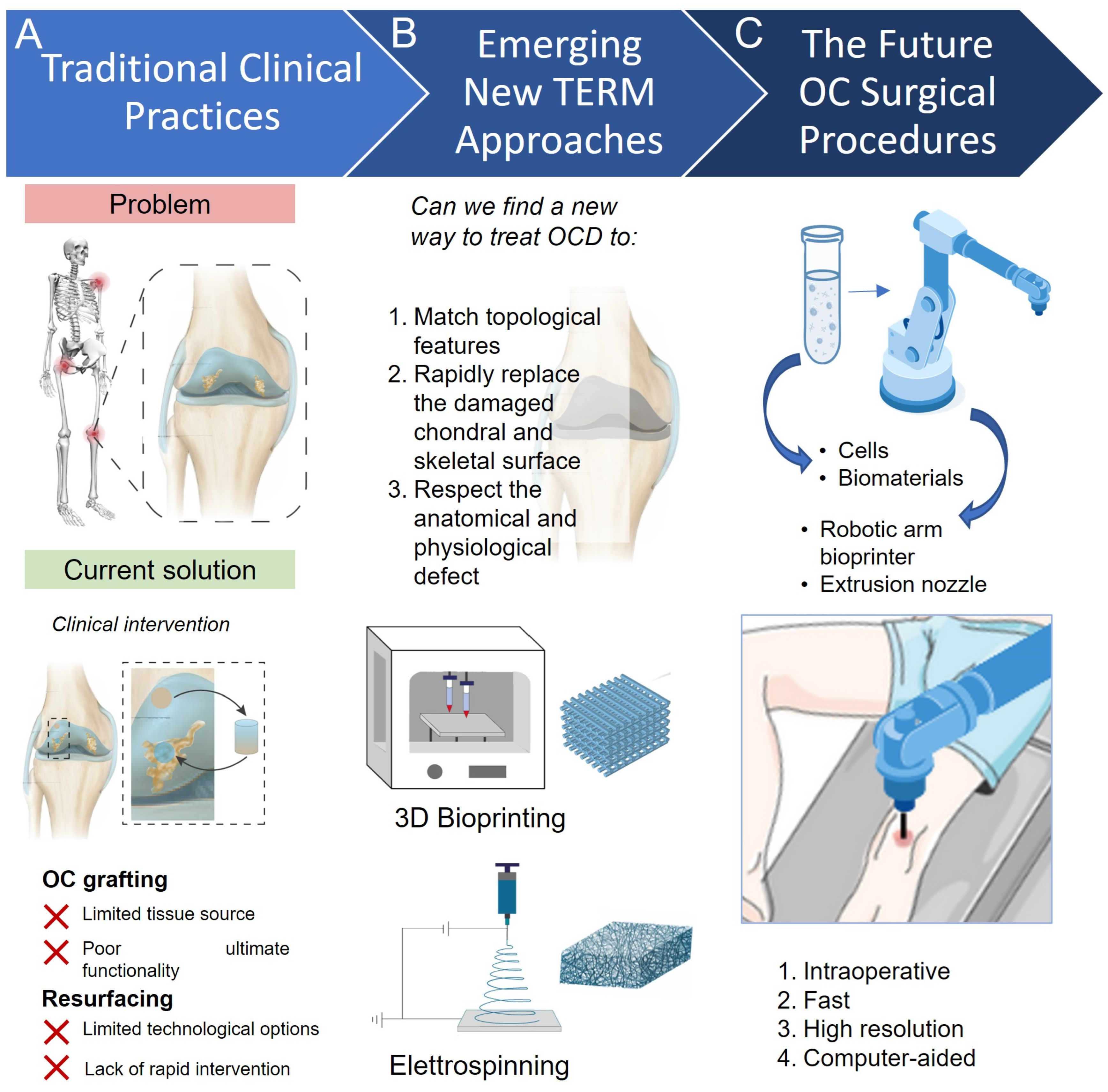
4. Resurfacing in Orthopedics
5. Technologies for Bone Resurfacing
5.1. Electrospinning
5.2. 3D Bioprinting
5.2.1. Inkjet–Based Bioprinting
5.2.2. Laser–Assisted Bioprinting
5.2.3. Extrusion–Based Bioprinting
6. New Platforms for Functional Tissue Resurfacing
6.1. Electrospinning to Re-Engineer Osteochondral Surfaces
6.2. 3D Bioprinting Approaches for Bone Resurfacing
6.3. Advance Technology In-Vivo via Tissue Engineering Osteo—Chondral Resurfacing
| Animal | Therapy (T) and Findings (F) | Ref. | ||
|---|---|---|---|---|
| Electrospinning Resurfacing | Calf | T | A cartilage graft (PLGA/PCL) enhanced with chemotactic factor (IGF-1) | [67] |
| F | The defect regeneration is improved by promoting cell-mediated integrative cartilage. | |||
| Ovine | T | A cell-free PCL electrospun scaffold made with aligned microfibers | [68] | |
| F | The aligned scaffold exhibited high levels of cell colonization, demonstrating that the aligned fibers improve cell viability. | |||
| Rabbit | T | An aligned porous (PLLA) electrospun-coated scaffold | [63] | |
| F | The biological effect is significantly increased, and the combination of aligned porous hierarchical structure exhibits high regenerative properties. | |||
| Porcine | T | A hierarchical scaffold designed to mimic the articular cartilage structure (multiple techniques) | [64] | |
| F | The retention, osteointegration, and prolonged degradation of the scaffold were acceptable with beneficial effects. | |||
| 3D Bioprinting Resurfacing | Rat | T | A microfluidic extruder to compartmentalize OCD | [94] |
| F | The possibility of mimicking the biological and mechanical gradient structure of cartilage interface is demonstrated. | |||
| Rat | T | A construct with collagenous bio-ink for cartilage regeneration | [85] | |
| F | A high concentration of collagen generates new tissue rich in GAGs and type II collagen. | |||
| Rabbit | T | An anisotropic pore gradient-structured cartilage 3D scaffold combining printing of hydrogel and PCL fibers with BMSC and HIF1α/FAK. | [65] | |
| F | The scaffold generated and maintained stable cartilage phenotype in different layers, and the ECM implant composition induced cartilage similar to native tissue. | |||
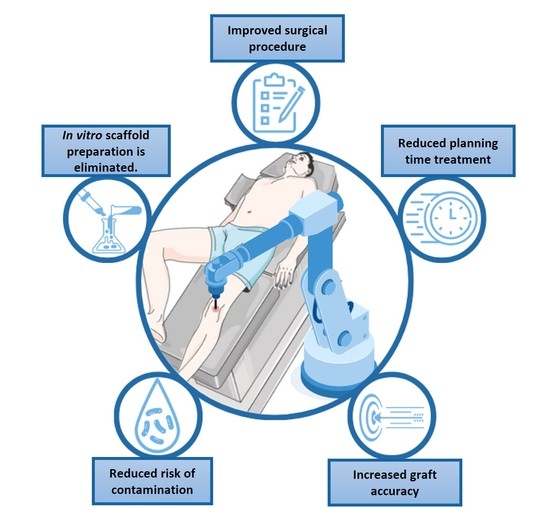
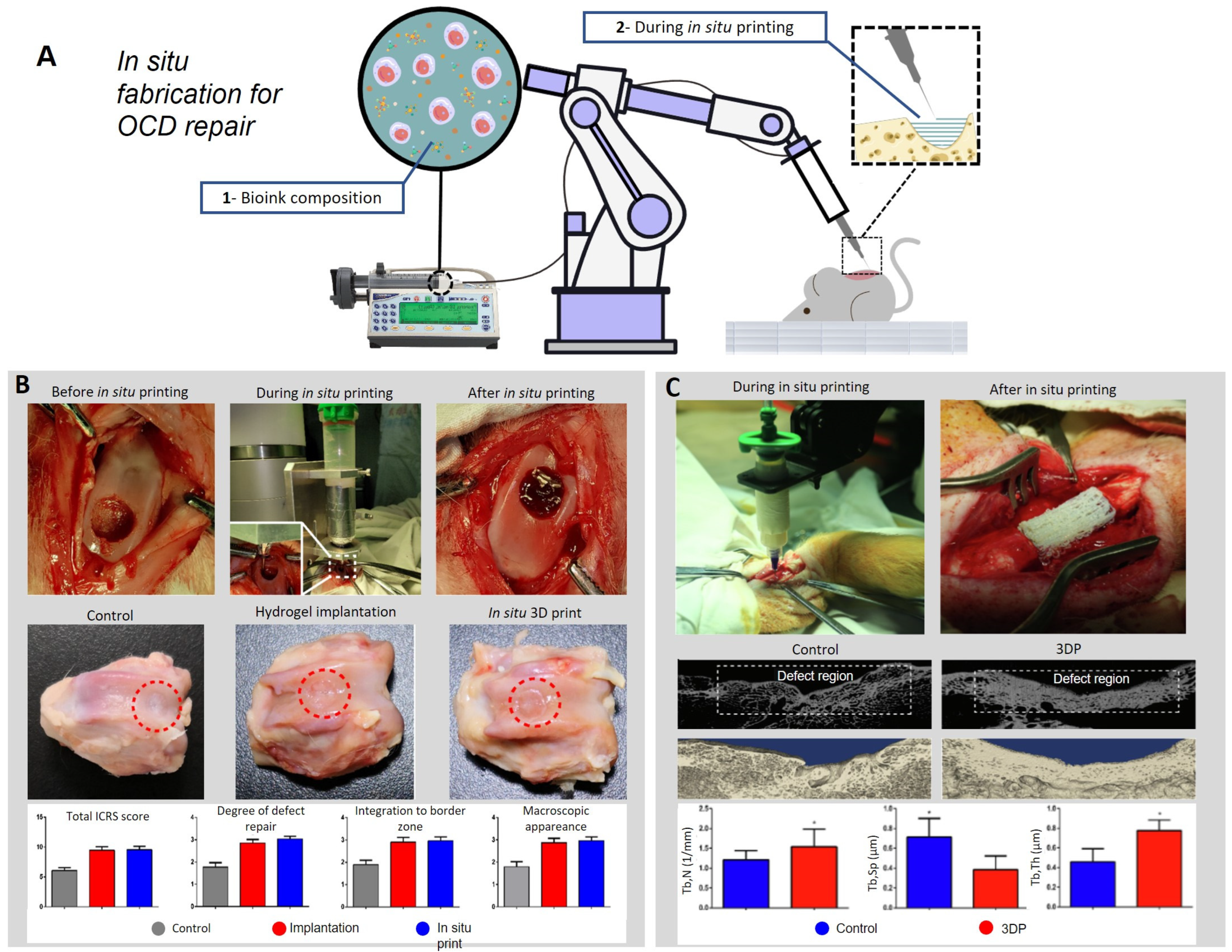
| In situ approaches | Rabbit | T | A robotic arm is used for in situ 3D printing process, depositing the bio-ink directly inside the defect. | [89] |
| F | The regenerated tissue faithfully reproduces the native tissue composition and morphology, demonstrating that the technology can improve the surgical procedure in clinical application. | |||
7. Summary, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Goldring, S.R.; Goldring, M.B. Changes in the Osteochondral Unit during Osteoarthritis: Structure, Function and Cartilage Bone Crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Vilela, C.A.; da Silva Morais, A.; Pina, S.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Espregueira-Mendes, J. Clinical Trials and Management of Osteochondral Lesions. Adv. Exp. Med. Biol. 2018, 1058, 391–413. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. (Eds.) Principles of Tissue Engineering, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, ISBN 9781119130536. [Google Scholar]
