The Impact of Left Ventricular Assist Device Outflow Graft Positioning on Aortic Hemodynamics: Improving Flow Dynamics to Mitigate Aortic Insufficiency
Abstract
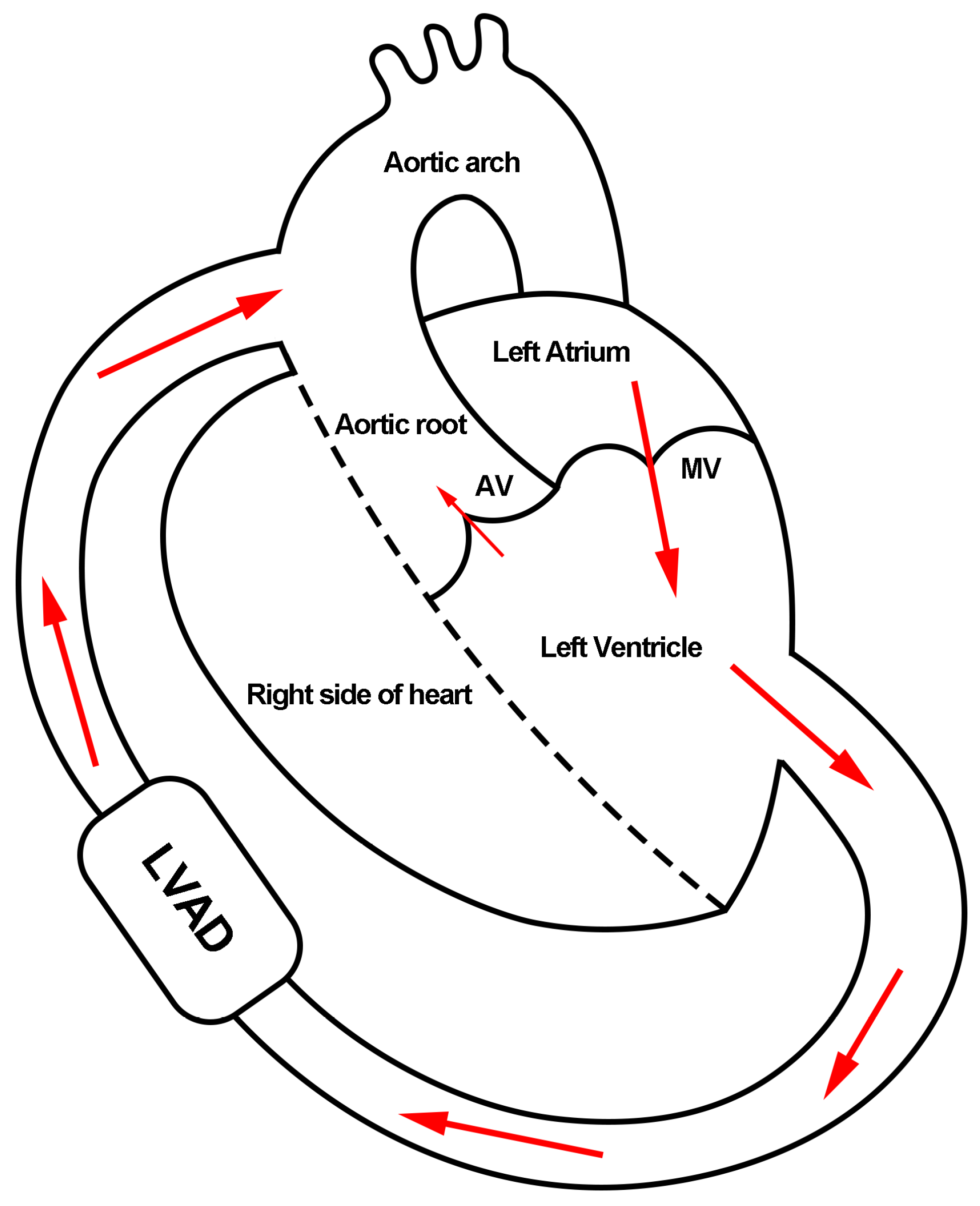
:1. Introduction
2. Materials and Methods
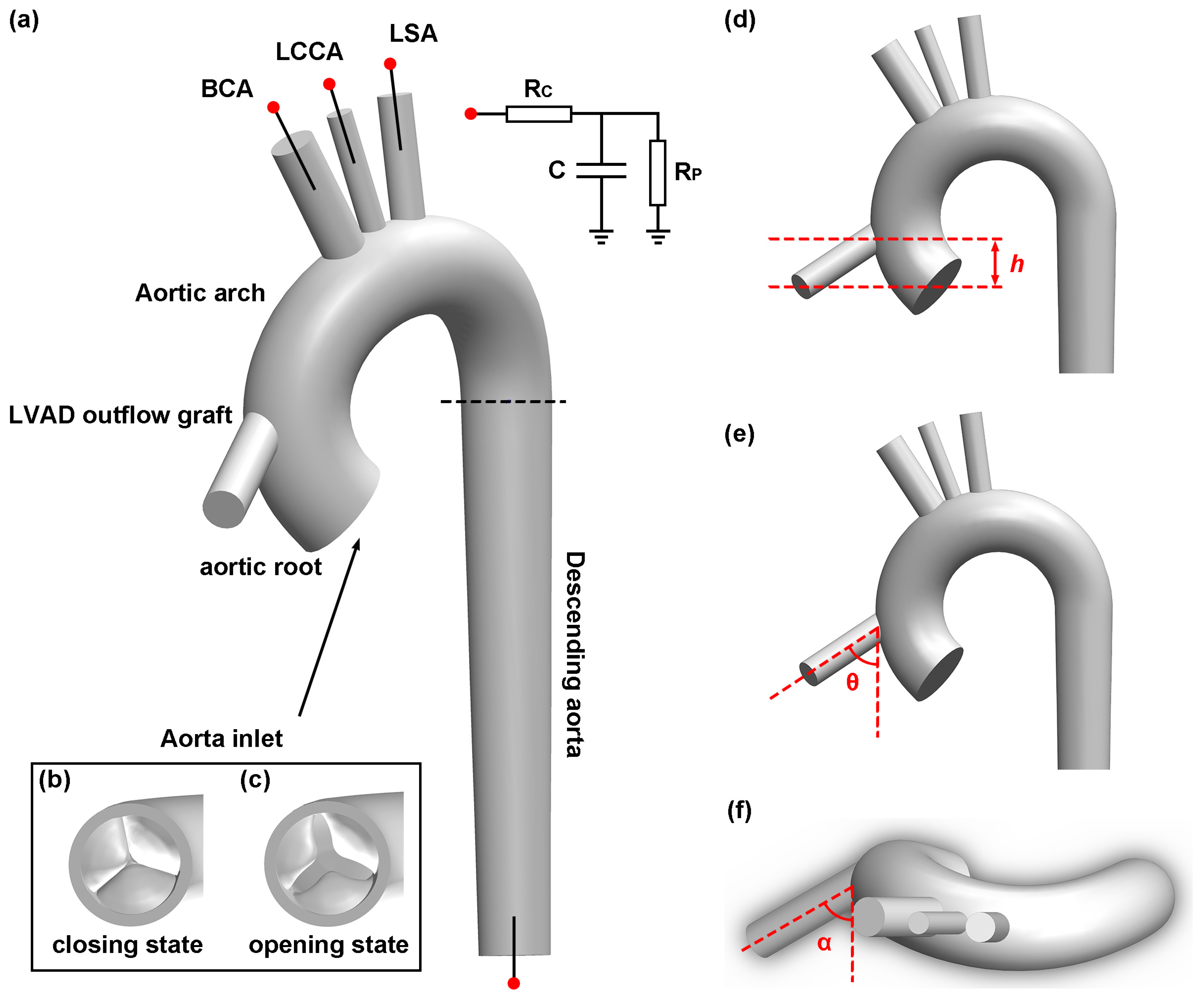
2.1. Geometry Construction
2.2. Mathematical Modelling and Governing Equations
2.3. Mesh Generation and Independence Test
2.4. Boundary Condition
2.5. Quantities of Interest
- -
- TAWSS is applied as the temporal average of WSS vector intensity for one cardiac cycle to evaluate the wall shear stress on the aortic wall and aortic valve leaflet shown in the following equation [46]:
- -
- OSI is introduced to describe the degree of deviation of the WSS from its mean direction [46]. The definition of OSI is shown in the following equation:
- -
- The vortex structure plays a crucial role in hemodynamics, as pathologically altered vortices may reduce the velocity of blood flow and lead to blood clots or thrombosis [47]. The Q-criterion method is applied due to its ability to provide a clear visualization of the three-dimensional vortex structure. The second invariant of the velocity gradient tensor is:
3. Results
3.1. Effect of Outflow Graft Location on Velocity Distribution
3.2. Effect of Outflow Graft Location on Vortex Structure
3.3. Effect of Outflow Graft Location on TAWSS Distribution
3.4. Effect of Outflow Graft Location on OSI Distribution
4. Discussion
4.1. Aortic Valve Morphology
4.2. Height of LVAD Outflow Graft
4.3. Inclination Angle of LVAD Outflow Graft
4.4. Azimuthal Angle of LVAD Outflow Graft
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Gruessner, A.; Agopian, V.G.; Khalpey, Z.; Riaz, I.B.; Kaplan, B.; Halazun, K.J.; Busuttil, R.W.; Gruessner, R.W.G. Survival Benefit of Solid-Organ Transplant in the United States. JAMA Surg. 2015, 150, 252. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.; Smith, J.M.; Ahn, Y.; Skeans, M.A.; Messick, E.; Bradbrook, K.; Gauntt, K.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2020 Annual Data Report: Heart. Am. J. Transplant. 2022, 22, 350–437. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Fang, J.C.; Ewald, G.A.; Allen, L.A.; Butler, J.; Westlake Canary, C.A.; Colvin-Adams, M.; Dickinson, M.G.; Levy, P.; Stough, W.G.; Sweitzer, N.K.; et al. Advanced (Stage D) Heart Failure: A Statement from the Heart Failure Society of America Guidelines Committee. J. Card. Fail. 2015, 21, 519–534. [Google Scholar] [CrossRef]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth Annual INTERMACS Report: Special Focus on Framing the Impact of Adverse Events. J. Heart Lung Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef]
