Substrate Stiffness of Bone Microenvironment Controls Functions of Pre-Osteoblasts and Fibroblasts In Vitro
Abstract
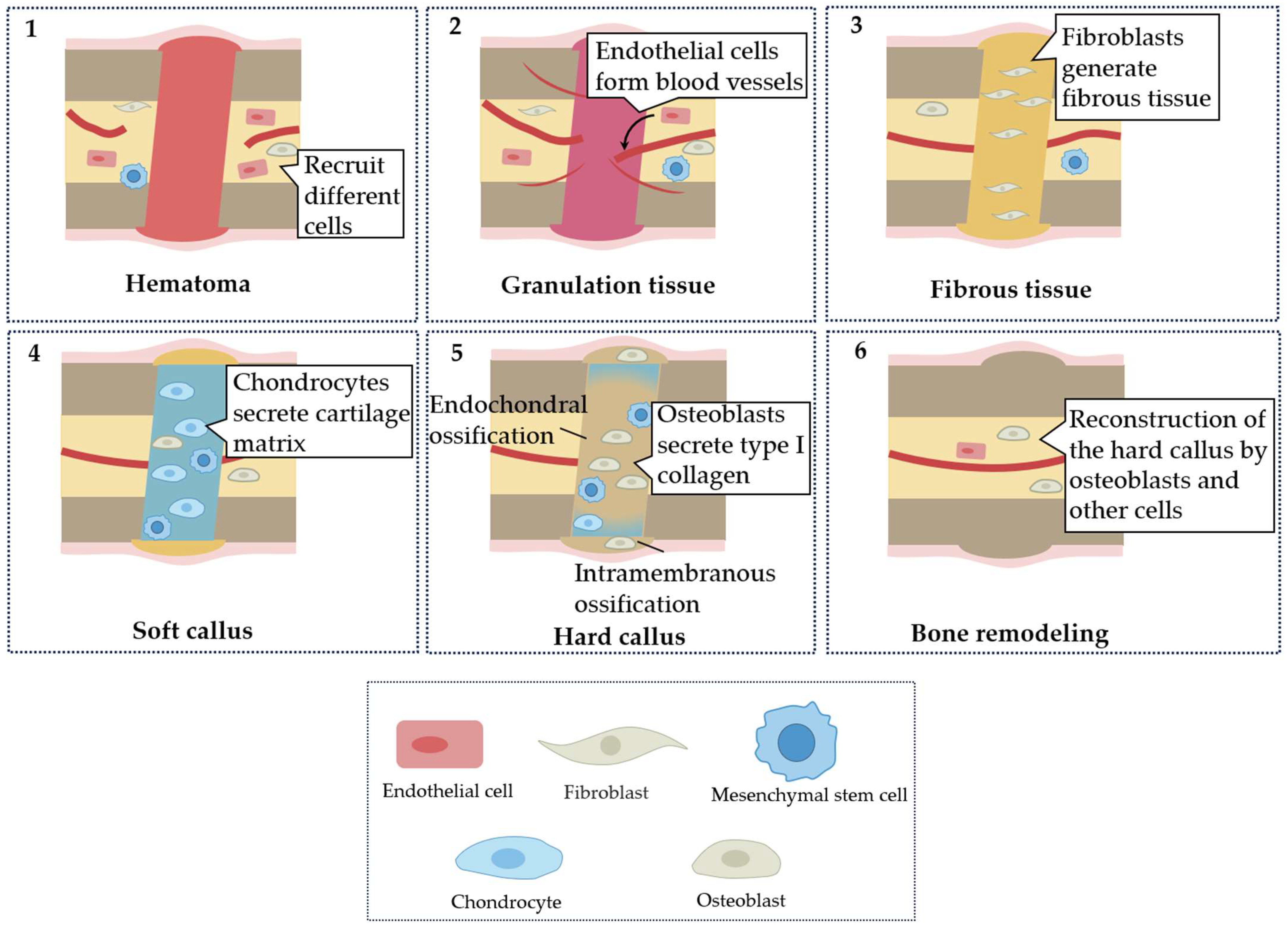
:1. Introduction
2. Materials and Methods
3. Result
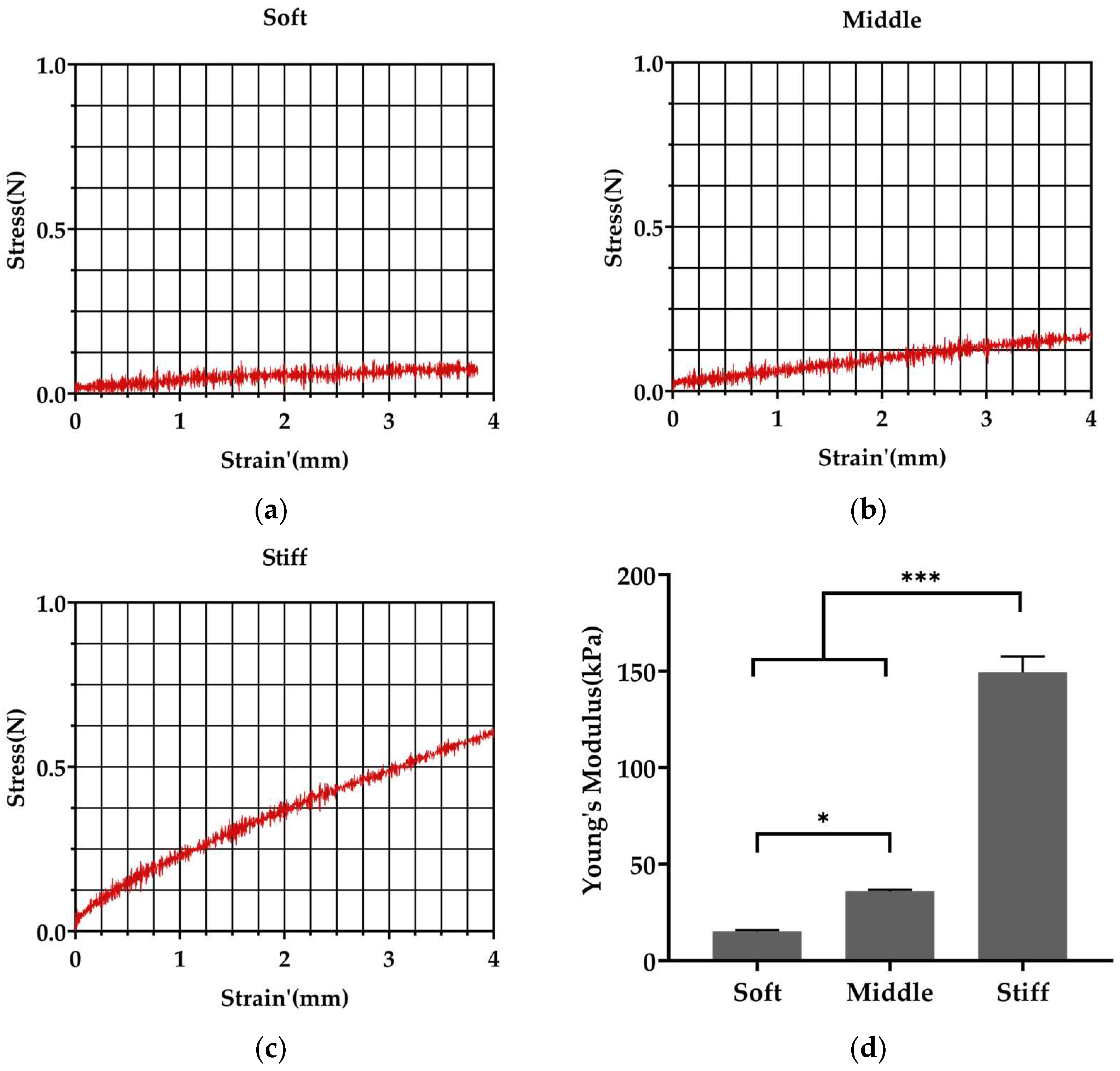
3.1. Fabrication of Polyacrylamide Substrates with Varied Stiffness
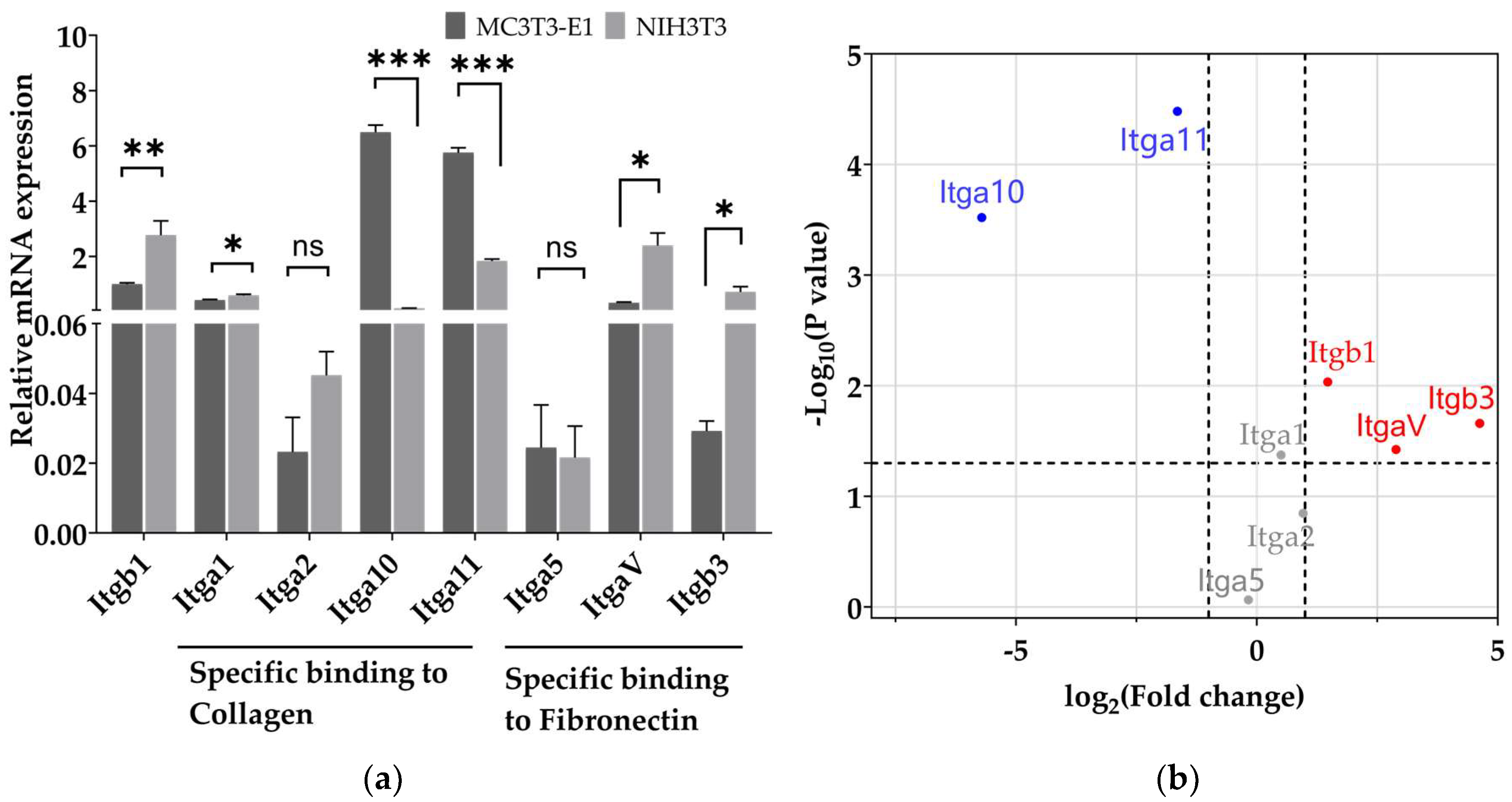
3.2. Pre-Osteoblasts Exhibit Higher Level of mRNA Expression of the Integrin Subunits Binding to Type I Collagen Than Fibroblasts
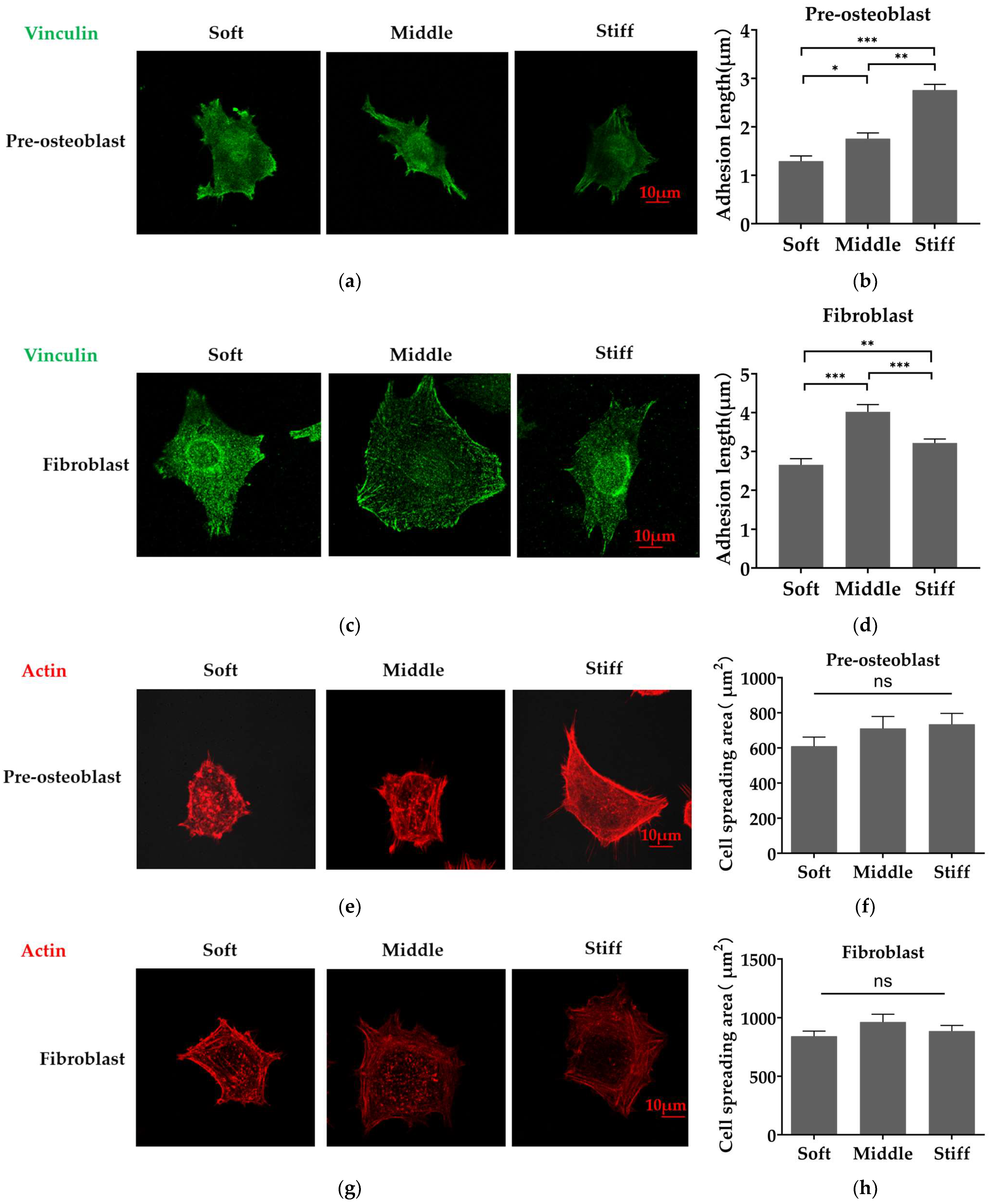
3.3. Stiffer Substrate Increases the Length of Focal Adhesion in Pre-Osteoblasts and Reduces the Length of Focal Adhesion in Fibroblasts
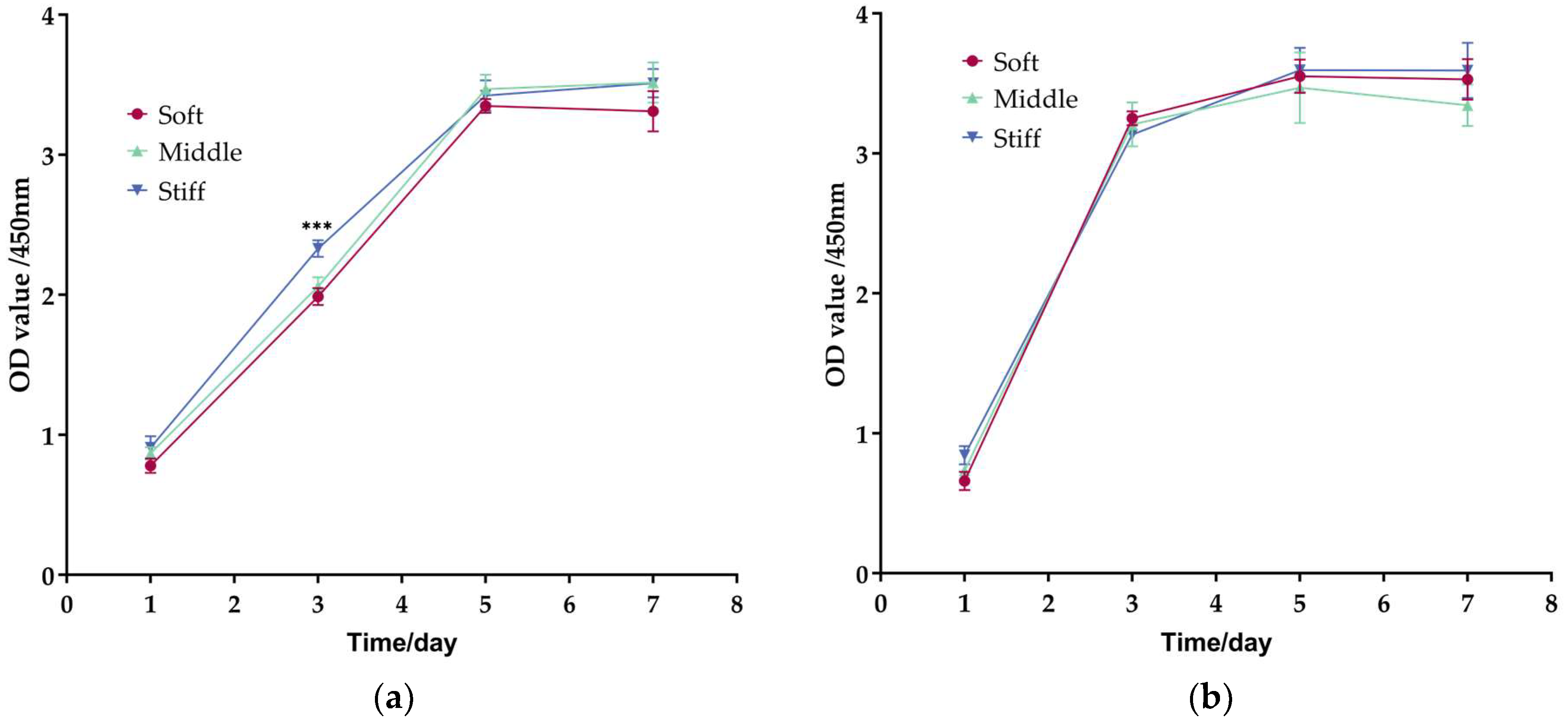
3.4. Proliferation of Fibroblasts Is Faster Than Pre-Osteoblasts on Three Substrates
3.5. Substrate Stiffness Modulates Cell Differentiation and Matrix Secretion of Pre-Osteoblasts and Fibroblasts
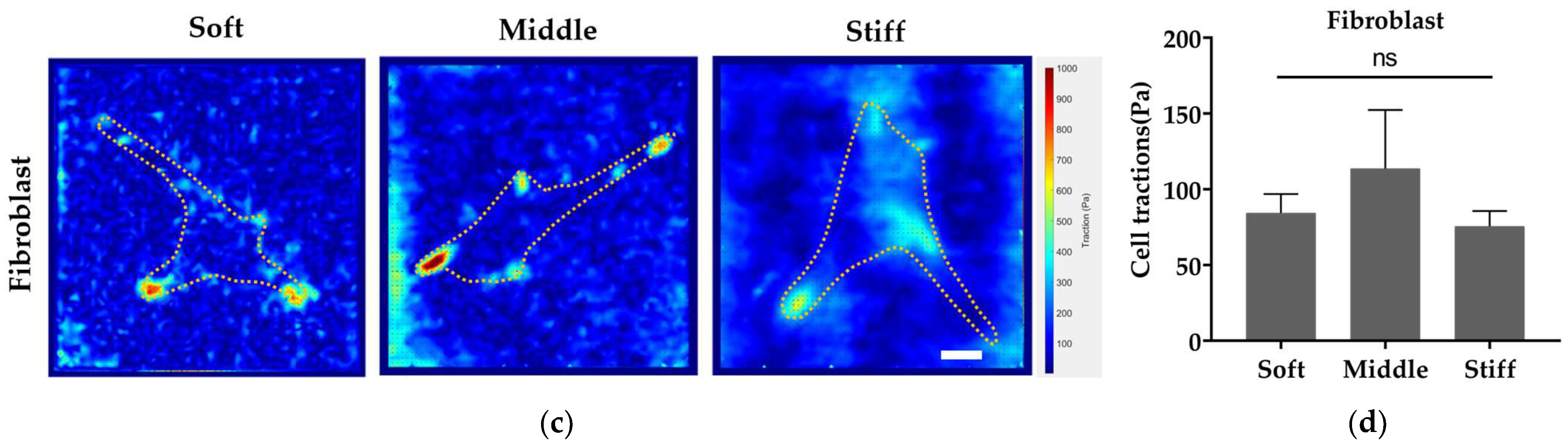
3.6. Stiffer Substrate Enhances Cell Traction Force of Pre-Osteoblast While Reducing Cell Traction Force of Fibroblast
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [PubMed]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef]
- Alauddin, M.S.; Abdul Hayei, N.A.; Sabarudin, M.A.; Mat Baharin, N.H. Barrier Membrane in Regenerative Therapy: A Narrative Review. Membranes 2022, 12, 444. [Google Scholar] [PubMed]