- Iafrate, L.; Benedetti, M.C.; Donsante, S.; Rosa, A.; Corsi, A.; Oreffo, R.O.C.; Riminucci, M.; Ruocco, G.; Scognamiglio, C.; Cidonio, G. Modelling Skeletal Pain Harnessing Tissue Engineering. Vitr. Model. 2022, 1, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An Educational Review of Cartilage Repair: Precepts & Practice—Myths & Misconceptions—Progress & Prospects. Osteoarthr. Cartil. 2015, 23, 334–350. [Google Scholar] [CrossRef]
- Vyas, C.; Mishbak, H.; Cooper, G.; Peach, C.; Pereira, R.F.; Bartolo, P. Biological Perspectives and Current Biofabrication Strategies in Osteochondral Tissue Engineering. Biomanuf. Rev. 2020, 5, 2. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Qiao, S.; Zhang, W. Carbon-Based Nanomaterials for Bone and Cartilage Regeneration: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4718–4735. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular Cartilage and Osteochondral Tissue Engineering Techniques: Recent Advances and Challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Engineering Large, Anatomically Shaped Osteochondral Constructs with Robust Interfacial Shear Properties. npj Regen. Med. 2021, 6, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. 3D Diffusion Model within the Collagen Apatite Porosity: An Insight to the Nanostructure of Human Trabecular Bone. PLoS ONE 2017, 12, e0189041. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. A 3D Model of the Effect of Tortuosity and Constrictivity on the Diffusion in Mineralized Collagen Fibril. Sci. Rep. 2019, 9, 2658. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. Percolation Networks inside 3D Model of the Mineralized Collagen Fibril. Sci. Rep. 2021, 11, 11398. [Google Scholar] [CrossRef]
- Okesola, B.O.; Mendoza-Martinez, A.K.; Cidonio, G.; Derkus, B.; Boccorh, D.K.; Osuna de la Peña, D.; Elsharkawy, S.; Wu, Y.; Dawson, J.I.; Wark, A.W.; et al. De Novo Design of Functional Coassembling Organic–Inorganic Hydrogels for Hierarchical Mineralization and Neovascularization. ACS Nano 2021, 15, 11202–11217. [Google Scholar] [CrossRef]
- Marinozzi, F.; Bini, F.; De Paolis, A.; De Luca, R.; Marinozzi, A. Effects of Hip Osteoarthritis on Mechanical Stimulation of Trabecular Bone: A Finite Element Study. J. Med. Biol. Eng. 2015, 35, 535–544. [Google Scholar] [CrossRef]
- Marinozzi, F.; Bini, F.; De Paolis, A.; Zuppante, F.; Bedini, R.; Marinozzi, A. A Finite Element Analysis of Altered Load Distribution within Femoral Head in Osteoarthritis. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2015, 3, 84–90. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. Prediction of Stress and Strain Patterns from Load Rearrangement in Human Osteoarthritic Femur Head: Finite Element Study with the Integration of Muscular Forces and Friction Contact. In New Developments on Computational Methods and Imaging in Biomechanics and Biomedical Engineering; Lecture Notes in Computational Vision and Biomechanics; Springer: Cham, Switzerland, 2019; Volume 999, pp. 49–64. [Google Scholar]