- Jorde, U.P.; Uriel, N.; Nahumi, N.; Bejar, D.; Gonzalez-Costello, J.; Thomas, S.S.; Han, J.; Morrison, K.A.; Jones, S.; Kodali, S.; et al. Prevalence, Significance, and Management of Aortic Insufficiency in Continuous Flow Left Ventricular Assist Device Recipients. Circ. Heart Fail. 2014, 7, 310–319. [Google Scholar] [CrossRef]
- Truby, L.K.; Garan, A.R.; Givens, R.C.; Wayda, B.; Takeda, K.; Yuzefpolskaya, M.; Colombo, P.C.; Naka, Y.; Takayama, H.; Topkara, V.K. Aortic Insufficiency During Contemporary Left Ventricular Assist Device Support. JACC Heart Fail. 2018, 6, 951–960. [Google Scholar] [CrossRef]
- Vu, V.; May-Newman, K. Aortic Insufficiency in LVAD Patients. In Ventricular Assist Devices—Advances and Applications in Heart Failure [Working Title]; IntechOpen: London, UK, 2022. [Google Scholar]
- Iizuka, K.; Nishinaka, T.; Ichihara, Y.; Miyamoto, T.; Yamazaki, K. Outflow Graft Anastomosis Site Design Could Be Correlated to Aortic Valve Regurgitation under Left Ventricular Assist Device Support. J. Artif. Organs 2018, 21, 150–155. [Google Scholar] [CrossRef]
- Iizuka, K.; Nishinaka, T.; Akiyama, D.; Sumikura, H.; Mizuno, T.; Tsukiya, T.; Takewa, Y.; Yamazaki, K.; Tatsumi, E. The Angle of the Outflow Graft to the Aorta Can Affect Recirculation Due to Aortic Insufficiency under Left Ventricular Assist Device Support. J. Artif. Organs 2018, 21, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.D.; Laín, S. A Review on Computational Fluid Dynamics Modelling in Human Thoracic Aorta. Cardiovasc. Eng. Technol. 2013, 4, 103–130. [Google Scholar] [CrossRef]
- Ong, C.W.; Wee, I.; Syn, N.; Ng, S.; Leo, H.L.; Richards, A.M.; Choong, A.M.T.L. Computational Fluid Dynamics Modeling of Hemodynamic Parameters in the Human Diseased Aorta: A Systematic Review. Ann. Vasc. Surg. 2020, 63, 336–381. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Toda, K.; Miyagawa, S.; Yoshikawa, Y.; Hata, H.; Yoshioka, D.; Kainuma, S.; Kawamura, T.; Kawamura, A.; Nakatani, S.; et al. Impact of Turbulent Blood Flow in the Aortic Root on de Novo Aortic Insufficiency during Continuous-flow Left Ventricular-assist Device Support. Artif. Organs 2020, 44, 883–891. [Google Scholar] [CrossRef]