- Li, M.; Zhang, A.; Li, J.; Zhou, J.; Zheng, Y.; Zhang, C.; Xia, D.; Mao, H.; Zhao, J. Osteoblast/fibroblast coculture derived bioactive ECM with unique matrisome profile facilitates bone regeneration. Bioact. Mater. 2020, 5, 938–948. [Google Scholar]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar]
- Malizos, K.N.; Papatheodorou, L.K. The healing potential of the periosteum molecular aspects. Injury 2005, 36 (Suppl. 3), S13–S19. [Google Scholar] [CrossRef]
- Chang, H.; Liu, X.Q.; Hu, M.; Zhang, H.; Li, B.C.; Ren, K.F.; Boudou, T.; Albiges-Rizo, C.; Picart, C.; Ji, J. Substrate Stiffness Combined with Hepatocyte Growth Factor Modulates Endothelial Cell Behavior. Biomacromolecules 2016, 17, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Laney, W.R. Glossary of Oral and Maxillofacial Implants. Int. J. Oral Maxillofac. Implant. 2017, 32, Gi-200. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [PubMed]
- Di Carlo, S.E.; Peduto, L. The perivascular origin of pathological fibroblasts. J. Clin. Investig. 2018, 128, 54–63. [Google Scholar] [PubMed]
- Julien, A.; Kanagalingam, A.; Martínez-Sarrà, E.; Megret, J.; Luka, M.; Ménager, M.; Relaix, F.; Colnot, C. Direct contribution of skeletal muscle mesenchymal progenitors to bone repair. Nat. Commun. 2021, 12, 2860. [Google Scholar] [CrossRef]
