Protection and Restoration of Damaged Hair via a Polyphenol Complex by Promoting Mechanical Strength, Antistatic, and Ultraviolet Protection Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Characterization
2.2. Preparation of PPC Nanoparticles and PPC-Incorporated Shampoo
2.3. Investigation of the Electrical Performance and Coatability of the PPC Nanoparticles
2.4. Evaluation of Anti-Electrostatic and Mechanical Properties of PPC-Coated Hair Samples
2.5. Application of the PPC for ROS Scavenging
3. Results and Discussion
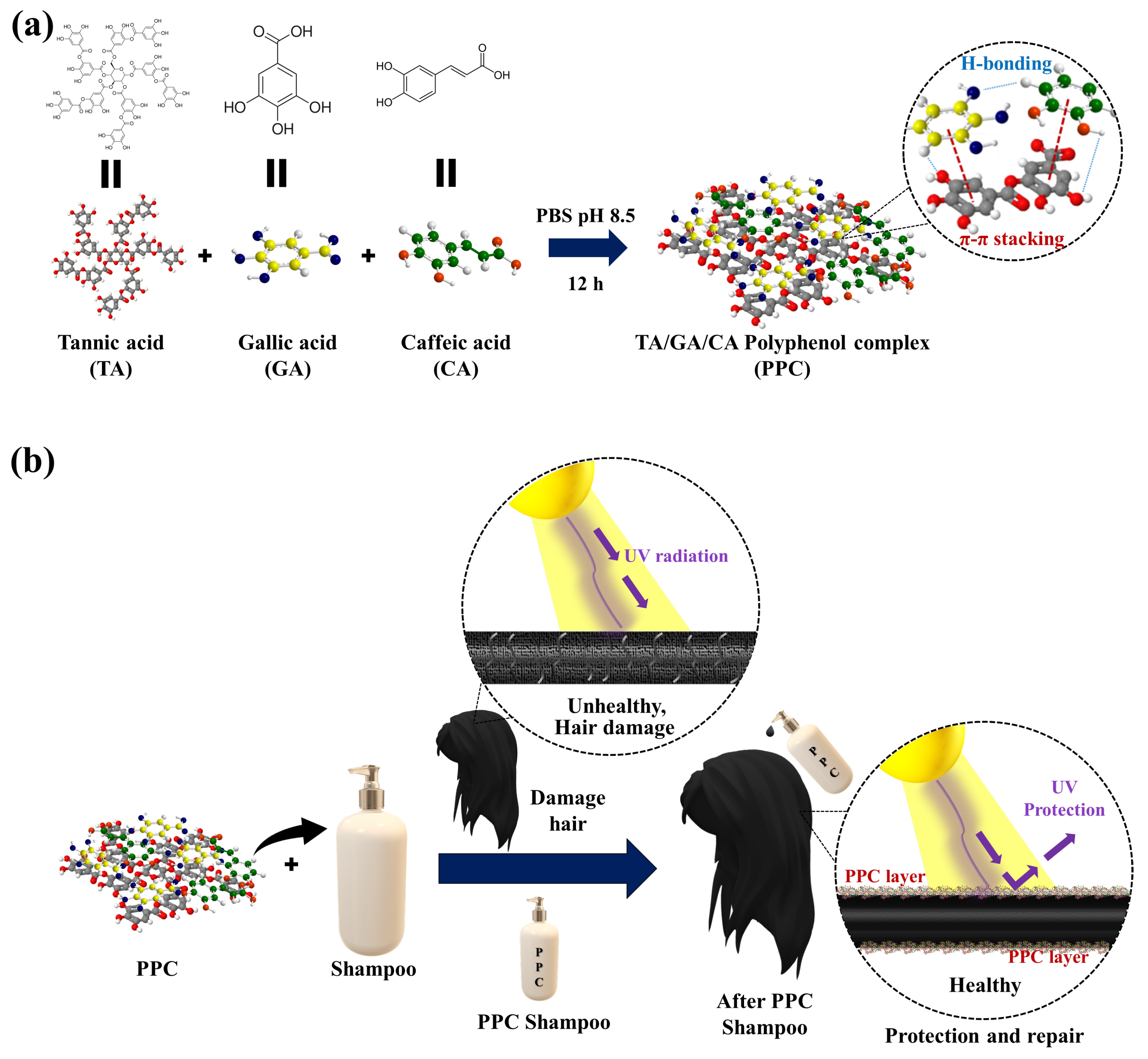
3.1. Design of PPC Shampoo for Hair Protection and Repair
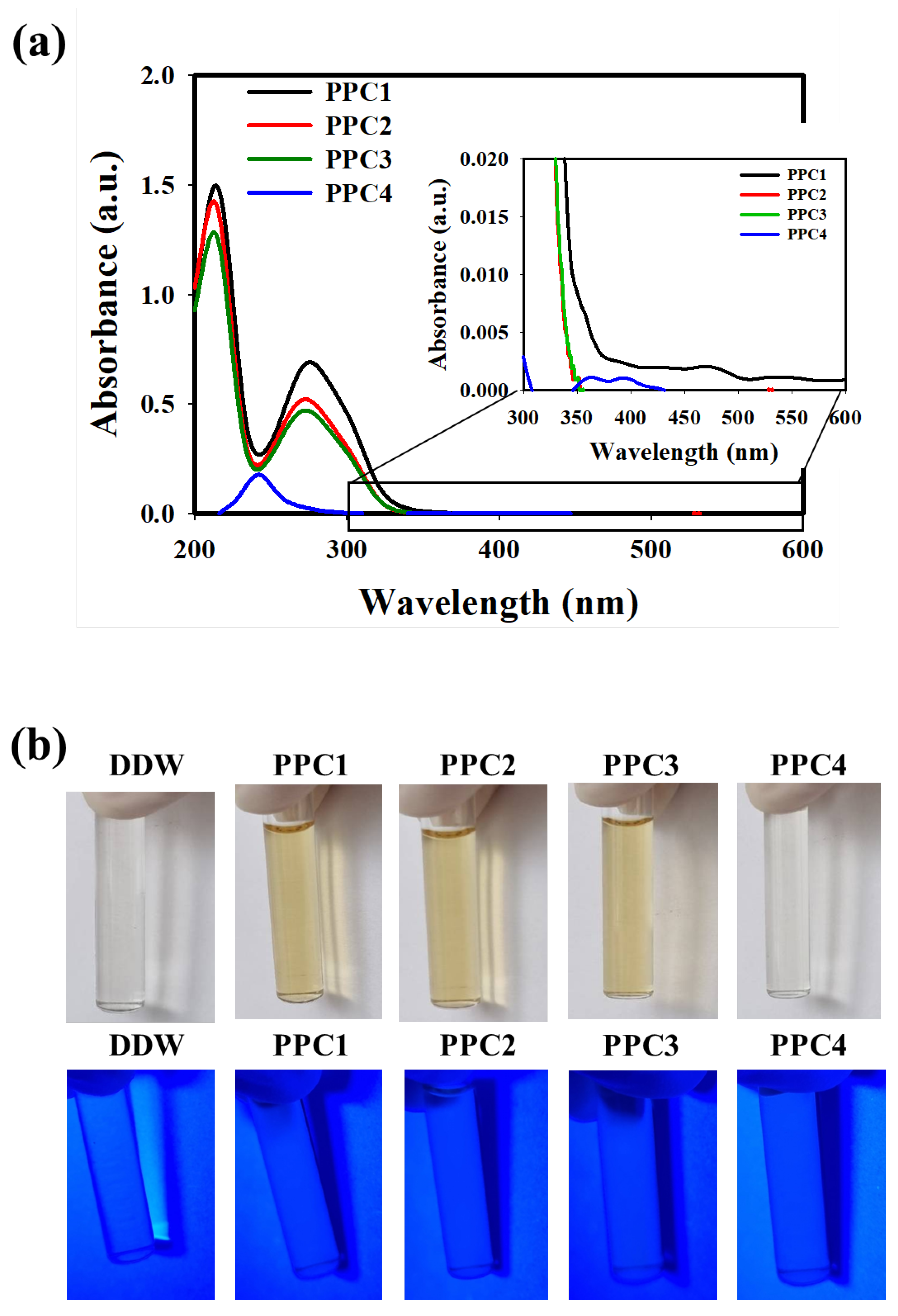
3.2. Characterization of the As-Synthesized PPCs
3.3. Performance of the PPC Shampoo at Hair Protection and Restoration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, H.J.; Lee, W.-S. An Ultrastuctural Study of Hair Fiber Damage and Restoration following Treatment with Permanent Hair Dye. Int. J. Dermatol. 2002, 41, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Kim, J.-H.; Park, S.-D.; Shim, J.-J.; Lee, J.-L. Lactobacillus Paracasei HY7015 and Lycopus Lucidus Turcz. Extract Promotes Human Dermal Papilla Cell Cytoprotective Effect and Hair Regrowth Rate in C57BL/6 Mice. Molecules 2022, 27, 8235. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, Y.-D.; Hyun, H.-J.; Pi, L.; Jin, X.; Lee, W.-S. Hair Shaft Damage from Heat and Drying Time of Hair Dryer. Ann. Dermatol. 2011, 23, 455. [Google Scholar] [CrossRef] [Green Version]
- Dario, M.F.; Baby, A.R.; Velasco, M.V.R. Effects of Solar Radiation on Hair and Photoprotection. J. Photochem. Photobiol. B 2015, 153, 240–246. [Google Scholar] [CrossRef]
- Cavagnino, A.; Starck, A.; Bobier, A.; Baraibar, M.A. Protein Carbonylation as a Reliable Read-Out of Urban Pollution Damage/Protection of Hair Fibers. Cosmetics 2022, 9, 98. [Google Scholar] [CrossRef]