- Shad, R.; Kaiser, A.D.; Kong, S.; Fong, R.; Quach, N.; Bowles, C.; Kasinpila, P.; Shudo, Y.; Teuteberg, J.; Woo, Y.J.; et al. Patient-Specific Computational Fluid Dynamics Reveal Localized Flow Patterns Predictive of Post–Left Ventricular Assist Device Aortic Incompetence. Circ. Heart Fail. 2021, 14, e008034. [Google Scholar] [CrossRef] [PubMed]
- Kasinpila, P.; Kong, S.; Fong, R.; Shad, R.; Kaiser, A.D.; Marsden, A.L.; Woo, Y.J.; Hiesinger, W. Use of Patient-Specific Computational Models for Optimization of Aortic Insufficiency after Implantation of Left Ventricular Assist Device. J. Thorac. Cardiovasc. Surg. 2021, 162, 1556–1563. [Google Scholar] [CrossRef]
- May-Newman, K.; Hillen, B.; Dembitsky, W. Effect of Left Ventricular Assist Device Outflow Conduit Anastomosis Location on Flow Patterns in the Native Aorta. ASAIO J. 2006, 52, 132–139. [Google Scholar] [CrossRef]
- Karmonik, C.; Partovi, S.; Loebe, M.; Schmack, B.; Ghodsizad, A.; Robbin, M.R.; Noon, G.P.; Kallenbach, K.; Karck, M.; Davies, M.G.; et al. Influence of LVAD Cannula Outflow Tract Location on Hemodynamics in the Ascending Aorta: A Patient-Specific Computational Fluid Dynamics Approach. ASAIO J. 2012, 58, 562–567. [Google Scholar] [CrossRef]
- Brown, A.G.; Shi, Y.; Arndt, A.; Müller, J.; Lawford, P.; Hose, D.R. Importance of Realistic LVAD Profiles for Assisted Aortic Simulations: Evaluation of Optimal Outflow Anastomosis Locations. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 669–680. [Google Scholar] [CrossRef]
- Inci, G.; Sorgüven, E. Effect of LVAD Outlet Graft Anastomosis Angle on the Aortic Valve, Wall, and Flow. ASAIO J. 2012, 58, 373–381. [Google Scholar] [CrossRef]
- Caruso, M.V.; Gramigna, V.; Rossi, M.; Serraino, G.F.; Renzulli, A.; Fragomeni, G. A Computational Fluid Dynamics Comparison between Different Outflow Graft Anastomosis Locations of Left Ventricular Assist Device (LVAD) in a Patient-Specific Aortic Model: BEST LVAD OUTFLOW GRAFT LOCATION IN ASCENDING AORTA: CFD STUDY. Int. J. Numer. Methods Biomed. Eng. 2015, 31, e02700. [Google Scholar] [CrossRef] [PubMed]