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36 (Suppl. 3), S5–S7. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [PubMed]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2011, 13, 27–38. [Google Scholar] [PubMed]
- Jang, T.S.; Park, S.J.; Lee, J.E.; Yang, J.; Park, S.H.; Jun, M.B.G.; Kim, Y.W.; Aranas, C.; Choi, J.P.; Zou, Y.; et al. Topography-Supported Nanoarchitectonics of Hybrid Scaffold for Systematically Modulated Bone Regeneration and Remodeling. Adv. Funct. Mater. 2022, 32, 2206863. [Google Scholar] [CrossRef]
- Zhu, J.; Clark, R.A.F. Fibronectin at select sites binds multiple growth factors and enhances their activity: Expansion of the collaborative ECM-GF paradigm. J. Investig. Dermatol. 2014, 134, 895–901. [Google Scholar]
- Smith, L.R.; Cho, S.; Discher, D.E. Stem Cell Differentiation is Regulated by Extracellular Matrix Mechanics. Physiology 2018, 33, 16–25. [Google Scholar]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Lv, H.; Li, L.; Sun, M.; Zhang, Y.; Chen, L.; Rong, Y.; Li, Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, e12338. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimighaei, R.; Sala-Newby, G.B.; Hudson, C.; Kimura, T.E.; Hathway, T.; Hawkins, J.; McNeill, M.C.; Richardson, R.; Newby, A.C.; Bond, M. Combined role for YAP-TEAD and YAP-RUNX2 signalling in substrate-stiffness regulation of cardiac fibroblast proliferation. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119329. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.E.; Tong, X.; Yang, F. Extracellular matrix type modulates mechanotransduction of stem cells. Acta Biomater. 2019, 96, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [Green Version]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell-extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [PubMed]
- Du, J.; Chen, X.; Liang, X.; Zhang, G.; Xu, J.; He, L.; Zhan, Q.; Feng, X.Q.; Chien, S.; Yang, C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 9466–9471. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Bazellieres, E.; Allen, M.D.; Andreu, I.; Oria, R.; Sunyer, R.; Gomm, J.J.; Marshall, J.F.; Jones, J.L.; Trepat, X.; et al. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 2014, 13, 631–637. [Google Scholar]
- Plotnikov, S.V.; Sabass, B.; Schwarz, U.S.; Waterman, C.M. High-resolution traction force microscopy. Methods Cell Biol. 2014, 123, 367–394. [Google Scholar]
- Tse, J.R.; Engler, A.J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 2010, 47, 10–16. [Google Scholar]
- Boudou, T.; Ohayon, J.; Picart, C.; Tracqui, P. An extended relationship for the characterization of Young’s modulus and Poisson’s ratio of tunable polyacrylamide gels. Biorheology 2006, 43, 721–728. [Google Scholar]
- Ghiasi, M.S.; Chen, J.E.; Rodriguez, E.K.; Vaziri, A.; Nazarian, A. Computational modeling of human bone fracture healing affected by different conditions of initial healing stage. BMC Musculoskelet. Disord. 2019, 20, 562. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [PubMed]