- Binsi, P.K.; Muhamed Ashraf, P.; Parvathy, U.; Zynudheen, A.A. Photo-protective Effect of Cuttlefish Ink Melanin on Human Hair. J. Appl. Polym. Sci. 2022, 139, 51631. [Google Scholar] [CrossRef]
- Tang, Y.; Dyer, J.M.; Deb-Choudhury, S.; Li, Q. Trace Metal Ions in Hair from Frequent Hair Dyers in China and the Associated Effects on Photo-Oxidative Damage. J. Photochem. Photobiol. B 2016, 156, 35–40. [Google Scholar] [CrossRef]
- Trüeb, R.M. The Impact of Oxidative Stress on Hair. Int. J. Cosmet. Sci. 2015, 37, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Porter, D.; Guan, J.; Vollrath, F. Spider Silk: Super Material or Thin Fibre? Adv. Mater. 2013, 25, 1275–1279. [Google Scholar] [CrossRef]
- Chan, N.J.-A.; Gu, D.; Tan, S.; Fu, Q.; Pattison, T.G.; O’Connor, A.J.; Qiao, G.G. Spider-Silk Inspired Polymeric Networks by Harnessing the Mechanical Potential of β-Sheets through Network Guided Assembly. Nat. Commun. 2020, 11, 1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available Technologies on Improving the Stability of Polyphenols in Food Processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Ju, J.; Jin, S.; Kim, S.; Choi, J.H.; Lee, H.A.; Son, D.; Lee, H.; Shin, M. Addressing the Shortcomings of Polyphenol-Derived Adhesives: Achievement of Long Shelf Life for Effective Hemostasis. ACS Appl. Mater. Interfaces 2022, 14, 25115–25125. [Google Scholar] [CrossRef] [PubMed]
- Casagualda, C.; Mancebo-Aracil, J.; Moreno-Villaécija, M.; López-Moral, A.; Alibés, R.; Busqué, F.; Ruiz-Molina, D. Mussel-Inspired Lego Approach for Controlling the Wettability of Surfaces with Colorless Coatings. Biomimetics 2022, 8, 3. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.; Mei, L.; Zeng, X. Polyphenol-Based Hydrogels: Pyramid Evolution from Crosslinked Structures to Biomedical Applications and the Reverse Design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol Scaffolds in Tissue Engineering. Mater. Horiz. 2021, 8, 145–167. [Google Scholar] [CrossRef]