- Callington, A.; Long, Q.; Mohite, P.; Simon, A.; Mittal, T.K. Computational Fluid Dynamic Study of Hemodynamic Effects on Aortic Root Blood Flow of Systematically Varied Left Ventricular Assist Device Graft Anastomosis Design. J. Thorac. Cardiovasc. Surg. 2015, 150, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.A.S.; Hormes, M.; Laumen, M.; Timms, D.L.; Linde, T.; Schmitz-Rode, T.; Moritz, A.; Dzemali, O.; Steinseifer, U. The Impact of Aortic/Subclavian Outflow Cannulation for Cardiopulmonary Bypass and Cardiac Support: A Computational Fluid Dynamics Study. Artif. Organs 2009, 33, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Karmonik, C.; Partovi, S.; Loebe, M.; Schmack, B.; Weymann, A.; Lumsden, A.B.; Karck, M.; Ruhparwar, A. Computational Fluid Dynamics in Patients with Continuous-Flow Left Ventricular Assist Device Support Show Hemodynamic Alterations in the Ascending Aorta. J. Thorac. Cardiovasc. Surg. 2014, 147, 1326–1333.e1. [Google Scholar] [CrossRef] [PubMed]
- Travis, A.R.; Giridharan, G.A.; Pantalos, G.M.; Dowling, R.D.; Prabhu, S.D.; Slaughter, M.S.; Sobieski, M.; Undar, A.; Farrar, D.J.; Koenig, S.C. Vascular Pulsatility in Patients with a Pulsatile- or Continuous-Flow Ventricular Assist Device. J. Thorac. Cardiovasc. Surg. 2007, 133, 517–524. [Google Scholar] [CrossRef]
- John, R.; Mantz, K.; Eckman, P.; Rose, A.; May-Newman, K. Aortic Valve Pathophysiology during Left Ventricular Assist Device Support. J. Heart Lung Transplant. 2010, 29, 1321–1329. [Google Scholar] [CrossRef]
- Cowger, J.; Pagani, F.D.; Haft, J.W.; Romano, M.A.; Aaronson, K.D.; Kolias, T.J. The Development of Aortic Insufficiency in Left Ventricular Assist Device-Supported Patients. Circ. Heart Fail. 2010, 3, 668–674. [Google Scholar] [CrossRef]
- Pak, S.-W.; Uriel, N.; Takayama, H.; Cappleman, S.; Song, R.; Colombo, P.C.; Charles, S.; Mancini, D.; Gillam, L.; Naka, Y.; et al. Prevalence of de Novo Aortic Insufficiency during Long-Term Support with Left Ventricular Assist Devices. J. Heart Lung Transplant. 2010, 29, 1172–1176. [Google Scholar] [CrossRef]
- Hatano, M.; Kinugawa, K.; Shiga, T.; Kato, N.; Endo, M.; Hisagi, M.; Nishimura, T.; Yao, A.; Hirata, Y.; Kyo, S.; et al. Less Frequent Opening of the Aortic Valve and a Continuous Flow Pump Are Risk Factors for Postoperative Onset of Aortic Insufficiency in Patients With a Left Ventricular Assist Device. Circ. J. 2011, 75, 1147–1155. [Google Scholar] [CrossRef]