- Xu, J.; Ji, J.; Jiao, J.; Zheng, L.; Hong, Q.; Tang, H.; Zhang, S.; Qu, X.; Yue, B. 3D Printing for Bone-Cartilage Interface Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Xu, C.; Zhou, Q.; Cheng, Y. Advances of Nanotechnology in Osteochondral Regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1576. [Google Scholar] [CrossRef] [PubMed]
- Condron, N.B.; Kester, B.S.; Tokish, J.M.; Zumstein, M.A.; Gobezie, R.; Scheibel, M.; Cole, B.J. Nonoperative and Operative Soft-Tissue, Cartilage, and Bony Regeneration and Orthopaedic Biologics of the Shoulder: An Orthoregeneration Network (ON) Foundation Review. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 3200–3218. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Ribeiro, V.P.; Reis, R.L. Advances on Gradient Scaffolds for Osteochondral Tissue Engineering. Prog. Biomed. Eng. 2021, 3, 033101. [Google Scholar] [CrossRef]
- Fu, J.N.; Wang, X.; Yang, M.; Chen, Y.R.; Zhang, J.Y.; Deng, R.H.; Zhang, Z.N.; Yu, J.K.; Yuan, F.Z. Scaffold-Based Tissue Engineering Strategies for Osteochondral Repair. Front. Bioeng. Biotechnol. 2022, 9, 1–21. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef]
- Van der Heide, D.; Cidonio, G.; Stoddart, M.J.; D’Este, M. 3D Printing of Inorganic-Biopolymer Composites for Bone Regeneration. Biofabrication 2022, 14, 042003. [Google Scholar] [CrossRef]
- Amstutz, H.C.; Grigoris, P.; Dorey, F.J. Evolution and Future of Surface Replacement of the Hip. J. Orthop. Sci. 1998, 3, 169–186. [Google Scholar] [CrossRef]
- Marsh, M.; Newman, S. Trends and Developments in Hip and Knee Arthroplasty Technology. J. Rehabil. Assist. Technol. Eng. 2021, 8, 205566832095204. [Google Scholar] [CrossRef]
- Athiviraham, A.; Kodali, P.; Miniaci, A. Joint Resurfacing of the Shoulder and Knee in Athletes. In Sports Injuries: Prevention, Diagnosis, Treatment and Rehabilitation, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2419–2431. [Google Scholar] [CrossRef]
- Peebles, L.A.; Arner, J.W.; Haber, D.B.; Provencher, M.T. Glenohumeral Resurfacing in Young, Active Patients With End-Stage Osteoarthritis of the Shoulder. Arthrosc. Tech. 2020, 9, e1315–e1322. [Google Scholar] [CrossRef]
- Hodge, W.A.; Fitts, S.M. Hip Resurfacing: A Fair and Balanced Review. Semin. Arthroplast. JSES 2006, 17, 35–41. [Google Scholar] [CrossRef]
- Lawrie, C.M.; Barrack, R.L. Hip Resurfacing Arthroplasty—What Has History Taught Us? Ann. Jt. 2020, 5, 20. [Google Scholar] [CrossRef]
- Marker, D.R.; Strimbu, K.; McGrath, M.S.; Zywiel, M.G.; Mont, M.A. Resurfacing versus Conventional Total Hip Arthroplasty: Review of Comparative Clinical and Basic Science Studies. Bull. NYU Hosp. Jt. Dis. 2009, 67, 120–127. [Google Scholar] [PubMed]
- Hellman, M.D.; Ford, M.C.; Barrack, R.L. Is There Evidence to Support an Indication for Surface Replacement Arthroplasty? A Systematic Review. Bone Jt. J. 2019, 101B, 32–40. [Google Scholar] [CrossRef]