- Andersen, A.; Chen, Y.; Birkedal, H. Bioinspired Metal–Polyphenol Materials: Self-Healing and Beyond. Biomimetics 2019, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Contreras, R.; Chavez-Granados, P.A.; Jurado, C.A.; Aranda-Herrera, B.; Afrashtehfar, K.I.; Nurrohman, H. Natural Bioactive Epigallocatechin-Gallate Promote Bond Strength and Differentiation of Odontoblast-like Cells. Biomimetics 2023, 8, 75. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-Inspired Chemistry toward Hierarchical Superhydrophobic, Antibacterial and Biocompatible Nanofibrous Membranes for Effective UV-Shielding, Self-Cleaning and Oil-Water Separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef]
- Xiong, L.; Jin, S.; Zhang, F.; Li, K.; Li, J.; Mei, C.; Han, J.; Xiao, H.; Seidi, F. Bioinspired Fabrication of Self-Recovery, Adhesive, and Flexible Conductive Hydrogel Sensor Driven by Dynamic Borate Ester Bonds and Tannic Acid-Mediated Noncovalent Network. Eur. Polym. J. 2022, 180, 111636. [Google Scholar] [CrossRef]
- Janošević Ležaić, A.; Bajuk-Bogdanović, D.; Krstić, J.; Jovanović, Z.; Mravik, Ž.; Kovač, J.; Gavrilov, N. What Role Does Carbonized Tannic Acid Play in Energy Storage Composites? Fuel 2022, 312, 122930. [Google Scholar] [CrossRef]
- Aydogdu Emir, A.; Yildiz, E.; Aydogdu, Y.; Sumnu, G.; Sahin, S. Gallic Acid Encapsulated Pea Flour-based Nanofibers Produced by Electrospinning as a Potential Active Food Packaging Material. Legume Sci. 2021, 3, e90. [Google Scholar] [CrossRef]
- Quilez-Molina, A.I.; Marini, L.; Athanassiou, A.; Bayer, I.S. UV-Blocking, Transparent, and Antioxidant Polycyanoacrylate Films. Polymers 2020, 12, 2011. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Figueroa, I.H.; Medrano-Ruiz, L.G.; Moreno-Vásquez, M.d.J.; Ovando-Martínez, M.; Gámez-Meza, N.; Del-Toro-Sánchez, C.L.; Castro-Enríquez, D.D.; López-Ahumada, G.A.; Dórame-Miranda, R.F. Use of Coffee Bean Bagasse Extracts in the Brewing of Craft Beers: Optimization and Antioxidant Capacity. Molecules 2022, 27, 7755. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhou, L.; Chiou, K.; Huang, J. Multifunctional Graphene Hair Dye. Chem 2018, 4, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Han, L.; Ren, J.; Wei, H.; Jia, L. Coating Process and Stability of Metal-Polyphenol Film. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 197–205. [Google Scholar] [CrossRef]
- Kim, S.G.; Robby, A.I.; Lee, B.C.; Lee, G.; Park, S.Y. Mitochondria-Targeted ROS- and GSH-Responsive Diselenide-Crosslinked Polymer Dots for Programmable Paclitaxel Release. J. Ind. Eng. Chem. 2021, 99, 98–106. [Google Scholar] [CrossRef]
- Jo, H.J.; Robby, A.I.; Kim, S.G.; Lee, G.; Lee, B.C.; Park, S.Y. Reusable Biosensor-Based Polymer Dot-Coated Electrode Surface for Wireless Detection of Bacterial Contamination. Sens. Actuators B Chem. 2021, 346, 130503. [Google Scholar] [CrossRef]
- Min Kim, T.; Ryplida, B.; Lee, G.; Young Park, S. Cancer Cells Targeting H2O2-Responsive MXene-Integrated Hyaluronic Acid Polymer Dots Coated Sensor. J. Ind. Eng. Chem. 2023, 120, 188–194. [Google Scholar] [CrossRef]
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R. Thermogravimetric Analysis of Caffeic and Rosmarinic Acid Containing Chitosan Complexes. Carbohydr. Polym. 2019, 222, 115003. [Google Scholar] [CrossRef] [PubMed]
- Dorniani, D.; Kura, A.U.; Ahmad, Z.; Halim Shaari, A.; Hussein, M.Z.; Fakurazi, S. Preparation of Fe3O4 Magnetic Nanoparticles Coated with Gallic Acid for Drug Delivery. Int. J. Nanomed. 2012, 7, 5745–5756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanath, V.; Leo, V.V.; Sabna Prabha, S.; Prabhakumari, C.; Potty, V.P.; Jisha, M.S. Thermal Properties of Tannin Extracted from Anacardium Occidentale L. Using TGA and FT-IR Spectroscopy. Nat. Prod. Res. 2016, 30, 223–227. [Google Scholar] [CrossRef]