- da Rocha e Silva, J.G.; Meyer, A.L.; Eifert, S.; Garbade, J.; Mohr, F.W.; Strueber, M. Influence of Aortic Valve Opening in Patients with Aortic Insufficiency after Left Ventricular Assist Device Implantation. Eur. J. Cardiothorac. Surg. 2016, 49, 784–787. [Google Scholar] [CrossRef]
- Acharya, D.; Kazui, T.; Al Rameni, D.; Acharya, T.; Betterton, E.; Juneman, E.; Loyaga-Rendon, R.; Lotun, K.; Shetty, R.; Chatterjee, A. Aortic Valve Disorders and Left Ventricular Assist Devices. Front. Cardiovasc. Med. 2023, 10, 1098348. [Google Scholar] [CrossRef] [PubMed]
- Tolpen, S.; Janmaat, J.; Reider, C.; Kallel, F.; Farrar, D.; May-Newman, K. Programmed Speed Reduction Enables Aortic Valve Opening and Increased Pulsatility in the LVAD-Assisted Heart. ASAIO J. 2015, 61, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Nitta, D.; Hatano, M.; Kinoshita, O.; Nawata, K.; Ono, M. Advantage of Pulsatility in Left Ventricular Reverse Remodeling and Aortic Insufficiency Prevention During Left Ventricular Assist Device Treatment. Circ. J. 2015, 79, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Ising, M.S.; Sobieski, M.A.; Slaughter, M.S.; Koenig, S.C.; Giridharan, G.A. Feasibility of Pump Speed Modulation for Restoring Vascular Pulsatility with Rotary Blood Pumps. ASAIO J. 2015, 61, 526–532. [Google Scholar] [CrossRef]
- Noly, P.-E.; Pagani, F.D.; Noiseux, N.; Stulak, J.M.; Khalpey, Z.; Carrier, M.; Maltais, S. Continuous-Flow Left Ventricular Assist Devices and Valvular Heart Disease: A Comprehensive Review. Can. J. Cardiol. 2020, 36, 244–260. [Google Scholar] [CrossRef]
- Xiao, N.; Alastruey, J.; Figueroa, C.A. A Systematic Comparison between 1-D and 3-D Hemodynamics in Compliant Arterial Models. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 204–231. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Kamensky, D.; Xu, F.; Kiendl, J.; Wang, C.; Wu, M.C.H.; Mineroff, J.; Reali, A.; Bazilevs, Y.; Sacks, M.S. Dynamic and Fluid–Structure Interaction Simulations of Bioprosthetic Heart Valves Using Parametric Design with T-Splines and Fung-Type Material Models. Comput. Mech. 2015, 55, 1211–1225. [Google Scholar] [CrossRef]
- Shroff, G.S.; Ocazionez, D.; Akkanti, B.; Vargas, D.; Garza, A.; Gupta, P.; Patel, J.A.; Patel, M.K.; Gregoric, I.D. CT Imaging of Complications Associated with Continuous-Flow Left Ventricular Assist Devices (LVADs). Semin. Ultrasound. CT MRI 2017, 38, 616–628. [Google Scholar] [CrossRef]