- Wang, Q.; Xie, J.; Zhou, C.; Lai, W. Substrate stiffness regulates the differentiation profile and functions of osteoclasts via cytoskeletal arrangement. Cell Prolif. 2022, 55, e13172. [Google Scholar] [PubMed]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar]
- Miller, F.D.; Kaplan, D.R. Mobilizing endogenous stem cells for repair and regeneration: Are we there yet? Cell Stem Cell 2012, 10, 650–652. [Google Scholar]
- Du, J.; Zu, Y.; Li, J.; Du, S.; Xu, Y.; Zhang, L.; Jiang, L.; Wang, Z.; Chien, S.; Yang, C. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci. Rep. 2016, 6, 20395. [Google Scholar]
- Flanagan, L.A.; Ju, Y.E.; Marg, B.; Osterfield, M.; Janmey, P.A. Neurite branching on deformable substrates. Neuroreport 2002, 13, 2411–2415. [Google Scholar] [CrossRef] [Green Version]
- Gronthos, S.; Stewart, K.; Graves, S.E.; Hay, S.; Simmons, P.J. Integrin expression and function on human osteoblast-like cells. J. Bone Miner. Res. 1997, 12, 1189–1197. [Google Scholar] [CrossRef]
- Oates, T.W.; Maller, S.C.; West, J.; Steffensen, B. Human gingival fibroblast integrin subunit expression on titanium implant surfaces. J. Periodontol. 2005, 76, 1743–1750. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y.-R.V.; Tseng, K.-F.; Lai, H.-Y.; Lin, C.-H.; Lee, O.K. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 2011, 26, 730–738. [Google Scholar] [PubMed]