- Ryplida, B.; Lee, K.D.; In, I.; Park, S.Y. Light-Induced Swelling-Responsive Conductive, Adhesive, and Stretchable Wireless Film Hydrogel as Electronic Artificial Skin. Adv. Funct. Mater. 2019, 29, 1903209. [Google Scholar] [CrossRef]
- Jo, H.J.; Ryu, J.S.; Robby, A.I.; Kim, Y.S.; Chung, H.J.; Park, S.Y. Rapid and Selective Electrochemical Sensing of Bacterial Pneumonia in Human Sputum Based on Conductive Polymer Dot Electrodes. Sens. Actuators B Chem. 2022, 368, 132084. [Google Scholar] [CrossRef]






| Sample Code | TA | GA | CA | DLS (nm) |
|---|---|---|---|---|
| PPC1 | 100 | 20 | 0.5 | 850 ± 10 |
| PPC2 | 100 | 20 | - | 650 ± 20 |
| PPC3 | 100 | - | 0.5 | 400 ± 12 |
| PPC4 | - | 20 | 0.5 | 350 ± 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, H.J.; Kim, T.M.; An, I.-s.; Bae, H.J.; Park, S.Y. Protection and Restoration of Damaged Hair via a Polyphenol Complex by Promoting Mechanical Strength, Antistatic, and Ultraviolet Protection Properties. Biomimetics 2023, 8, 296. https://doi.org/10.3390/biomimetics8030296
Won HJ, Kim TM, An I-s, Bae HJ, Park SY. Protection and Restoration of Damaged Hair via a Polyphenol Complex by Promoting Mechanical Strength, Antistatic, and Ultraviolet Protection Properties. Biomimetics. 2023; 8(3):296. https://doi.org/10.3390/biomimetics8030296
Chicago/Turabian StyleWon, Hyun Jeong, Tae Min Kim, In-sook An, Heung Jin Bae, and Sung Young Park. 2023. "Protection and Restoration of Damaged Hair via a Polyphenol Complex by Promoting Mechanical Strength, Antistatic, and Ultraviolet Protection Properties" Biomimetics 8, no. 3: 296. https://doi.org/10.3390/biomimetics8030296
APA StyleWon, H. J., Kim, T. M., An, I.-s., Bae, H. J., & Park, S. Y. (2023). Protection and Restoration of Damaged Hair via a Polyphenol Complex by Promoting Mechanical Strength, Antistatic, and Ultraviolet Protection Properties. Biomimetics, 8(3), 296. https://doi.org/10.3390/biomimetics8030296