- Meaike, J.J.; Patterson, D.C.; Anthony, S.G.; Parsons, B.O.; Cagle, P.J. Soft Tissue Resurfacing for Glenohumeral Arthritis: A Systematic Review. Shoulder Elb. 2020, 12, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Ciuffreda, M.; Mannering, N.; D’Andrea, V.; Cimmino, M.; Denaro, V. Patellar Resurfacing in Total Knee Arthroplasty: Systematic Review and Meta-Analysis. J. Arthroplast. 2018, 33, 620–632. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning Process and Applications of Electrospun Fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Guettler, J.H.; Demetropoulos, C.K.; Yang, K.H.; Jurist, K.A. Osteochondral Defects in the Human Knee: Influence of Defect Size on Cartilage Rim Stress and Load Redistribution to Surrounding Cartilage. Am. J. Sports Med. 2004, 32, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-S.; Kim, C.-W.; Jung, D.-W. Management of Focal Chondral Lesion in the Knee Joint. Knee Surg. Relat. Res. 2011, 23, 185–196. [Google Scholar] [CrossRef]
- Sahoo, B.; Panda, P.K. Preparation and Characterization of Barium Titanate Nanofibers by Electrospinning. Ceram. Int. 2012, 38, 5189–5193. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on Morphology of Electrospun Poly(Vinyl Alcohol) Mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Yang, G.Z.; Li, H.P.; Yang, J.H.; Wan, J.; Yu, D.G. Influence of Working Temperature on The Formation of Electrospun Polymer Nanofibers. Nanoscale Res. Lett. 2017, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Menceloǧlu, Y.Z.; Acatay, K.; Vargel, I.; Pişkin, E. In Vitro and in Vivo Degradation of Non-Woven Materials Made of Poly(ε-Caprolactone) Nanofibers Prepared by Electrospinning under Different Conditions. J. Biomater. Sci. Polym. Ed. 2005, 16, 1537–1555. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in Three-Dimensional Nanofibrous Macrostructures via Electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and Characterization of PCL/Gelatin Composite Nanofibrous Scaffold for Tissue Engineering Applications by Electrospinning Method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wang, H.; Wang, X. Self-Crimping Bicomponent Nanofibers Electrospun from Polyacrylonitrile and Elastomeric Polyurethane. Adv. Mater. 2005, 17, 2699–2703. [Google Scholar] [CrossRef]
- Ko, Y.M.; Choi, D.Y.; Jung, S.C.; Kim, B.H. Characteristics of Plasma Treated Electrospun Polycaprolactone (PCL) Nanofiber Scaffold for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2015, 15, 192–195. [Google Scholar] [CrossRef]
- Manso, M.; Valsesia, A.; Ceccone, G.; Rossi, F. Activation of PCL Surface by Ion Beam Treatment to Enhance Protein Adsorption. J. Bioact. Compat. Polym. 2004, 19, 287–300. [Google Scholar] [CrossRef]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The Preparation and Characterization of Polycaprolactone/Graphene Oxide Biocomposite Nanofiber Scaffolds and Their Application for Directing Cell Behaviors. Carbon N. Y. 