- Li, J.; Chen, H.; Xu, Y.; Hu, J.; Xie, F.Q.; Yang, C. Integrin endocytosis on elastic substrates mediates mechanosensing. J. Biomech. 2016, 49, 2644–2654. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Zhou, S.; Tang, X.; Zhang, Y.; Lin, F.; Xiong, C.; Yang, C. Quantitative Analyses of Dynamic Features of Fibroblasts on Different Protein-Coated Compliant Substrates. ACS Biomater. Sci. Eng. 2017, 3, 2987–2998. [Google Scholar]
- Mullen, C.A.; Vaughan, T.J.; Billiar, K.L.; McNamara, L.M. The effect of substrate stiffness, thickness, and cross-linking density on osteogenic cell behavior. Biophys. J. 2015, 108, 1604–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Zhou, M.; Zhang, Q.; Yong, L.; Zhang, T.; Tian, T.; Ma, Q.; Lin, S.; Zhu, B.; Cai, X. Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif. 2018, 51, e12478. [Google Scholar] [CrossRef] [Green Version]
- Oria, R.; Wiegand, T.; Escribano, J.; Elosegui-Artola, A.; Uriarte, J.J.; Moreno-Pulido, C.; Platzman, I.; Delcanale, P.; Albertazzi, L.; Navajas, D.; et al. Force loading explains spatial sensing of ligands by cells. Nature 2017, 552, 219–224. [Google Scholar] [CrossRef]