- Menter, F. Zonal Two Equation K-w Turbulence Models For Aerodynamic Flows. In Proceedings of the 23rd Fluid Dynamics, Plasmadynamics, and Lasers Conference, Nashville, TN, USA, 6–8 July 1992; Fluid Dynamics and Co-Located Conferences. American Institute of Aeronautics and Astronautics: Reston, VA, USA, 1993. [Google Scholar]
- Roache, P.J. Quantification of uncertainty in computational fluid dynamics. Annu. Rev. Fluid Mech. 1997, 29, 123–160. [Google Scholar] [CrossRef]
- Celik, I.; Ghia, U.; Roache, P.J.; Freitas, C.; Coloman, H.; Raad, P. Procedure for Estimation and Reporting of Uncertainty Due to Discretization in CFD Applications. J. Fluids Eng. 2008, 130, 078001. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, B.; Yu, C. The Hemodynamic Effects of the LVAD Outflow Cannula Location on the Thrombi Distribution in the Aorta: A Primary Numerical Study. Comput. Methods Programs Biomed. 2016, 133, 217–227. [Google Scholar] [CrossRef]
- Westerhof, N.; Lankhaar, J.-W.; Westerhof, B.E. The Arterial Windkessel. Med. Biol. Eng. Comput. 2009, 47, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Capoccia, M. Development and Characterization of the Arterial Windkessel and Its Role During Left Ventricular Assist Device Assistance. Artif. Organs 2015, 39, E138–E153. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ku, D.N. Pulsatile Flow in the Human Left Coronary Artery Bifurcation: Average Conditions. J. Biomech. Eng. 1996, 118, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Von Spiczak, J.; Crelier, G.; Giese, D.; Kozerke, S.; Maintz, D.; Bunck, A.C. Quantitative Analysis of Vortical Blood Flow in the Thoracic Aorta Using 4D Phase Contrast MRI. PLoS ONE 2015, 10, e0139025. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Hussain, F. On the Identification of a Vortex. J. Fluid Mech. 1995, 285, 69–94. [Google Scholar] [CrossRef]
- Butcher, J.T.; Tressel, S.; Johnson, T.; Turner, D.; Sorescu, G.; Jo, H.; Nerem, R.M. Transcriptional Profiles of Valvular and Vascular Endothelial Cells Reveal Phenotypic Differences. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 69–77. [Google Scholar] [CrossRef]
- Dayawansa, N.H.; Baratchi, S.; Peter, K. Uncoupling the Vicious Cycle of Mechanical Stress and Inflammation in Calcific Aortic Valve Disease. Front. Cardiovasc. Med. 2022, 9, 783543. [Google Scholar] [CrossRef]