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Ren, W.P.; Song, W.; Esquivel, A.O.; Jackson, N.M.; Nelson, M.; Flynn, J.C.; Markel, D.C. Effect of Erythromycin-Doped Calcium Polyphosphate Scaffold Composite in a Mouse Pouch Infection Model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1140–1147. [Google Scholar] [CrossRef]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication Strategies for 3D in Vitro Models and Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Gurkan, U.A.; El Assal, R.; Yildiz, S.E.; Sung, Y.; Trachtenberg, A.J.; Kuo, W.P.; Demirci, U. Engineering Anisotropic Biomimetic Fibrocartilage Microenvironment by Bioprinting Mesenchymal Stem Cells in Nanoliter Gel Droplets. Mol. Pharm. 2014, 11, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Chan Choi, Y.; Cho, Y.W. Piezoelectric Inkjet Printing of Polymers: Stem Cell Patterning on Polymer Substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of Fused Deposition Modeling Process Parameters: A Review of Current Research and Future Prospects. Adv. Manuf. 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The Cell in the Ink: Improving Biofabrication by Printing Stem Cells for Skeletal Regenerative Medicine. Biomaterials 2019, 209, 10–24. [Google Scholar] [CrossRef]
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol–Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Gu, Y.; Wu, Y.; Bunpetch, V.; Zhang, S. Lithography-Based 3D Bioprinting and Bioinks for Bone Repair and Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Starly, B.; Daly, A.C.; Burdick, J.A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M.; et al. The Bioprinting Roadmap. Biofabrication 2020, 12, 022002. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, N.I.; Hibino, N.; Nakayama, K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng. Part B Rev. 2017, 23, 237–244. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the Definition of an Evolving Field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Shi, J.; Shen, S.; Teng, H.; Yang, J.; Wang, X.; Jiang, Q. In Situ Repair of Bone and Cartilage Defects Using 3D Scanning and 3D Printing. Sci. Rep. 2017, 7, 9416. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Healthc. Mater. 2015, 4, 1359–1368. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Li, J.; Jiang, Y.; Li, L.; Yao, Q.; Ke, Q.; Xu, H. Aligned Porous Fibrous Membrane with a Biomimetic Surface to Accelerate Cartilage Regeneration. Chem. Eng. J. 2019, 370, 1027–1038. [Google Scholar] [CrossRef]
- Steele, J.A.M.; Moore, A.C.; St-Pierre, J.-P.; McCullen, S.D.; Gormley, A.J.; Horgan, C.C.; Black, C.R.; Meinert, C.; Klein, T.; Saifzadeh, S.; et al. In Vitro and in Vivo Investigation of a Zonal Microstructured Scaffold for Osteochondral Defect Repair. Biomaterials 2022, 286, 121548. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Q.; Zhang, Y.; Dai, K.; Wei, Y. 3D-Bioprinted Gradient-Structured Scaffold Generates Anisotropic Cartilage with Vascularization by Pore-Size-Dependent Activation of HIF1α/FAK Signaling Axis. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102426. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhenyu, L.I.