- Nikoloudaki, G.; Snider, P.; Simmons, O.; Conway, S.J.; Hamilton, D.W. Periostin and matrix stiffness combine to regulate myofibroblast differentiation and fibronectin synthesis during palatal healing. Matrix Biol. 2020, 94, 31–56. [Google Scholar]
- Kim, S.S.; Nikoloudaki, G.E.; Michelsons, S.; Creber, K.; Hamilton, D.W. Fibronectin synthesis, but not alpha-smooth muscle expression, is regulated by periostin in gingival healing through FAK/JNK signaling. Sci. Rep. 2019, 9, 2708. [Google Scholar] [PubMed] [Green Version]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [PubMed]
- Giannone, G.; Mège, R.-M.; Thoumine, O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009, 19, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [PubMed]
- Wang, P.Y.; Tsai, W.B.; Voelcker, N.H. Screening of rat mesenchymal stem cell behaviour on polydimethylsiloxane stiffness gradients. Acta Biomater. 2012, 8, 519–530. [Google Scholar] [PubMed]


| Soft | Middle | Stiff | |
|---|---|---|---|
| 40% Acrylamide (mL) | 1.25 | 1.88 | 3.00 |
| 2% Bisacrylamide (mL) | 0.75 | 1.50 | 3.00 |
| 10% Ammonium persulfate (mL) | 0.1 | 0.1 | 0.1 |
| TEMED (mL) | 0.1 | 0.1 | 0.1 |
| ddH2O (mL) | 7.8 | 6.42 | 3.8 |
| Target Gene | Forward Primer (5′-3′) | Reverse Primer (3′-5′) |
|---|---|---|
| Gapdh | AAGGTCGGTGTGAACGGATTTGG | CGTTGAATTTGCCGTGAGTGGAG |
| Runx2 | TTTAGGGCGCATTCCTCATC | TGTCCTTGTGGATTAAAAGGACTTG |
| ALP | CGGGACTGGTACTCGGATAA | ATTCCACGTCGGTTCTGTTC |
| OPN | ATCTCACCATTCGGATGAGTCT | TGTAGGGACGATTGGAGTGAAA |