- Li, C.; Xu, S.; Gotlieb, A.I. The Progression of Calcific Aortic Valve Disease through Injury, Cell Dysfunction, and Disruptive Biologic and Physical Force Feedback Loops. Cardiovasc. Pathol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- DePaola, N.; Gimbrone, M.A.; Davies, P.F.; Dewey, C.F. Vascular Endothelium Responds to Fluid Shear Stress Gradients. Arterioscler. Thromb. J. Vasc. Biol. 1992, 12, 1254–1257. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Yang, A.-S.; Tseng, L.-Y.; Chai, J.-W. Investigation of Pulsatile Flowfield in Healthy Thoracic Aorta Models. Ann. Biomed. Eng. 2010, 38, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Does Low and Oscillatory Wall Shear Stress Correlate Spatially with Early Atherosclerosis? A Systematic Review. Cardiovasc. Res. 2013, 99, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Fried, J.; Garan, A.R.; Shames, S.; Masoumi, A.; Yuzefpolskaya, M.; Takeda, K.; Takayama, H.; Uriel, N.; Naka, Y.; Colombo, P.C.; et al. Aortic Root Thrombosis in Patients Supported with Continuous-Flow Left Ventricular Assist Devices. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2018, 37, 1425–1432. [Google Scholar] [CrossRef]
- Worthen, G.S.; Smedly, L.A.; Tonnesen, M.G.; Ellis, D.; Voelkel, N.F.; Reeves, J.T.; Henson, P.M. Effects of Shear Stress on Adhesive Interaction between Neutrophils and Cultured Endothelial Cells. J. Appl. Physiol. 1987, 63, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Davies, P.F.; Shi, C.; Depaola, N.; Helmke, B.P.; Polacek, D.C. Hemodynamics and the Focal Origin of Atherosclerosis: A Spatial Approach to Endothelial Structure, Gene Expression, and Function. Ann. N. Y. Acad. Sci. 2001, 947, 7–16; discussion 16–17. [Google Scholar] [CrossRef]
- Numata, S.; Itatani, K.; Kanda, K.; Doi, K.; Yamazaki, S.; Morimoto, K.; Manabe, K.; Ikemoto, K.; Yaku, H. Blood Flow Analysis of the Aortic Arch Using Computational Fluid Dynamics. Eur. J. Cardiothorac. Surg. 2016, 49, 1578–1585. [Google Scholar] [CrossRef]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the Early Lesion of “degenerative” Valvular Aortic Stenosis. Histological and Immunohistochemical Studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef]