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.E.; Wei, Y. Influence of Solvents on the Formation of Ultrathin Uniform Poly(Vinyl Pyrrolidone) Nanofibers with Electrospinning. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Boushell, M.K.; Mosher, C.Z.; Suri, G.K.; Doty, S.B.; Strauss, E.J.; Hunziker, E.B.; Lu, H.H. Polymeric Mesh and Insulin-like Growth Factor 1 Delivery Enhance Cell Homing and Graft−cartilage Integration. Ann. N. Y. Acad. Sci. 2019, 1442, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Gluais, M.; Clouet, J.; Fusellier, M.; Decante, C.; Moraru, C.; Dutilleul, M.; Veziers, J.; Lesoeur, J.; Dumas, D.; Abadie, J.; et al. In Vitro and in Vivo Evaluation of an Electrospun-Aligned Microfibrous Implant for Annulus Fibrosus Repair. Biomaterials 2019, 205, 81–93. [Google Scholar] [CrossRef]
- Liang, X.; Qi, Y.; Pan, Z.; He, Y.; Liu, X.; Cui, S.; Ding, J. Design and Preparation of Quasi-Spherical Salt Particles as Water-Soluble Porogens to Fabricate Hydrophobic Porous Scaffolds for Tissue Engineering and Tissue Regeneration. Mater. Chem. Front. 2018, 2, 1539–1553. [Google Scholar] [CrossRef]
- Pot, M.W.; Faraj, K.A.; Adawy, A.; Van Enckevort, W.J.P.; Van Moerkerk, H.T.B.; Vlieg, E.; Daamen, W.F.; Van Kuppevelt, T.H. Versatile Wedge-Based System for the Construction of Unidirectional Collagen Scaffolds by Directional Freezing: Practical and Theoretical Considerations. ACS Appl. Mater. Interfaces 2015, 7, 8495–8505. [Google Scholar] [CrossRef]
- Brown, T.D.; Slotosch, A.; Thibaudeau, L.; Taubenberger, A.; Loessner, D.; Vaquette, C.; Dalton, P.D.; Hutmacher, D.W. Design and Fabrication of Tubular Scaffolds via Direct Writing in a Melt Electrospinning Mode. Biointerphases 2012, 7, 1–16. [Google Scholar] [CrossRef]
- Groen, W.M.; Diloksumpan, P.; van Weeren, P.R.; Levato, R.; Malda, J. From Intricate to Integrated: Biofabrication of Articulating Joints. J. Orthop. Res. 2017, 35, 2089–2097. [Google Scholar] [CrossRef]
- Hollenstein, J.; Terrier, A.; Cory, E.; Chen, A.C.; Sah, R.L.; Pioletti, D.P. Mechanical Evaluation of a Tissue-Engineered Zone of Calcification in a Bone–Hydrogel Osteochondral Construct. Comput. Methods Biomech. Biomed. Engin. 2015, 18, 332–337. [Google Scholar] [CrossRef]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef] [PubMed]
- Cidonio, G.; Glinka, M.; Kim, Y.-H.; Kanczler, J.M.; Lanham, S.A.; Ahlfeld, T.; Lode, A.; Dawson, J.I.; Gelinsky, M.; Oreffo, R.O.C. Nanoclay-Based 3D Printed Scaffolds Promote Vascular Ingrowth Ex Vivo and Generate Bone Mineral Tissue in Vitro and in Vivo. Biofabrication 2020, 12, 035010. [Google Scholar] [CrossRef] [PubMed]
- Cidonio, G.; Cooke, M.; Glinka, M.; Dawson, J.I.; Grover, L.; Oreffo, R.O.C. Printing Bone in a Gel: Using Nanocomposite Bioink to Print Functionalised Bone Scaffolds. Mater. Today Bio 2019, 4, 100028. [Google Scholar] [CrossRef] [PubMed]
- Cidonio, G.; Glinka, M.; Kim, Y.-H.; Dawson, J.I.; Oreffo, R.O.C. Nanocomposite Clay-Based Bioinks for Skeletal Tissue Engineering. In Computer-Aided Tissue Engineering: Methods and Protocols; Rainer, A., Moroni, L., Eds.; Springer US: New York, NY, USA, 2021; pp. 63–72. ISBN 978-1-0716-0611-7. [Google Scholar]