| Acta2 | ATGCCTCTGGACGTACAACTG | CACACCATCTCCAGAGTCCA |
| Fn1 | TACCAAGGTCAATCCACACCCC | CAGATGGCAAAAGAAAGCAGAGG |
| Itga1 | CGCTGTGAATCAGACGAGGT | CCCACAGGGCTCATTCTTGT |
| Itga2 | TGTCTGGCGTATAATGTTGGC | TGCTGTACTGAATACCCAAACTG |
| Itga5 | TGCAGTGGTTCGGAGCAAC | TTTTCTGTGCGCCAGCTATAC |
| Itga10 | AGGCCGAATTTGGATACAGTG | GAGCAACGATAAACATCCCCTC |
| Itga11 | TGCCCCAATGGAAACCAATG | CATGCCAGTGGTGTAGTAGGA |
| ItgaV | CGGGTCCCGAGGGAAGTTA | TGGATGAGCATTCACATTTGAGA |
| Itgb1 | ATGCCAAATCTTGCGGAGAAT | TTTGCTGCGATTGGTGACATT |
| Itgb3 | GGCGTTGTTGTTGGAGAGTC | CTTCAGGTTACATCGGGGTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Chen, B.; Gao, M.; Xu, Y.; Yang, X.; Yang, C.; Pan, S. Substrate Stiffness of Bone Microenvironment Controls Functions of Pre-Osteoblasts and Fibroblasts In Vitro. Biomimetics 2023, 8, 344. https://doi.org/10.3390/biomimetics8040344
Gao S, Chen B, Gao M, Xu Y, Yang X, Yang C, Pan S. Substrate Stiffness of Bone Microenvironment Controls Functions of Pre-Osteoblasts and Fibroblasts In Vitro. Biomimetics. 2023; 8(4):344. https://doi.org/10.3390/biomimetics8040344
Chicago/Turabian StyleGao, Shenghan, Bo Chen, Min Gao, Yue Xu, Xueyi Yang, Chun Yang, and Shaoxia Pan. 2023. "Substrate Stiffness of Bone Microenvironment Controls Functions of Pre-Osteoblasts and Fibroblasts In Vitro" Biomimetics 8, no. 4: 344. https://doi.org/10.3390/biomimetics8040344