- O’Brien, K.D.; Reichenbach, D.D.; Marcovina, S.M.; Kuusisto, J.; Alpers, C.E.; Otto, C.M. Apolipoproteins B, (a), and E Accumulate in the Morphologically Early Lesion of ‘Degenerative’ Valvular Aortic Stenosis. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 523–532. [Google Scholar] [CrossRef]
- Kobsa, S.; Akiyama, K.; Nemeth, S.K.; Kurlansky, P.A.; Naka, Y.; Takeda, K.; Itatani, K.; Werth, E.G.; Brown, L.M.; Ferrari, G.; et al. Correlation between Aortic Valve Protein Levels and Vector Flow Mapping of Wall Shear Stress and Oscillatory Shear Index in Patients Supported with Continuous-Flow Left Ventricular Assist Devices. J. Heart Lung Transplant. 2023, 42, 64–75. [Google Scholar] [CrossRef]
- Itatani, K.; Okada, T.; Uejima, T.; Tanaka, T.; Ono, M.; Miyaji, K.; Takenaka, K. Intraventricular Flow Velocity Vector Visualization Based on the Continuity Equation and Measurements of Vorticity and Wall Shear Stress. Jpn. J. Appl. Phys. 2013, 52, 07HF16. [Google Scholar] [CrossRef]











| Case | Height (cm) | Inclination Angle (Degree) | Azimuthal Angle (Degree) |
|---|---|---|---|
| 2 cm|60°|60° | 2 | 60 | 60 |
| 2 cm|60°|120° | 2 | 60 | 120 |
| 2 cm|90°|60° | 2 | 90 | 60 |
| 2 cm|90°|120° | 2 | 90 | 120 |
| 4 cm|60°|60° | 4 | 60 | 60 |
| 4 cm|60°|120° | 4 | 60 | 120 |
| 4 cm|90°|60° | 4 | 90 | 60 |
| 4 cm|60°|120° | 4 | 90 | 120 |
| Windkessel Resistance | Windkessel Compliance | Windkessel Resistance | |
|---|---|---|---|
| BCA | |||
| LCCA | |||
| LSA | |||
| Descending aorta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Ong, C.W.; Mi, Y.; Seetharaman, A.; Ling, R.R.; Ramanathan, K.; Leo, H.L. The Impact of Left Ventricular Assist Device Outflow Graft Positioning on Aortic Hemodynamics: Improving Flow Dynamics to Mitigate Aortic Insufficiency. Biomimetics 2023, 8, 465. https://doi.org/10.3390/biomimetics8060465
Gu Z, Ong CW, Mi Y, Seetharaman A, Ling RR, Ramanathan K, Leo HL. The Impact of Left Ventricular Assist Device Outflow Graft Positioning on Aortic Hemodynamics: Improving Flow Dynamics to Mitigate Aortic Insufficiency. Biomimetics. 2023; 8(6):465. https://doi.org/10.3390/biomimetics8060465
Chicago/Turabian StyleGu, Zhuohan, Chi Wei Ong, Yongzhen Mi, Ashwin Seetharaman, Ryan Ruiyang Ling, Kollengode Ramanathan, and Hwa Liang Leo. 2023. "The Impact of Left Ventricular Assist Device Outflow Graft Positioning on Aortic Hemodynamics: Improving Flow Dynamics to Mitigate Aortic Insufficiency" Biomimetics 8, no. 6: 465. https://doi.org/10.3390/biomimetics8060465