- Scognamiglio, C.; Soloperto, A.; Ruocco, G.; Cidonio, G. Bioprinting Stem Cells: Building Physiological Tissues One Cell at a Time. Am. J. Physiol. Cell Physiol. 2020, 319, C465–C480. [Google Scholar] [CrossRef]
- Cidonio, G.; Costantini, M.; Pierini, F.; Scognamiglio, C.; Agarwal, T.; Barbetta, A. 3D Printing of Biphasic Inks: Beyond Single-Scale Architectural Control. J. Mater. Chem. C 2021, 9, 12489–12508. [Google Scholar] [CrossRef]
- Arguchinskaya, N.V.; Beketov, E.E.; Isaeva, E.V.; Sergeeva, N.S.; Shegay, P.V.; Ivanov, S.A.; Kaprin, A.D. Materials for Creating Tissue-Engineered Constructs Using 3D Bioprinting: Cartilaginous and Soft Tissue Restoration. Vestn. Transpl. I Iskusstv. Organov 2021, 23, 60–74. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-Alginate as Bioink for Three-Dimensional (3D) Cell Printing Based Cartilage Tissue Engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef]
- Puetzer, J.L.; Bonassar, L.J. High Density Type i Collagen Gels for Tissue Engineering of Whole Menisci. Acta Biomater. 2013, 9, 7787–7795. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Beketov, E.E.; Isaeva, E.V.; Yakovleva, N.D.; Demyashkin, G.A.; Arguchinskaya, N.V.; Kisel, A.A.; Lagoda, T.S.; Malakhov, E.P.; Kharlov, V.I.; Osidak, E.O.; et al. Bioprinting of Cartilage with Bioink Based on High-Concentration Collagen and Chondrocytes. Int. J. Mol. Sci. 2021, 22, 1351. [Google Scholar] [CrossRef]
- Bramfeld, H.; Sabra, G.; Centis, V.; Vermette, P. Scaffold Vascularization: A Challenge for Three-Dimensional Tissue Engineering. Curr. Med. Chem. 2010, 17, 3944–3967. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and Challenges of Translational 3D Bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, T.; Yang, L.; Luo, S.; Wang, Z.; Ye, C. In Situ Cell Electrospun Using a Portable Handheld Electrospinning Apparatus for the Repair of Wound Healing in Rats. Int. Wound J. 2022, 19, 1693–1704. [Google Scholar] [CrossRef]
- Ma, K.; Zhao, T.; Yang, L.; Wang, P.; Jin, J.; Teng, H.; Xia, D.; Zhu, L.; Li, L.; Jiang, Q.; et al. Application of Robotic-Assisted in Situ 3D Printing in Cartilage Regeneration with HAMA Hydrogel: An in Vivo Study. J. Adv. Res. 2020, 23, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Liao, M.; Dai, L.; Tang, Y.; Zhang, H.; Coates, P.; Sefat, F.; Zheng, L.; Song, J.; et al. Aligned Electrospun Cellulose Scaffolds Coated with RhBMP-2 for Both in Vitro and in Vivo Bone Tissue Engineering. Carbohydr. Polym. 2019, 213, 27–38. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, M.; Yan, F.; Xie, Y.; Liu, Z.; Huang, H.; Yang, Z.; Yao, S.; Cai, L. A Radial 3D Polycaprolactone Nanofiber Scaffold Modified by Biomineralization and Silk Fibroin Coating Promote Bone Regeneration in Vivo. Int. J. Biol. Macromol. 2021, 172, 19–29. [Google Scholar] [CrossRef]
- Li, L.; Shi, J.; Ma, K.; Jin, J.; Wang, P.; Liang, H.; Cao, Y.; Wang, X.; Jiang, Q. Robotic in Situ 3D Bio-Printing Technology for Repairing Large Segmental Bone Defects. J. Adv. Res. 2021, 30, 75–84. [Google Scholar] [CrossRef]
- Lipskas, J.; Deep, K.; Yao, W. Robotic-Assisted 3D Bio-Printing for Repairing Bone and Cartilage Defects through a Minimally Invasive Approach. Sci. Rep. 2019, 9, 3746. [Google Scholar] [CrossRef]
- Idaszek, J.; Marco, C.; Tommy, K.A.; Jakub, J.; Colosi, C.; Testa, S.; Fornetti, E.; Bernardini, S.; Seta, M.; Kasarełło, K. 3D Bioprinting of Hydrogel Constructs with Cell and Material Gradients for the Regeneration of Full-Thickness Chondral Defect Using a Microfluidic Printing Head. Biofabrication 2019, 11, 044101. [Google Scholar] [CrossRef]
- Erickson, B.J.; Chalmers, P.N.; Yanke, A.B.; Cole, B.J. Surgical Management of Osteochondritis Dissecans of the Knee. Curr. Rev. Musculoskelet. Med. 2013, 6, 102–114. [Google Scholar] [CrossRef]
- Zhou, L.; Gjvm, V.O.; Malda, J.; Stoddart, M.J.; Lai, Y.; Richards, R.G.; Ki-wai Ho, K.; Qin, L. Innovative Tissue-Engineered Strategies for Osteochondral Defect Repair and Regeneration: Current Progress and Challenges. Adv. Healthc. Mater. 2020, 9, 2001008. [Google Scholar] [CrossRef]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in Skin Regeneration: Application of Electrospun Scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A Three-Dimensional Nanofibrous Scaffold for Cartilage Tissue Engineering Using Human Mesenchymal Stem Cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, S.; Morsi, Y.; El-Hamshary, H.; El-Newhy, M.; Fan, C.; Mo, X. Superabsorbent 3D Scaffold Based on Electrospun Nanofibers for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 24415–24425. [Google Scholar] [CrossRef]
- Marcotulli, M.; Tirelli, M.C.; Volpi, M.; Jaroszewicz, J.; Scognamiglio, C.; Kasprzycki, P.; Karnowski, K.; Święszkowski, W.; Ruocco, G.; Costantini, M.; et al. Microfluidic 3D Printing of Emulsion Ink for Engineering Porous Functionally Graded Materials. Adv. Mater. Technol. 2022, 8, 2201244. [Google Scholar] [CrossRef]
- Bartha, L.; Vajda, A.; Duska, Z.; Rahmeh, H.; Hangody, L. Autologous Osteochondral Mosaicplasty Grafting. J. Orthop. Sports Phys. Ther. 2006, 36, 739–750. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bini, F.; D’Alessandro, S.; Pica, A.; Marinozzi, F.; Cidonio, G. Harnessing Biofabrication Strategies to Re-Surface Osteochondral Defects: Repair, Enhance, and Regenerate. Biomimetics 2023, 8, 260. https://doi.org/10.3390/biomimetics8020260
Bini F, D’Alessandro S, Pica A, Marinozzi F, Cidonio G. Harnessing Biofabrication Strategies to Re-Surface Osteochondral Defects: Repair, Enhance, and Regenerate. Biomimetics. 2023; 8(2):260. https://doi.org/10.3390/biomimetics8020260
Chicago/Turabian StyleBini, Fabiano, Salvatore D’Alessandro, Andrada Pica, Franco Marinozzi, and Gianluca Cidonio. 2023. "Harnessing Biofabrication Strategies to Re-Surface Osteochondral Defects: Repair, Enhance, and Regenerate" Biomimetics 8, no. 2: 260. https://doi.org/10.3390/biomimetics8020260
APA StyleBini, F., D’Alessandro, S., Pica, A., Marinozzi, F., & Cidonio, G. (2023). Harnessing Biofabrication Strategies to Re-Surface Osteochondral Defects: Repair, Enhance, and Regenerate. Biomimetics, 8(2), 260. https://doi.org/10.3390/biomimetics8020260